Outcomes of MagLev LVAD Support in Patients Requiring Preoperative Continuous Renal Replacement Therapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Preoperative Characteristics of MagLev Patients with vs. Without CRRT
3.2. Peri-Operative Patient Morbidity
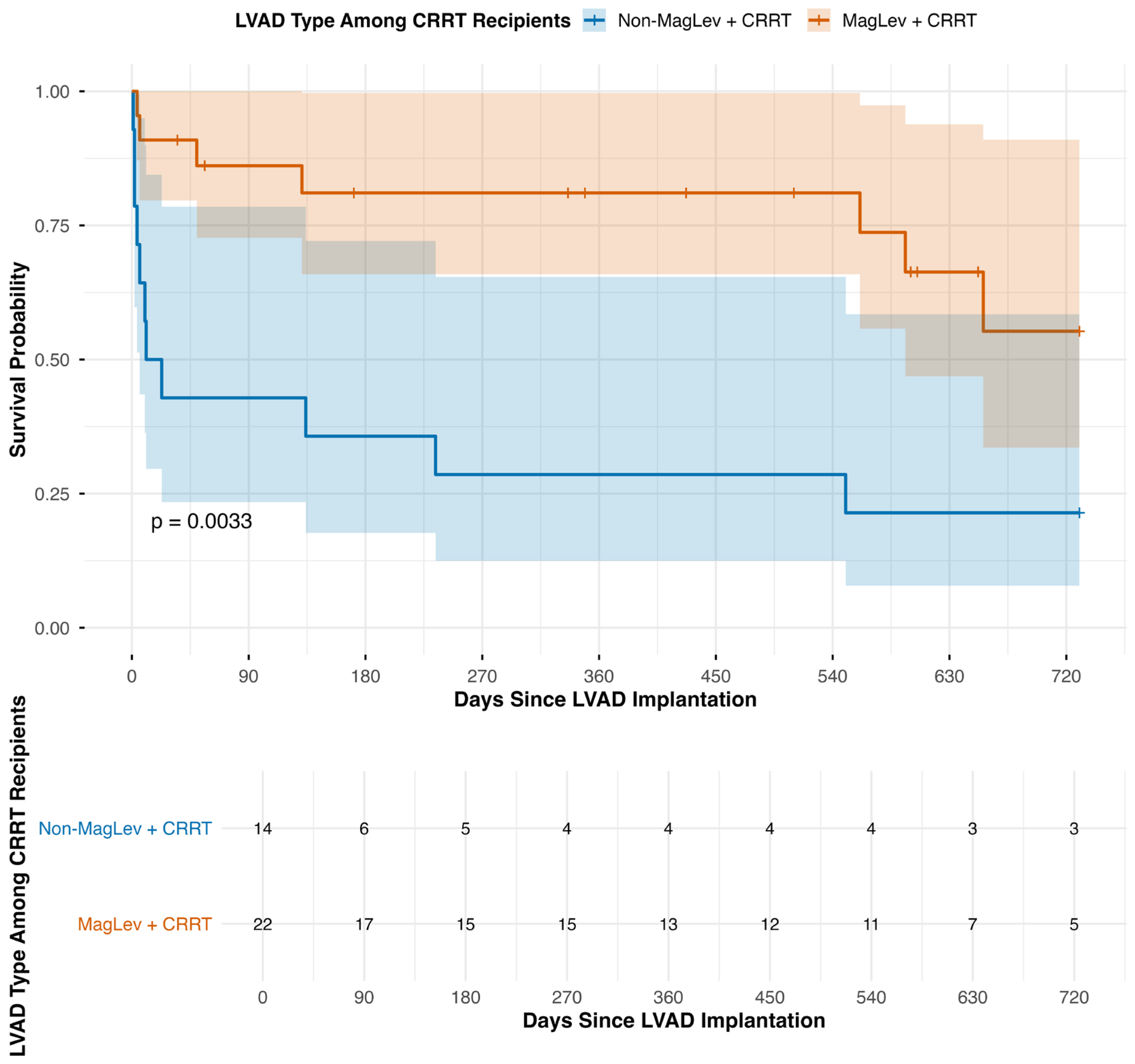
3.3. Long-Term Mortality and Post-Operative Renal Function
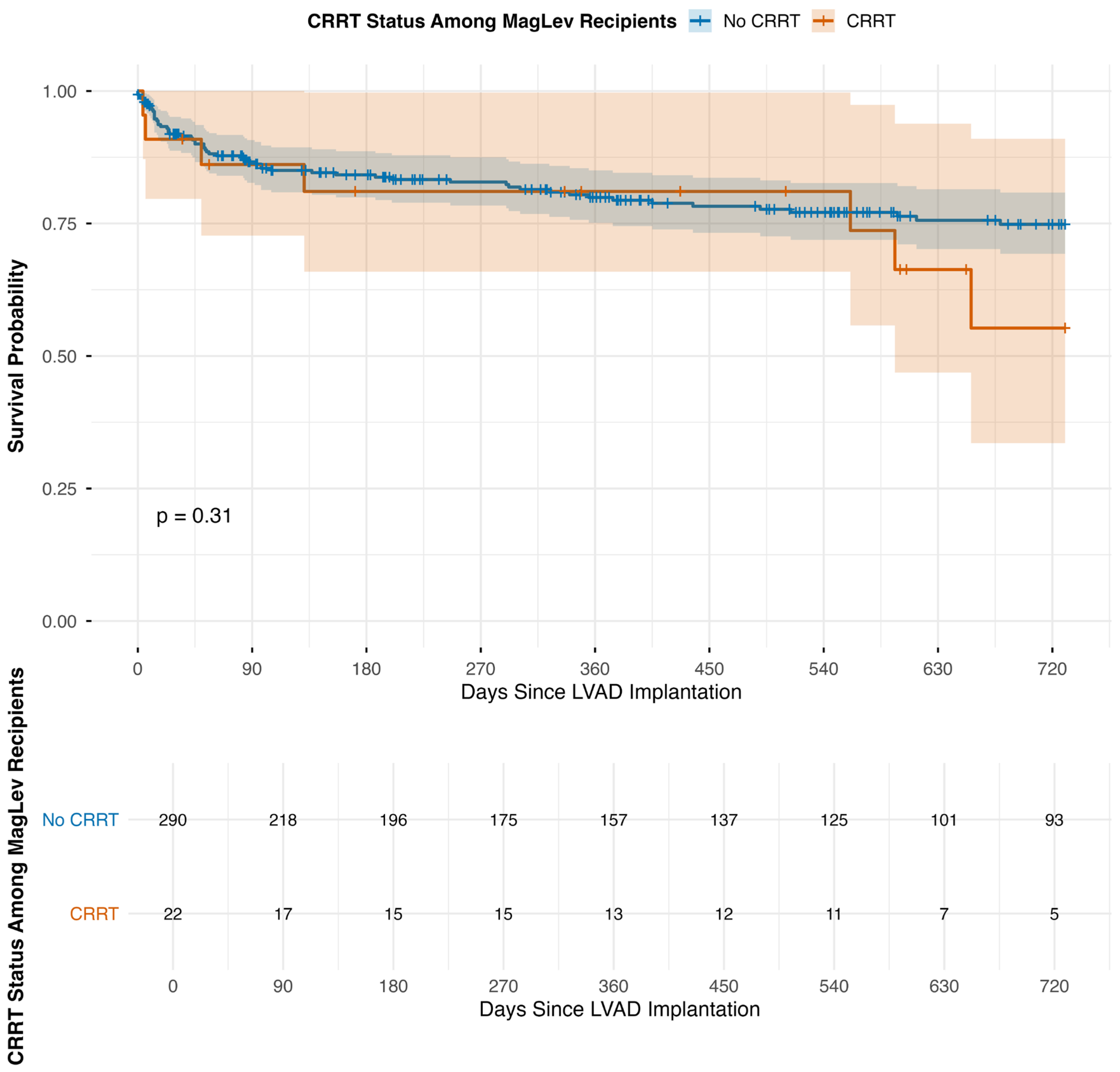
| Group | Total Number of Patients | Deaths at 30 Days | Deaths at 1 Year | Deaths at 2 Years | 30-Day Mortality (Incident/N) (%) | 1-Year Mortality (Incident/N) (%) | 2-Year Mortality (Incident/N) (%) |
|---|---|---|---|---|---|---|---|
| A. MagLev LVAD Patients by CRRT Status | |||||||
| MagLev (Non-CRRT) | 290 | 23 | 52 | 60 | 7.9% | 17.9% | 20.7% |
| MagLev + CRRT | 22 | 2 | 4 | 7 | 9.1% | 18.2% | 31.8% |
| B. All CRRT Recipients by LVAD Type | |||||||
| Non-MagLev + CRRT | 14 | 8 | 10 | 11 | 57.1% | 71.4% | 78.6% |
| MagLev + CRRT | 22 | 2 | 4 | 7 | 8.7% | 17.4% | 30.4% |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSD | Adult Cardiac Surgery Database |
| AKI | Acute Kidney Injury |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CRRT | Continuous renal replacement therapy |
| ECMO | Extracorporeal Membrane Oxygenation |
| eGFR | Estimated glomerular filtration rate |
| IABP | Intra-aortic balloon pump |
| INTERMACS | Interagency Registry for Mechanically Assisted Circulatory Support |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| LVAD | Left ventricular assist device |
| LVEDD | Left ventricular end-diastolic diameter |
| MagLev | Magnetically levitated |
| MCS | Mechanical circulatory support |
| NYHA | New York Heart Association |
| RVAD | Right ventricular assist device |
| RVEF | Right ventricular ejection fraction |
| STS | Society of Thoracic Surgeons |
References
- Mehra, M.R.; Goldstein, D.J.; Uriel, N. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Hailpern, S.M.; Katz, R. Outcomes Associated with Left Ventricular Assist Devices Among Recipients with and Without End-stage Renal Disease. JAMA Intern. Med. 2018, 178, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Roehm, B.; Vest, A.R.; Weiner, D.E. Left Ventricular Assist Devices, Kidney Disease, and Dialysis. Am. J. Kidney Dis. 2018, 71, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Kormos, R.L. Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J. Heart Lung Transplant. 2013, 32, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Lamba, H. Left Ventricular Assist Devices and Renal Ramifications. J. Am. Heart Assoc. 2023, 12, e028450. [Google Scholar] [CrossRef] [PubMed]
- Dalia, T.; Chan, W.C.; Sauer, A.J. Outcomes in Patients with Chronic Kidney Disease and End-stage Renal Disease and Durable Left Ventricular Assist Device: Insights From the United States Renal Data System Database. J. Card. Fail. 2022, 28, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Geisberg, C.; Howser, R. Relationship between renal function and left ventricular assist device use. Ann. Thorac. Surg. 2006, 81, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Sandner, S.E.; Zimpfer, D.; Zrunek, P. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann. Thorac. Surg. 2009, 87, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, M.; Kazory, A. Left Ventricular Assist Device and the Kidney: Getting to the Heart of the Matter. Blood Purif. 2019, 48, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Croix, G.R.; Lacy, S.; Chaparro, S. Impact of Renal Dysfunction on Outcomes after Left Ventricular Assist Device: A Systematic Review. Int. J. Heart Fail. 2021, 3, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Pamboukian, S.V.; Teuteberg, J.J. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J. Heart Lung Transplant. 2013, 32, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Colombo, P.C.; Cleveland, J.C. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation 2017, 135, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Goldstein, D.J.; Cleveland, J.C. Five-Year Outcomes in Patients with Fully Magnetically Levitated vs. Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R. The burden of haemocompatibility with left ventricular assist systems: A complex weave. Eur. Heart J. 2017, 40, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, C.; Tran, D.; George, P. Left ventricular assist device implantation may be feasible in appropriately selected patients with severe renal insufficiency. J. Thorac. Cardiovasc. Surg. 2020, 159, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Shah, P.; Lala, A. INTERMACS profiles and outcomes of ambulatory advanced heart failure patients: A report from the REVIVAL Registry. J. Heart Lung Transplant. 2020, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Lamba, H.K.; Musfee, F.I.; Chatterjee, S. The influence of preoperative dialysis on survival after continuous-flow left ventricular assist device implantation. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, L.; Thamsen, B.; de Zelicourt, D.; Granegger, M.; Boes, S.; Schmid Daners, M.; Meboldt, M.; Kurtcuoglu, V. Fluid Dynamics in the HeartMate 3: Influence of the Artificial Pulse Feature and Residual Cardiac Pulsation. Artif. Organs 2019, 43, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Stohr, E.J.; Ji, R.; Mondellini, G.; Braghieri, L.; Akiyama, K.; Castagna, F.; Pinsino, A.; Cockcroft, J.R.; Silverman, R.H.; Trocio, S.; et al. Pulsatility and flow patterns across macro- and microcirculatory arteries of continuous-flow left ventricular assist device patients. J. Heart Lung Transplant. 2023, 42, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristic | Non-CRRT N = 290 1 | CRRT N = 22 1 | p-Value 2 |
|---|---|---|---|
| Age (years) | 51.56 ± 12.78 | 48.35 ± 14.01 | 0.431 |
| Sex (Male %) | 0.630 | ||
| Female | 83 (29%) | 5 (23%) | |
| Male | 202 (71%) | 17 (77%) | |
| Missing | 5 | 0 | |
| BMI (kg/m2) | 28.65 ± 8.43 | 27.61 ± 5.70 | 0.936 |
| Missing | 0 | 1 | |
| Diabetes Mellitus | 127 (45%) | 7 (32%) | 0.273 |
| Missing | 5 | 0 | |
| Hypertension | 222 (78%) | 15 (68%) | 0.297 |
| Missing | 5 | 0 | |
| Chronic Lung Disease | 0.037 | ||
| No | 143 (49%) | 10 (45%) | |
| Mild/Moderate | 110 (38%) | 6 (27%) | |
| Severe | 22 (7.6%) | 6 (27%) | |
| Other/Unknown | 15 (5.2%) | 0 (0%) | |
| Cerebrovascular Disease | 81 (28%) | 7 (32%) | 0.807 |
| Missing | 5 | 0 | |
| Peripheral Arterial Disease | 12 (4.2%) | 3 (14%) | 0.083 |
| Missing | 5 | 0 | |
| Immunocompromised State | 16 (5.6%) | 3 (14%) | 0.146 |
| Missing | 5 | 0 | |
| Renal Characteristics | |||
| Creatinine (mg/dL) * | 1.65 ± 0.96 | 1.95 ± 0.85 | 0.032 |
| BUN (mmol/L) | 11.76 ± 6.80 | 8.50 ± 6.51 | 0.005 |
| Missing | 0 | 1 | |
| eGFR (mL/min/1.73 m2) | 53 (38, 76) | 40 (32, 66) | 0.074 |
| eGFR Category (mL/min/1.73 m2) | 0.2 | ||
| ≥60 | 118 (41%) | 7 (32%) | |
| 45–59 | 63 (22%) | 2 (9.1%) | |
| 30–44 | 68 (24%) | 9 (41%) | |
| <30 | 36 (13%) | 4 (18%) | |
| Unknown | 5 | 0 | |
| Cardiac Characteristics | |||
| Heart Failure Etiology | 0.115 | ||
| Congenital/Other | 3 (1.0%) | 0 (0%) | |
| Ischemic | 60 (21%) | 5 (23%) | |
| Non-ischemic | 218 (75%) | 14 (64%) | |
| Other Cardiomyopathy | 4 (1.4%) | 1 (4.5%) | |
| Unknown | 5 (1.7%) | 2 (9.1%) | |
| NYHA Class III or IV | 0.067 | ||
| No | 19 (6.6%) | 4 (18%) | |
| Yes | 271 (93%) | 18 (82%) | |
| INTERMACS Profile 1–2 | 0.019 | ||
| No | 83 (29%) | 1 (4.8%) | |
| Yes | 207 (71%) | 20 (95%) | |
| Missing | 0 | 1 | |
| LVEDD (cm) | 6.80 ± 1.13 | 6.33 ± 0.91 | 0.020 |
| Missing | 1 | 1 | |
| RVEF Group | 0.662 | ||
| Normal | 51 (18%) | 2 (9.1%) | |
| Mild/Moderate | 156 (54%) | 12 (55%) | |
| Severe | 38 (13%) | 3 (14%) | |
| Unknown | 45 (16%) | 5 (23%) | |
| Cardiac Output (L/min) | 4.02 ± 1.19 | 5.02 ± 1.74 | 0.011 |
| Missing | 32 | 2 | |
| Cardiac Arrhythmia | 0.372 | ||
| No | 94 (32%) | 8 (36%) | |
| Yes | 132 (46%) | 12 (55%) | |
| Unknown | 64 (22%) | 2 (9.1%) | |
| Resuscitation ≤ 1 h | 0.312 | ||
| No | 281 (99%) | 21 (95%) | |
| Yes—Within 1 h of the start of the procedure | 4 (1.4%) | 1 (4.5%) | |
| Missing | 5 | 0 | |
| Preoperative MCS | |||
| ECMO | 16 (5.5%) | 5 (24%) | 0.009 |
| Missing | 0 | 1 | |
| IABP | 34 (12%) | 4 (19%) | 0.304 |
| Missing | 0 | 1 | |
| RVAD | 3 (1.0%) | 1 (4.8%) | 0.245 |
| Missing | 0 | 1 | |
| LVAD Planned Strategy | 0.083 | ||
| Bridge to Transplant | 88 (30%) | 7 (32%) | |
| Destination Therapy | 202 (70%) | 14 (64%) | |
| Other/Unknown | 0 (0%) | 1 (4.5%) |
| (A) MagLev LVADs: CRRT vs. Non-CRRT | |||
| Outcome | Non-CRRT N = 290 1 | CRRT N = 22 1 | p-Value 2 |
| Stroke | 13 (5.8%) | 1 (5.3%) | >0.999 |
| Reoperation due to bleeding | 19 (8.5%) | 1 (5.3%) | >0.999 |
| Stage 3 AKI * | 58 (26%) | — | — |
| Mechanical ventilation > 24 h | 133 (60%) | 14 (74%) | 0.328 |
| Postoperative Dialysis Requirement | 44 (76%) | 2 (67%) | >0.999 |
| (B) CRRT Cohort: MagLev vs. Non-MagLev | |||
| Outcome | Non-MagLev N = 14 1 | MagLev N = 22 1 | p-Value 2 |
| Stroke | 1 (11%) | 1 (5.3%) | >0.999 |
| Reoperation due to bleeding | 2 (22%) | 1 (5.3%) | 0.234 |
| Stage 3 AKI * | 0 (0%) | — | — |
| Mechanical ventilation > 24 h | 6 (67%) | 14 (74%) | >0.999 |
| Postoperative Dialysis Requirement | 0 (0%) | 2 (67%) | >0.999 |
| Variable | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Preoperative CRRT | 1.22 (0.50–2.95) | 0.6605 |
| INTERMACS 1–2 | 1.71 (0.92–3.16) | 0.0885 |
| Preoperative ECMO | 0.96 (0.34–2.70) | 0.9427 |
| Preoperative Creatinine | 0.59 (0.35–0.99) | 0.0448 |
| BUN (mmol/L) | 1.05 (0.99–1.11) | 0.0790 |
| LVEDD (cm) | 0.87 (0.69–1.10) | 0.2382 |
| Cardiac Output (L/min) | 1.17 (0.97–1.41) | 0.1076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.L.; Rust, C.J.; Kusher, I.M.; El Sammak, S.; Tang, A.; Preston, J.D.; Randhawa, S.S.; Halkos, M.E.; Bishawi, M.M.; Daneshmand, M.A.; et al. Outcomes of MagLev LVAD Support in Patients Requiring Preoperative Continuous Renal Replacement Therapy. J. Clin. Med. 2025, 14, 8502. https://doi.org/10.3390/jcm14238502
He CL, Rust CJ, Kusher IM, El Sammak S, Tang A, Preston JD, Randhawa SS, Halkos ME, Bishawi MM, Daneshmand MA, et al. Outcomes of MagLev LVAD Support in Patients Requiring Preoperative Continuous Renal Replacement Therapy. Journal of Clinical Medicine. 2025; 14(23):8502. https://doi.org/10.3390/jcm14238502
Chicago/Turabian StyleHe, Christopher L., Clayton J. Rust, Ian M. Kusher, Sally El Sammak, Ailin Tang, Joshua D. Preston, Supreet S. Randhawa, Michael E. Halkos, Muath M. Bishawi, Mani A. Daneshmand, and et al. 2025. "Outcomes of MagLev LVAD Support in Patients Requiring Preoperative Continuous Renal Replacement Therapy" Journal of Clinical Medicine 14, no. 23: 8502. https://doi.org/10.3390/jcm14238502
APA StyleHe, C. L., Rust, C. J., Kusher, I. M., El Sammak, S., Tang, A., Preston, J. D., Randhawa, S. S., Halkos, M. E., Bishawi, M. M., Daneshmand, M. A., & Chan, J. L. (2025). Outcomes of MagLev LVAD Support in Patients Requiring Preoperative Continuous Renal Replacement Therapy. Journal of Clinical Medicine, 14(23), 8502. https://doi.org/10.3390/jcm14238502






