Linking Thyroid Function, Morphology, Autoimmunity, Body Mass Index, and Reproductive Aging to Women’s Sexual Health: Evidence from a Population Study in Kaunas
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical and Sociodemographic Variables
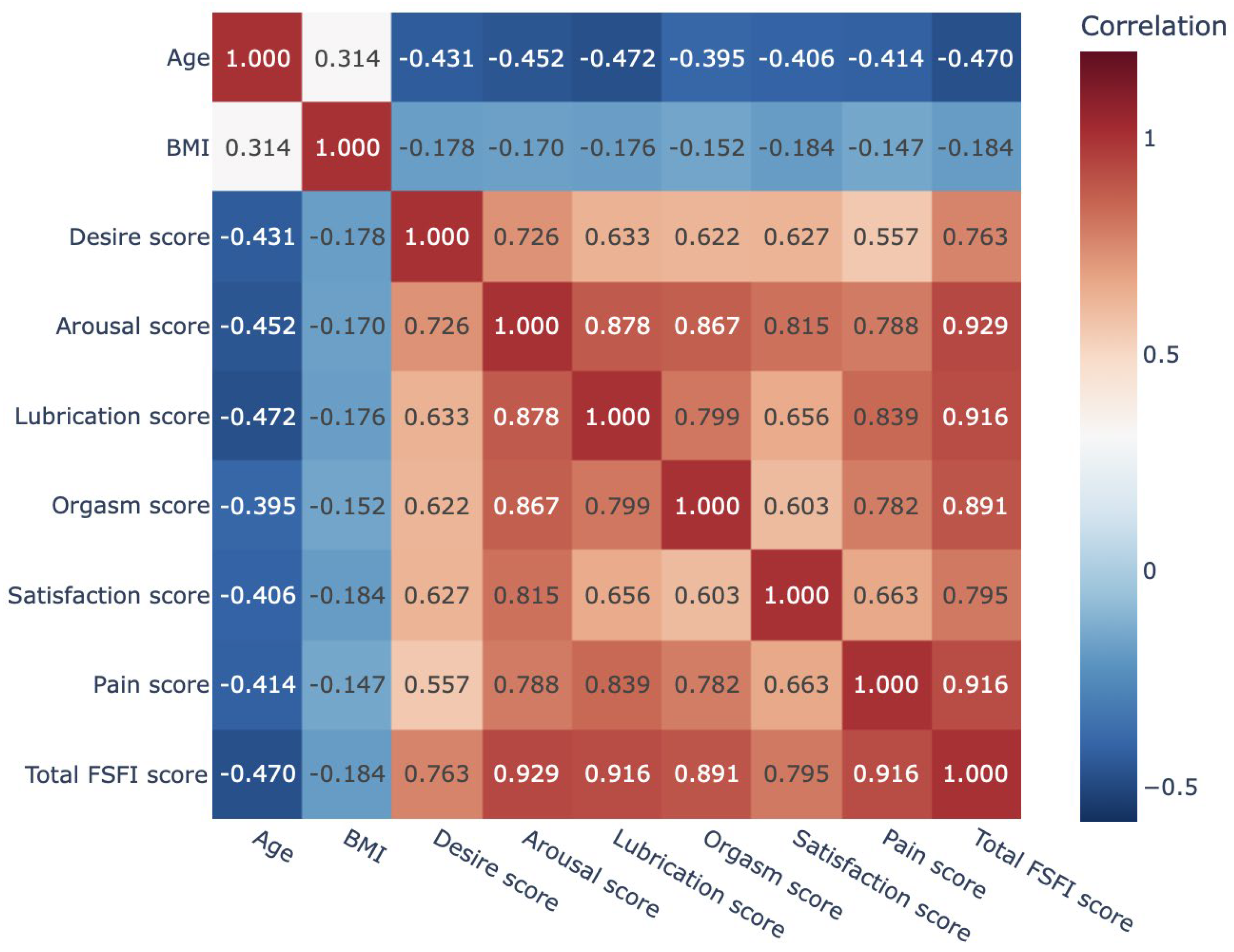
3.2. Analysis of Female Sexual Function Index (FSFI) Domain Scores and Their Associations with Age and Body Mass Index
3.3. Association of Thyroid Function and Morphology Characteristics with Sexual Dysfunction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Anti-TPO | anti-thyroid peroxidase antibodies |
| HT | Hashimoto’s thyroiditis |
| BMI | body mass index |
| FSFI | Female Sexual Function Index |
| fT4 | free thyroxine |
| SD | sexual dysfunction |
| TSH | thyroid-stimulating hormone |
References
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R., Jr. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital. Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Ghodusi, M.; Rezaei, P.; Kabirian Abyaneh, S.; Sureshjani, E.H.; Sheikhi, R.A. Sexual Function and Factors Affecting Menopause: A Systematic Review. J. Menopausal Med. 2019, 25, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Jonusiene, G.; Zilaitiene, B.; Adomaitiene, V.; Aniuliene, R.; Bancroft, J. Sexual function, mood and menopause symptoms in Lithuanian postmenopausal women. Climacteric 2013, 16, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hasheminezhad, R.; Sedighi, T.; Zarei, H.; Shohaimi, S.; Mohammadi, M. The global prevalence of sexual dysfunction in obese and overweight women: A systematic review and meta-analysis. BMC Women’s Health 2023, 23, 375. [Google Scholar] [CrossRef]
- Di Nardo, M.; Conti, C.; Di Francesco, G.; Nicolardi, G.; Guagnano, M.T.; Porcelli, P. What is the “weight” of body mass index on sexual functioning in women? A mediation model. Eat. Weight. Disord. 2021, 26, 1801–1811. [Google Scholar] [CrossRef]
- Sato, Y.; Ozaki, Y.; Tomoe, H.; Ninomiya, N.; Sekiguchi, Y.; Yamamoto, M.; Takahashi, S. Cross-sectional study of the association between regular sexual activity and sexual function and genitourinary syndrome of menopause-related symptoms. Menopause 2025, 32, 592–600. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H. Effects of Hypothyroidism and Subclinical Hypothyroidism on Sexual Function: A Meta-Analysis of Studies Using the Female Sexual Function Index. Sex. Med. 2020, 8, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bortun, A.C.; Ivan, V.; Navolan, D.B.; Dehelean, L.; Borlea, A.; Stoian, D. Thyroid Autoimmune Disease—Impact on Sexual Function in Young Women. J. Clin. Med. 2021, 10, 369. [Google Scholar] [CrossRef]
- Gabrielson, A.T.; Sartor, R.A.; Hellstrom, W.J.G. The Impact of Thyroid Disease on Sexual Dysfunction in Men and Women. Sex. Med. Rev. 2019, 7, 57–70. [Google Scholar] [CrossRef]
- Pasquali, D.; Maiorino, M.I.; Renzullo, A.; Bellastella, G.; Accardo, G.; Esposito, D.; Barbato, F.; Esposito, K. Female sexual dysfunction in women with thyroid disorders. J. Endocrinol. Investig. 2013, 36, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Heidarian, P.; Jalili, F.; Babajani, F.; Shohaimi, S.; Nasirian, M.; Mohammadi, M. The sexual dysfunction in women with thyroid disorders: A meta-analysis. BMC Endocr. Disord. 2024, 24, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krysiak, R.; Drosdzol-Cop, A.; Skrzypulec-Plinta, V.; Okopien, B. Sexual function and depressive symptoms in young women with thyroid autoimmunity and subclinical hypothyroidism. Clin. Endocrinol. 2016, 84, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Sexual Function and Depressive Symptoms in Young Women with Euthyroid Hashimoto’s Thyroiditis Receiving Vitamin D, Selenomethionine and Myo-Inositol: A Pilot Study. Nutrients 2023, 15, 2815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbagallo, F.; Cannarella, R.; Condorelli, R.A.; Cucinella, L.; La Vignera, S.; Nappi, R.E.; Calogero, A.E. Thyroid diseases and female sexual dysfunctions. Sex. Med. Rev. 2024, 12, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Oppo, A.; Franceschi, E.; Atzeni, F.; Taberlet, A.; Mariotti, S. Effects of hyperthyroidism, hypothyroidism, and thyroid autoimmunity on female sexual function. J. Endocrinol. Investig. 2011, 34, 449–453. [Google Scholar] [CrossRef]
- Luo, H.; Yang, H.; Zhao, W.; Han, Q.; Zeng, L.; Tang, H.; Zhu, J. Elevated free triiodothyronine may lead to female sexual dysfunction in Chinese urban women: A hospital-based survey. Sci. Rep. 2017, 7, 1216. [Google Scholar] [CrossRef]
- WHO MONICA Project. WHO MONICA Project: Objectives and Design. Int. J. Epidemiol. 1989, 18, S29–S37. [Google Scholar] [CrossRef]
- WHO MONICA Project. Available online: https://iris.who.int/handle/10665/48342 (accessed on 5 June 2018).
- Wiegel, M.; Meston, C.; Rosen, R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. J. Sex Marital. Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Cronbach’s alpha. BMJ 1997, 314, 572. [Google Scholar] [CrossRef]
- World Health Organization. Indicators for Assessing Iodine Deficiency Disorders and Their Control Through Salt Iodization; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Jackson-Koku, G. Beck Depression Inventory. Occup. Med. 2016, 66, 174–175. [Google Scholar] [CrossRef]
- Bieling, P.J.; Antony, M.M.; Swinson, R.P. The State-Trait Anxiety Inventory, Trait version: Structure and content re-examined. Behav. Res. Ther. 1998, 36, 777–788. [Google Scholar] [CrossRef]
- Harris, K.M.; Gaffey, A.E.; Schwartz, J.E.; Krantz, D.S.; Burg, M.M. The Perceived Stress Scale as a Measure of Stress: Decomposing Score Variance in Longitudinal Behavioral Medicine Studies. Ann. Behav. Med. 2023, 57, 846–854. [Google Scholar] [CrossRef]
- Cuenca Montesino, M.L.; Graña Gómez, J.L.; Peña Fernández, M.E.; Andreu Rodríguez, J.M. Psychometric properties of the Dyadic Adjustment Scale (DAS) in a community sample of couples. Psicothema 2013, 25, 536–541. [Google Scholar] [CrossRef]
- Cea García, J.; Márquez Maraver, F.; Rubio Rodríguez, M.C. Cross-sectional study on the impact of age, menopause and quality of life on female sexual function. J. Obstet. Gynaecol. 2022, 42, 1225–1232. [Google Scholar] [CrossRef]
- Bond, D.S.; Vithiananthan, S.; Leahey, T.M.; Thomas, J.G.; Sax, H.C.; Pohl, D.; Ryder, B.A.; Roye, G.D.; Giovanni, J.; Wing, R.R. Prevalence and degree of sexual dysfunction in a sample of women seeking bariatric surgery. Surg. Obes. Relat. Dis. 2009, 5, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Javadifar, N.; Pargar, F.; Musavi, P.; Haghighizadeh, M.H. Comparing Sexual Function in Females of Reproductive Age Referred to Rural and Urban Healthcare Centers in Ahvaz, Iran. Jundishapur J. Chronic Dis. Care 2016, 5, e37038. [Google Scholar] [CrossRef]
- Fabricio, A.M.F.; Sato, T.O.; Gomes da Silva, S.; Poli, G.G.; de Araujo Silva, C.M.; Padovez, R.F.C.M.; Rodrigues de Souza, D.P.; Driusso, P.; Beleza, A.C.S. Prevalence and factors associated with sexual dysfunction in brazilian women: A cross-sectional study. Int. Urogynecol. J. 2023, 34, 2507–2511. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Thu, W.P.P.; Ho, K.; Cauley, J.A.; Kramer, M.S.; Yong, E.L. Sexual inactivity and sexual dysfunction in midlife Singaporean women: A prospective cross-sectional study of prevalence and risk factors. Maturitas 2021, 152, 1–9. [Google Scholar] [CrossRef]
- Merghati-Khoei, E.; Sheikhan, F.; Shamsalizadeh, N.; Haghani, H.; Yousofnia Pasha, Y.R.; Killeen, T. Menopause negatively impacts sexual lives of middle-aged Iranian women: A cross-sectional study. J. Sex Marital. Ther. 2014, 40, 552–560. [Google Scholar] [CrossRef]
- Beigi, M.; Fahami, F. A Comparative study on sexual dysfunctions before and after menopause. Iran. J. Nurs. Midwifery Res. 2012, 17 (Suppl. S1), S72–S75. [Google Scholar]
- Salari, N.; Moradi, M.; Hosseinian-Far, A.; Khodayari, Y.; Mohammadi, M. Global prevalence of sexual dysfunction among women with metabolic syndrome: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2023, 22, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Fairbanks, F.; Kuhle, C.L.; Sood, R.; Kling, J.M.; Vencill, J.A.; Mara, K.C.; Kapoor, E. Association Between Body Mass Index and Female Sexual Dysfunction: A Cross-sectional Study from the Data Registry on Experiences of Aging, Menopause, and Sexuality. J. Sex. Med. 2020, 17, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Zakauskiene, U.; Macioniene, E.; Zabuliene, L.; Sukackiene, D.; Linkeviciute-Dumce, A.; Banys, V.; Bratcikoviene, N.; Karosiene, D.; Slekiene, V.; Kontrimas, V.; et al. Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania. Nutrients 2022, 14, 3817. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Rzeszutek, M.; Pięta, M.; Van Hoy, A.; Zawistowska, M.; Grymowicz, M.; Pięta, W.; Gołoś, S.; Walicka, M. Coping profiles, depression, and body image anxiety during the COVID-19 pandemic: Comparative analysis of females with thyroid diseases and a non-clinical sample. PLoS ONE 2023, 18, e0282302. [Google Scholar] [CrossRef]
- Brinch, F.A.; Døssing, H.; Nguyen, N.; Bonnema, S.J.; Hegedüs, L.; Godballe, C.; Sorensen, J.R. The Impact of Esophageal Compression on Goiter Symptoms before and after Thyroid Surgery. Eur. Thyroid J. 2019, 8, 16–23. [Google Scholar] [CrossRef]
- Alkabban, F.M.; Patel, B.C. Nontoxic Goiter. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482274/ (accessed on 15 October 2020).
- Carosa, E.; Sansone, A.; Jannini, E.A. Management of endocrine disease: Female sexual dysfunction for the endocrinologist. Eur. J. Endocrinol. 2020, 182, R101–R116. [Google Scholar] [CrossRef] [PubMed]
| Variable | Category/Description | n | % | Median (IQR) |
|---|---|---|---|---|
| Age (years) | 59 (7) | |||
| Age at menopause onset (years) | 50 (6) | |||
| BMI (kg/m2) BMI categories | 27.15 (6.86) | |||
| Underweight | 29 | 1.8 | ||
| Normal weight | 642 | 40.9 | ||
| Overweight | 498 | 31.7 | ||
| Class I obesity | 262 | 16.7 | ||
| Class II obesity | 104 | 6.6 | ||
| Class III obesity | 34 | 2.2 | ||
| Menopause status | Menopausal | 824 | 53.4 | |
| Premenopausal | 720 | 46.6 | ||
| Marital status | Married | 975 | 62.3 | |
| Single | 168 | 10.7 | ||
| Divorced | 233 | 14.9 | ||
| Cohabiting | 86 | 6.6 | ||
| Widowed | 103 | 5.5 | ||
| Thyroid function | Euthyroidism | 1223 | 96.9 | |
| Hypothyroidism | 25 | 2.0 | ||
| Hyperthyroidism | 14 | 1.1 | ||
| Thyroid morphology | Nodule(s) | 748 | 49.9 | |
| No nodule(s) | 752 | 50.1 | ||
| Autoimmunity | HT | 444 | 28.4 | |
| no HT | 1117 | 71.6 |
| FSFI Domain | Median (IQR) | SD |
|---|---|---|
| Desire | 3.00 (1.8) | - |
| Arousal | 3.30 (3.9) | - |
| Lubrication | 4.20 (5.7) | - |
| Orgasm | 3.60 (5.2) | - |
| Satisfaction | 4.00 (3.2) | - |
| Pain | 4.40 (6.0) | - |
| Total FSFI | 23.30 (24) | 64.10% |
| Characteristic | HT Group (n = 444) | Reference Group (n = 1117) | p-Value |
|---|---|---|---|
| Age (years) | 54 (18) | 51 (19) | 0.001 |
| Menopause (yes), n (%) | 262 (59.7) | 559 (50.9) | 0.002 |
| BMI (kg/m2) | 27.3 (7.82) | 25.53 (6.97) | <0.001 |
| Thyroid volume (mL) | 8.6 (5.82) | 8.71 (5.67) | 0.455 |
| TSH (μIU/mL) | 1.9 (1.47) | 1.41 (0.84) | <0.001 |
| FT4 (ng/dL) | 1.0 (0.21) | 0.98 (0.2) | 0.183 |
| Goiter n (%) | 31 (7.2) | 64 (6.0) | 0.377 |
| Thyroid nodules n (%) | 180 (40.5) | 568 (50.9) | <0.001 |
| Thyroid function: | |||
| Euthyroidism, n (%) | 332 (93.5) | 891 (98.2) | <0.001 |
| Hypothyroidism, n (%) | 18 (5.1) | 7 (0.8) | 0.028 |
| Hyperthyroidism, n (%) | 5 (1.4) | 9 (1.0) | 0.285 |
| FSFI Domain | HT Group (n = 444) | Reference Group (n = 1117) | Total n = 1561 | p-Value |
|---|---|---|---|---|
| Desire | 3.0 (1.8) | 3.0 (1.8) | 3.0 (1.8) | 0.119 |
| Arousal | 3.3 (3.6) | 3.3 (3.9) | 3.3 (3.9) | 0.100 |
| Lubrication | 3.9 (5.4) | 4.2 (5.7) | 4.2 (5.7) | 0.019 |
| Orgasm | 3.6 (4.8) | 3.6 (5.2) | 3.6 (5.2) | 0.397 |
| Satisfaction | 4.0 (3.0) | 4.0 (3.2) | 4.0 (3.2) | 0.414 |
| Pain | 4.0 (6.0) | 4.8 (6.0) | 4.4 (6.0) | 0.046 |
| Total FSFI | 21.5 (24) | 23.1 (25) | 22.5 (24) | 0.076 |
| SD (FSFI ≤ 26.55) n (%) | 312 (71.7) | 693 (64.2) | 1006 (64.1) | <0.001 |
| Thyroid Factor | SD (+) (FSFI ≤ 26.55) n (%) | SD (−) (FSFI > 26.55) n (%) | p-Value | OR (95% CI) | |
|---|---|---|---|---|---|
| HT | Yes No | 312 (71.7) 693 (64.2) | 123 (28.3) 387 (35.8) | 0.005 | 1.42 (1.11–1.81) |
| Goiter | Yes No | 73 (80.2) 911 (65.3) | 18 (19.8) 484 (34.7) | 0.004 | 2.16 (1.27–3.65) |
| Thyroid nodules | Yes No | 517 (69.4) 489 (63.3) | 228 (30.6) 284 (36.7) | 0.012 | 1.32 (1.06–1.63) |
| Risk Factor | Unadjusted OR (95% CI) * | p-Value | Adjusted OR (95% CI) * | p-Value |
|---|---|---|---|---|
| HT | 1.5 (1.17–1.92) | 0.001 | 1.29 (0.98–1.68) | 0.065 |
| Goiter | 2.0 (1.17–3.38) | 0.011 | 1.82 (1.03–3.2) | 0.040 |
| Thyroid nodules | 1.35 (1.09–1.69) | 0.007 | 0.81 (0.63–1.04) | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daukšienė, D.; Klimaitė, R.; Kondrotienė, A.; Matukaitienė, R.; Čeponis, J.; Rimkutė, A.; Dudonytė, L.; Steponavičiūtė, R.; Lukšienė, D.; Lesauskaitė, V.; et al. Linking Thyroid Function, Morphology, Autoimmunity, Body Mass Index, and Reproductive Aging to Women’s Sexual Health: Evidence from a Population Study in Kaunas. J. Clin. Med. 2025, 14, 8441. https://doi.org/10.3390/jcm14238441
Daukšienė D, Klimaitė R, Kondrotienė A, Matukaitienė R, Čeponis J, Rimkutė A, Dudonytė L, Steponavičiūtė R, Lukšienė D, Lesauskaitė V, et al. Linking Thyroid Function, Morphology, Autoimmunity, Body Mass Index, and Reproductive Aging to Women’s Sexual Health: Evidence from a Population Study in Kaunas. Journal of Clinical Medicine. 2025; 14(23):8441. https://doi.org/10.3390/jcm14238441
Chicago/Turabian StyleDaukšienė, Dalia, Raimonda Klimaitė, Aistė Kondrotienė, Radvilė Matukaitienė, Jonas Čeponis, Agnė Rimkutė, Laura Dudonytė, Rasa Steponavičiūtė, Dalia Lukšienė, Vaiva Lesauskaitė, and et al. 2025. "Linking Thyroid Function, Morphology, Autoimmunity, Body Mass Index, and Reproductive Aging to Women’s Sexual Health: Evidence from a Population Study in Kaunas" Journal of Clinical Medicine 14, no. 23: 8441. https://doi.org/10.3390/jcm14238441
APA StyleDaukšienė, D., Klimaitė, R., Kondrotienė, A., Matukaitienė, R., Čeponis, J., Rimkutė, A., Dudonytė, L., Steponavičiūtė, R., Lukšienė, D., Lesauskaitė, V., Veličkienė, D., Verkauskienė, R., & Žilaitienė, B. (2025). Linking Thyroid Function, Morphology, Autoimmunity, Body Mass Index, and Reproductive Aging to Women’s Sexual Health: Evidence from a Population Study in Kaunas. Journal of Clinical Medicine, 14(23), 8441. https://doi.org/10.3390/jcm14238441






