Long-Term Biochemical and Cardiovascular Profiles 3–6 Years After Preeclampsia: Impact of Angiogenic Imbalance During Pregnancy
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
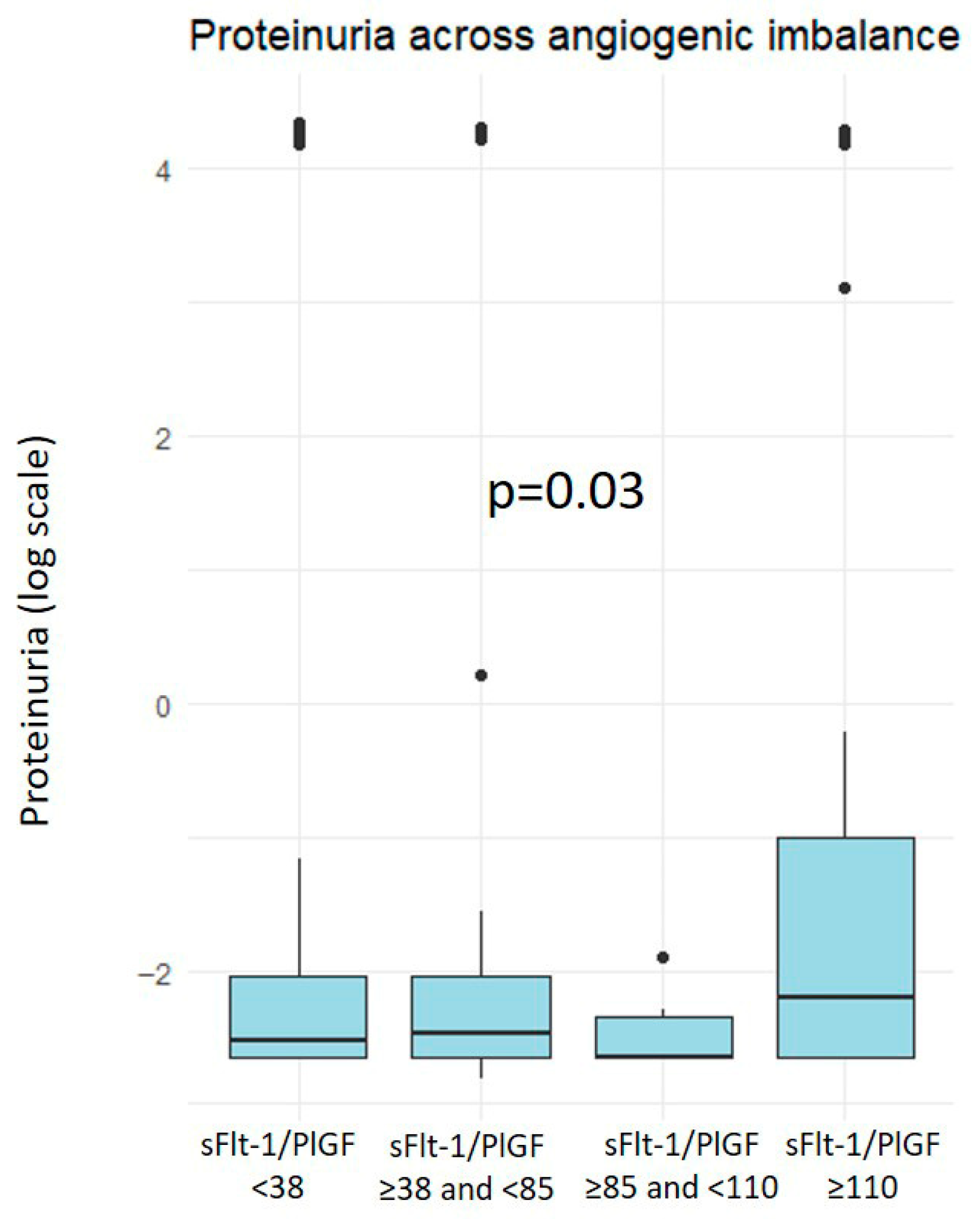
3. Results
4. Discussion
4.1. Main Findings
4.2. Comparison with Other Studies
4.3. Clinical Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| sFlt-1 | Soluble fms-like tyrosine kinase-1 |
| PlGF | Placental growth factor |
| TSH | Thyroid stimulating hormone |
| NT-proBNP | N-terminal pro B-type natriuretic peptide |
| Hs-TnT | High sensitivity Troponin T |
| IQRs | Interquartile ranges |
| STROBE | Strengthening the reporting of observational studies in epidemiology |
| BMI | Body mass index |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| LDL | Low-density lipoprotein |
| LDH | Lactate dehydrogenase |
| VLDL | Very low-density lipoprotein |
| HDL | High-density lipoprotein |
| sVCAM-1 | Soluble vascular cell adhesion molecule 1 |
| hsCRP | High sensitivity C-reactive protein |
| Hs-TNI | High sensitivity Troponin I |
References
- Countouris, M.E.; Bello, N.A. Advances in Our Understanding of Cardiovascular Diseases After Preeclampsia. Circ. Res. 2025, 136, 583–593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obs. Gynecol. 2022, 226, S786–S803. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Verlohren, S.; Brennecke, S.; Galindo, A.; Karumanchi, S.; Mirković, L.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2021, 27, 42–50. [Google Scholar] [CrossRef]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obs. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Minissian, M.B.; Wei, J.; Saade, G.R.; Smith, G.N. Contemporary clinical updates on the prevention of future cardiovascular disease in women who experience adverse pregnancy outcomes. Clin. Cardiol. 2020, 43, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Costa, M.L.; Dechend, R.; Jacobsen, D.P.; Sugulle, M. Hypertensive disorders of pregnancy and long-term maternal cardiovascular risk: Bridging epidemiological knowledge into personalized postpartum care and follow-up. Pregnancy Hypertens. 2024, 36, 101127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manau, P.; Platero, J.; Costa, N.; Garcia, Z.; Garrido-Giménez, C.; Pellicer, C.; Ullmo, J.; Nan, M.; Mora, J.; Garcia-Osuna, A.; et al. Ophthalmic Artery Doppler and Carotid Intima-Media Thickness 3 to 6 Years Postpartum in Women With and Without a History of Placental Insufficiency. Acta Obs. Gynecol. Scand. 2025. [Google Scholar] [CrossRef]
- Benschop, L.; Schalekamp-Timmermans, S.; Broere-Brown, Z.A.; van Lennep, J.E.R.; Jaddoe, V.W.; Roos-Hesselink, J.W.; Ikram, M.K.; Steegers, E.A.; Roberts, J.M.; Gandley, R.E. Placental Growth Factor as an Indicator of Maternal Cardiovascular Risk After Pregnancy. Circulation 2019, 139, 1698–1709. [Google Scholar] [CrossRef]
- Garrido-Gimenez, C.; Mendoza, M.; Cruz-Lemini, M.; Galian-Gay, L.; Sanchez-Garcia, O.; Granato, C.; Rodriguez-Sureda, V.; Rodriguez-Palomares, J.; Carreras-Moratonas, E.; Cabero-Roura, L.; et al. Angiogenic Factors and Long-Term Cardiovascular Risk in Women That Developed Preeclampsia During Pregnancy. Hypertension 2020, 76, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Gascon, M.; Bustamante, M.; Rivas, I.; Foraster, M.; Basagaña, X.; Cosín, M.; Eixarch, E.; Ferrer, M.; Gratacós, E.; et al. Cohort Profile: Barcelona Life Study Cohort (BiSC). Int. J. Epidemiol. 2024, 53, dyae063. [Google Scholar] [CrossRef] [PubMed]
- Ullmo, J.; Cruz-Lemini, M.; Sánchez-García, O.; Bos-Real, L.; De La Llama, P.F.; Calero, F.; Domínguez-Gallardo, C.; Garrido-Gimenez, C.; Trilla, C.; Carreras-Costa, F.; et al. Cardiac dysfunction and remodeling regulated by anti-angiogenic environment in patients with preeclampsia: The ANGIOCOR prospective cohort study protocol. BMC Pregnancy Childbirth 2021, 21, 816. [Google Scholar] [CrossRef]
- Fundació Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau. Randomizated Open-Label Control Trial to Evaluate If the Incorporation of sFlt1/PlGF Ratio in the Diagnosis and Classification of PE Improves Maternal and Perinatal Outcomes in Women With the Suspicion of the Disease (EuroPE Study). Available online: https://clinicaltrials.gov/study/NCT03231657 (accessed on 4 May 2025).
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obs. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Alonso-Ventura, V.; Li, Y.; Pasupuleti, V.; Roman, Y.M.; Hernandez, A.V.; Pérez-López, F.R. Effects of preeclampsia and eclampsia on maternal metabolic and biochemical outcomes in later life: A systematic review and meta-analysis. Metabolism 2020, 102, 154012. [Google Scholar] [CrossRef]
- Tuzcu, Z.B.; Asicioglu, E.; Sunbul, M.; Ozben, B.; Arikan, H.; Koc, M. Circulating endothelial cell number and markers of endothelial dysfunction in previously preeclamptic women. Am. J. Obs. Gynecol. 2015, 213, 533.e1–533.e5337. [Google Scholar] [CrossRef]
- Grand’Maison, S.; Pilote, L.; Okano, M.; Landry, T.; Dayan, N. Markers of Vascular Dysfunction After Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Hypertension 2016, 68, 1447–1458. [Google Scholar] [CrossRef]
- Sandvik, M.K.; Leirgul, E.; Nygård, O.; Ueland, P.M.; Berg, A.; Svarstad, E.; Vikse, B.E. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am. J. Obs. Gynecol. 2013, 209, 569.e1–569.e10. [Google Scholar] [CrossRef]
- Kattah, A. Preeclampsia and Kidney Disease: Deciphering Cause and Effect. Curr. Hypertens. Rep. 2020, 22, 91. [Google Scholar] [CrossRef]
- Stillman, I.E.; Karumanchi, S.A. The glomerular injury of preeclampsia. J. Am. Soc. Nephrol. 2007, 18, 2281–2284. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Stefańska, K.; Zieliński, M.; Sakowska, J.; Jankowiak, M.; Trzonkowski, P.; Marek-Trzonkowska, N.; Kwiatkowski, S. Podocytes-The Most Vulnerable Renal Cells in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 5051. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Panagiotopoulos, M.; Bellos, I.; Theodora, M.; Stavros, S.; Ntomali, E.; Papapanagiotou, A.; Daskalakis, G. Serum LDH values in hypertensive disorders of pregnancy and their association with maternal and neonatal morbidity: A meta-analysis. Int. J. Clin. Pract. 2021, 75, e14986. [Google Scholar] [CrossRef]
- Burwick, R.M.; Rincon, M.; Beeraka, S.S.; Gupta, M.; Feinberg, B.B. Evaluation of Hemolysis as a Severe Feature of Preeclampsia. Hypertension 2018, 72, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Lodge-Tulloch, N.A.; Paré, J.F.; Couture, C.; Bernier, E.; Cotechini, T.; Girard, S.; Graham, C.H. Maternal Innate Immune Reprogramming After Complicated Pregnancy. Am. J. Reprod. Immunol. 2024, 92, e13908. [Google Scholar] [CrossRef]
- Dockree, S.; Brook, J.; Shine, B.; James, T.; Green, L.; Vatish, M. Cardiac-specific troponins in uncomplicated pregnancy and pre-eclampsia: A systematic review. PLoS ONE 2021, 16, e0247946. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.P.; Røysland, R.; Strand, H.; Moe, K.; Sugulle, M.; Omland, T.; Staff, A.C. Circulating cardiovascular biomarkers during and after preeclampsia: Crosstalk with placental function. Pregnancy Hypertens. 2022, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ullmo, J.; Nan, M.N.; Cruz-Lemini, M.; Garrido-Gimenez, C.; Platero, J.; García-Osuna, Á.; García-Manau, P.; Twickler, M.; Llurba, E. Cardiovascular biomarkers and preeclampsia: A narrative review. Eur. J. Clin. Investig. 2025. [Google Scholar] [CrossRef]
- Muijsers, H.E.C.; Westermann, D.; Birukov, A.; van der Heijden, O.W.; Drost, J.T.; Kräker, K.; Haase, N.; Müller, D.N.; Herse, F.; Maas, A.H.; et al. High-sensitivity cardiac troponin I in women with a history of early-onset preeclampsia. J. Hypertens. 2020, 38, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Aldo, C.; Martina, Z.; Alberto, A.; Mario, P. Cardiovascular risk evaluation in pregnancy: Focus on cardiac specific biomarkers. Clin. Chem. Lab. Med. 2024, 62, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.M.; da Silva, T.E.M.; Silva, L.G.; Ferreira, A.P.S.; Paraguai, C.M.D.C.; Ferreira, C.N.; Dusse, L.M.S.; Mayrink, J.; Alpoim, P.N. Preeclampsia beyond pregnancy: Investigating the long-term increase in cardiovascular disease and metabolic syndrome (PERLA- Brazil study). Women Health 2025, 65, 328–339. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, B.B.; Nijdam, M.E.; Bruinse, H.W.; Roest, M.; Uiterwaal, C.S.; Grobbee, D.E.; Bots, M.L.; Franx, A. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obs. Gynecol. 2013, 121, 1040–1048. [Google Scholar] [CrossRef]
| No Previous PE (N = 250) | Previous PE (N = 113) | p Value | |
|---|---|---|---|
| Follow-up interval, years | 4.38 (4.04–4.63) | 4.38 (3.66–5.10) | 0.34 |
| Age at inclusion, years | 40.7 (37.3–44.1) | 42.3 (38.1–45.0) | 0.03 |
| BMI | 24.9 (22.2–28.8) | 27.7 (24.3–32.8) | <0.01 |
| Race or ethnic group * | 0.18 | ||
| White | 188 (75.2) | 71 (62.8) | |
| Black | 11 (4.4) | 5(4.4) | |
| Latin American | 45 (18.0) | 31 (27.4) | |
| South Asian | 2 (0.8) | 3 (2.7) | |
| East Asian | 2 (0.8) | 1 (0.9) | |
| Mixed Race | 2 (0.8) | 2 (1.8) | |
| Current Medical History | |||
| Current Chronic Hypertension | 18 (7.2) | 23 (20.4) | <0.01 |
| Type 1 or 2 diabetes mellitus | 5 (2.0) | 5 (4.4) | 0.19 |
| Antiphospholipid syndrome | 1 (0.4) | 0 | 0.50 |
| Autoimmune disease | 11 (4.4) | 3 (2.7) | 0.42 |
| Dyslipidemia | 21 (8.4) | 10 (8.9) | 0.89 |
| Current Cigarette smoking | 5 (2.0) | 6 (5.3) | 0.09 |
| Current Arterial Blood Pressure | |||
| Systolic Blood Pressure, mmHg | 110 (103–120) | 123 (113–133) | <0.01 |
| Diastolic Blood Pressure, mmHg | 72.0 (67.0–79.0) | 78.5 (73.0–83.0) | <0.01 |
| Mean Arterial Blood, mmHg | 84 (79–93) | 93 (86–100) | <0.01 |
| Pregnancy History | |||
| Breastfeeding | 234 (93.6) | 99 (87.6) | 0.06 |
| Primiparous | 136 (54.4) | 64 (56.6) | 0.69 |
| Previous spontaneous pregnancy loss | 56 (22.4) | 29 (25.7) | 0.50 |
| Previous Gestational diabetes | 31 (12.4) | 13 (11.5) | 0.79 |
| Pre-existing chronic hypertension | 10 (4.0) | 11 (9.7) | 0.03 |
| Pre-existing type 1 or 2 diabetes mellitus | 3 (1.2) | 4 (3.5) | 0.13 |
| Pre-existing antiphospholipid syndrome | 1 (0.4) | 0 | 0.50 |
| Pre-existing autoimmune disease | 6 (2.4) | 4 (3.5) | 0.54 |
| Angiogenic Biomarkers in Pregnancy | |||
| sFlt-1 during pregnancy, pg/mL | 2396 (1751–3631) | 6690 (3836–10,605) | <0.01 |
| PlGF during pregnancy, pg/mL | 354 (183–668) | 106 (57–184) | <0.01 |
| sFlt-1/PlGF during pregnancy | 6.24 (2.76–19.90) | 65.20 (25.40–193.00) | <0.01 |
| Gestational age at sFlt-1/PlGF determination, weeks | 33.8 (32.0–37.2) | 35.6 (33.5–37.6) | 0.01 |
| sFlt-1/PlGF < 38 | 186/214 (86.9) | 27/81 (33.3) | <0.01 |
| sFlt-1/PlGF ≥ 38 | 28/214 (13.1) | 54/81 (66.7) | <0.01 |
| Hematological and Biochemical Parameters | No Previous PE (n = 250) | Previous PE (n = 113) | Adjusted p Value * |
|---|---|---|---|
| Hemoglobin, g/L | 130 (122–136) | 131 (121–137) | 0.53 |
| Hematocrit, l/L | 0.39 (0.37–0.41) | 0.39 (0.37–0.41) | 0.60 |
| Leukocytes, U/mL | 6205 (5300–5570) | 6310 (5570–7380) | 0.94 |
| Platelets, U/mL | 262,500 (222,250–309,750) | 279,000 (243,000–313,000) | 0.12 |
| Glucose, mg/dL | 86.5 (82.0–92.0) | 89.0 (83.0–94.0) | 0.59 |
| Sodium, mmol/L | 139 (138–141) | 140 (138–141) | 0.25 |
| Potassium, mmol/L | 4.22 (4.04–4.39) | 4.23 (4.01–4.37) | 0.91 |
| Uric acid, mg/dL | 4.07 (3.51–4.65) | 4.03 (3.39–4.69) | 0.68 |
| Creatinine, mg/dL | 0.67 (0.62–0.74) | 0.65 (0.59–0.71) | 0.09 |
| Glycated hemoglobin, % | 5.3 (5.2–5.5) | 5.4 (5.3–5.6) | 0.25 |
| AST, U/L | 20.0 (17.0–23.8) | 20.0 (17.0–24.0) | 0.19 |
| ALT, U/L | 15 (12–20) | 16 (12–23) | 0.32 |
| Bilirubin, mg/dL | 0.59 (0.45–0.81) | 0.54 (0.43–0.67) | 0.05 |
| LDH, U/L | 162 (148–179) | 166 (152–187) | 0.36 |
| LDL, mg/dL | 111.0 (93.3–132.0) | 104.0 (93.0–130.0) | 0.03 |
| VLDL, mg/dL | 11.7 (9.1–16.2) | 14.3 (10.3–20.3) | 0.76 |
| HDL, mg/dL | 59.6 (50.0–68.4) | 55.7 (46.4–65.8) | 0.74 |
| Cholesterol, mg/dL | 184 (166–207) | 179 (162–207) | 0.11 |
| Triglycerides, mg/dL | 58.1 (48.7–80.7) | 70.8 (51.3–101.0) | 0.86 |
| Urinary Protein, g/L | 0.08 (0.07–0.13) | 0.08 (0.07–0.12) | 0.46 |
| Urinary Albumin, mg/L | 8.4 (5.0–14.7) | 8.0 (5.1–13.8) | 0.09 |
| TSH, mUI/L | 1.33 (1.04–1.96) | 1.41 (1.11–1.65) | 0.26 |
| Prolactin, mUI/L | 260 (210–449) | 309 (214–367) | 0.94 |
| Cardiovascular biomarkers | No previous PE (n = 163) | Previous PE (n = 66) | Adjusted p value * |
| PlGF, pg/mL | 10.00 (9.00–12.00) | 11.00 (9.68–12.00) | 0.46 |
| NT-ProBNP, ng/L | 42.2 (25.4–66.0) | 46.0 (26.0–76.2) | 0.36 |
| Hs-TnT, ng/L | 3.2 (3.0–5.0) | 4.0 (3.0–6.0) | 0.03 |
| sFlt-1/PlGF < 38 (n = 213) | sFlt-1/PlGF ≥ 38 (n = 82) | p Value | |
|---|---|---|---|
| Follow-up interval, years | 4.33 (3.93–4.60) | 4.38 (3.65–5.00) | 0.18 |
| Age at inclusion, years | 40.2 (37.0–44.3) | 41.6 (37.8–44.4) | 0.33 |
| BMI | 24.8 (22.2–29.4) | 28.3 (24.6–32.7) | <0.01 |
| Race or ethnic group * | 0.22 | ||
| White | 160 (75.2) | 54 (65.9) | |
| Black | 11 (5.2) | 2 (2.4) | |
| Latin American | 35 (16.4) | 23 (28.1) | |
| South Asian | 3 (1.4) | 2 (2.4) | |
| East Asian | 2 (0.9) | 0 | |
| Mixed Race | 2 (0.9) | 1 (1.2) | |
| Current Medical History | |||
| Current chronic hypertension | 20 (9.4) | 19 (23.2) | <0.01 |
| Type 1 or 2 diabetes mellitus | 4 (1.9) | 4 (4.9) | 0.16 |
| Antiphospholipid syndrome | 1 (0.5) | 0 | 0.53 |
| Autoimmune disease | 13 (6.1) | 5 (6.1) | 0.59 |
| Dyslipidemia | 18 (8.5) | 9 (11.0) | 0.50 |
| Current cigarette smoking | 8 (3.8) | 2 (2.4) | 0.58 |
| Current Arterial Blood Pressure | |||
| Systolic Blood Pressure, mmHg | 111 (103–121) | 122 (113–130) | <0.01 |
| Diastolic Blood Pressure, mmHg | 72.5 (67.0–81.0) | 77.5 (71.3–82.0) | <0.01 |
| Mean Arterial Blood, mmHg | 85.0 (79.0–94.0) | 92.0 (87.0–98.8) | <0.01 |
| Pregnancy history | |||
| Breastfeeding | 200 (93.9) | 72 (87.8) | 0.08 |
| Primiparous | 108 (50.7) | 53 (64.6) | 0.03 |
| Previous spontaneous pregnancy loss | 45 (21.1) | 16 (19.5) | 0.76 |
| Previous gestational diabetes | 28 (13.2) | 7 (8.5) | 0.27 |
| Pre-existing chronic hypertension | 11 (5.2) | 10 (12.2) | 0.04 |
| Pre-existing type 1 or 2 diabetes mellitus | 2 (0.9) | 3 (3.7) | 0.11 |
| Pre-existing antiphospholipid syndrome | 1 (0.5) | 0 | 0.53 |
| Pre-existing autoimmune disease | 8 (3.8) | 1 (1.2) | 0.26 |
| Angiogenic Biomarkers in Pregnancy | |||
| sFlt-1 during pregnancy, pg/ml | 2273 (1729–3156) | 8619 (5895–11,495) | <0.01 |
| PlGF during pregnancy, pg/ml | 417.0 (219.0–687.0) | 75.0 (47.3–112.0) | <0.01 |
| sFlt-1/PlGF during pregnancy, pg/ml | 5.56 (2.61–14.00) | 88.30 (60.00–213.00) | <0.01 |
| Gestational age at sFlt-1/PlGF determination, weeks | 33.3 (31.8–36.2) | 37.0 (34.6–38.3) | <0.01 |
| Hematological and Biochemical Parameters | sFlt-1/PlGF < 38 (n = 213) | sFlt-1/PlGF ≥ 38 (n = 82) | Adjusted p Value * |
|---|---|---|---|
| Hemoglobin, g/L | 130 (122–137) | 132 (121–136) | 0.68 |
| Hematocrit, l/L | 0.390 (0.370–0.400) | 0.390 (0.370–0.407) | 0.08 |
| Leukocytes, U/ml | 6250 (5430–7450) | 6150 (5348–7055) | 0.03 |
| Platelets, U/mcL | 264,000 (222,000–313,000) | 279,000 (222,000–3,077,501) | 0.74 |
| Glucose, mg/dL | 86 (82–91) | 89 (83–94) | 0.87 |
| Sodium, mmol/L | 139 (138–141) | 139 (138–140) | 0.97 |
| Potassium, mmol/L | 4.19 (4.02–4.37) | 4.25 (4.07–4.40) | 0.048 |
| Uric acid, mg/dL | 4.08 (3.53–4.69) | 3.95 (3.38–4.72) | 0.84 |
| Creatinine, mg/dL | 0.67 (0.62–0.74) | 0.65 (0.60–0.74) | 0.84 |
| Glycated hemoglobin, % | 5.3 (5.2–5.5) | 5.4 (5.3–5.6) | 0.26 |
| AST, U/L | 20 (17–23) | 20 (17–23) | 0.32 |
| ALT, U/L | 15.0 (12.0–21.0) | 16.0 (12.0–22.8) | 0.58 |
| Bilirubin, mg/dL | 0.570 (0.450–0.780) | 0.560 (0.443–0.728) | 0.84 |
| LDH, U/L | 163 (149–177) | 168 (150–189) | 0.046 |
| LDL, mg/dL | 110.0 (93.4–13.2) | 109.0 (93.6–134.0) | 0.40 |
| VLDL, mg/dL | 12.50 (9.27–18.20) | 13.50 (9.81–18.30) | 0.14 |
| HDL, mg/dL | 59.2 (49.5–68.1) | 57.1 (46.5–66.3) | 0.96 |
| Cholesterol, mg/dL | 183 (166–208) | 182 (166–208) | 0.26 |
| Triglycerides, mg/dL | 62.0 (48.7–90.3) | 66.8 (50.0–90.9) | 0.15 |
| Urinary Protein, g/L | 0.08 (0.07–0.13) | 0.09 (0.07–0.18) | 0.01 |
| Urinary Albumin, mg/L | 8.7 (5.2–15.5) | 7.3 (5.0–13.3) | 0.11 |
| TSH, mUI/L | 1.33 (1.02–1.67) | 1.40 (1.18–1.90) | 0.88 |
| Prolactin, mUI/L | 260 (217–415) | 309 (219–348) | 0.78 |
| Cardiovascular Biomarkers | sFlt-1/PlGF < 38 (n = 171) | sFlt-1/PlGF > 38 (n = 58) | Adjusted p * |
| PlGF, pg/mL | 10.0 (9.0–12.0) | 11.0 (9.5–12.0) | 0.91 |
| NT-ProBNP, ng/L | 45.0 (25.5–69.0) | 47.0 (26.5–78.5) | 0.14 |
| Hs-TnT, ng/L | 3.5 (3.0–5.0) | 4.0 (3.0–6.0) | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, N.; Platero, J.; Garcia-Manau, P.; Sanchez-Garcia, O.; Pellicer, C.; Jordi, M.; Garcia, Z.; Garrido-Gimenez, C.; Ullmo, J.; Nan, M.; et al. Long-Term Biochemical and Cardiovascular Profiles 3–6 Years After Preeclampsia: Impact of Angiogenic Imbalance During Pregnancy. J. Clin. Med. 2025, 14, 8389. https://doi.org/10.3390/jcm14238389
Costa N, Platero J, Garcia-Manau P, Sanchez-Garcia O, Pellicer C, Jordi M, Garcia Z, Garrido-Gimenez C, Ullmo J, Nan M, et al. Long-Term Biochemical and Cardiovascular Profiles 3–6 Years After Preeclampsia: Impact of Angiogenic Imbalance During Pregnancy. Journal of Clinical Medicine. 2025; 14(23):8389. https://doi.org/10.3390/jcm14238389
Chicago/Turabian StyleCosta, Noah, Judit Platero, Pablo Garcia-Manau, Olga Sanchez-Garcia, Clàudia Pellicer, Mariona Jordi, Zoraida Garcia, Carmen Garrido-Gimenez, Johana Ullmo, Madalina Nan, and et al. 2025. "Long-Term Biochemical and Cardiovascular Profiles 3–6 Years After Preeclampsia: Impact of Angiogenic Imbalance During Pregnancy" Journal of Clinical Medicine 14, no. 23: 8389. https://doi.org/10.3390/jcm14238389
APA StyleCosta, N., Platero, J., Garcia-Manau, P., Sanchez-Garcia, O., Pellicer, C., Jordi, M., Garcia, Z., Garrido-Gimenez, C., Ullmo, J., Nan, M., Mora, J., Garcia-Osuna, A., Choliz, M., Cruz-Lemini, M., del Carmen Medina, M., & Llurba, E. (2025). Long-Term Biochemical and Cardiovascular Profiles 3–6 Years After Preeclampsia: Impact of Angiogenic Imbalance During Pregnancy. Journal of Clinical Medicine, 14(23), 8389. https://doi.org/10.3390/jcm14238389








