Prevalence of Psoriatic Arthritis in Patients with Moderate-to-Severe Psoriasis in the Era of Biologics and Small Molecule Therapies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Population
2.3. Data Collection
2.4. Variables
2.5. Study Size
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Sociodemographic Characteristics of Patients with Moderate-to-Severe Psoriasis
3.2. Prevalence of PsA
3.3. Characteristics of PsA Patients
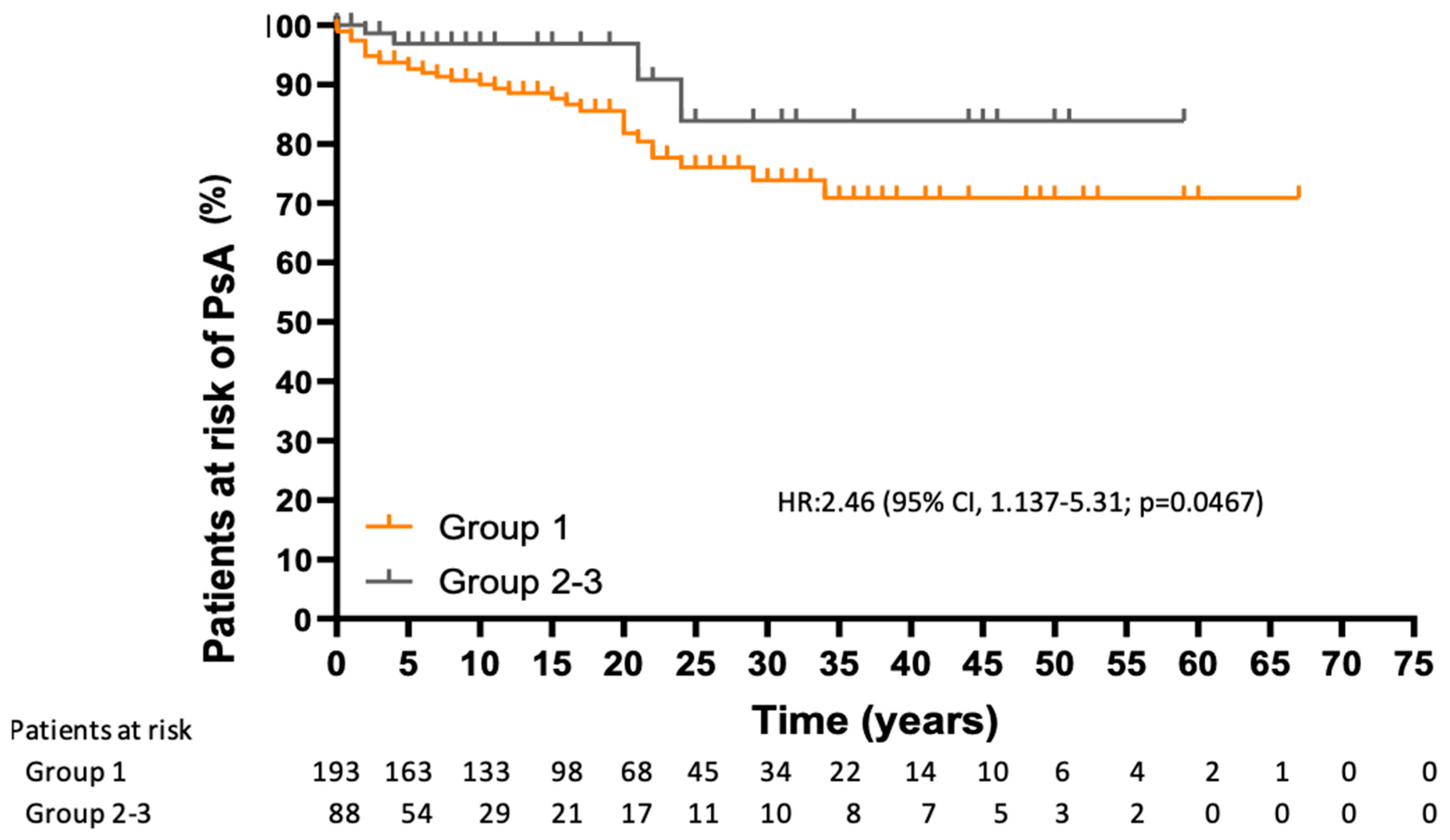
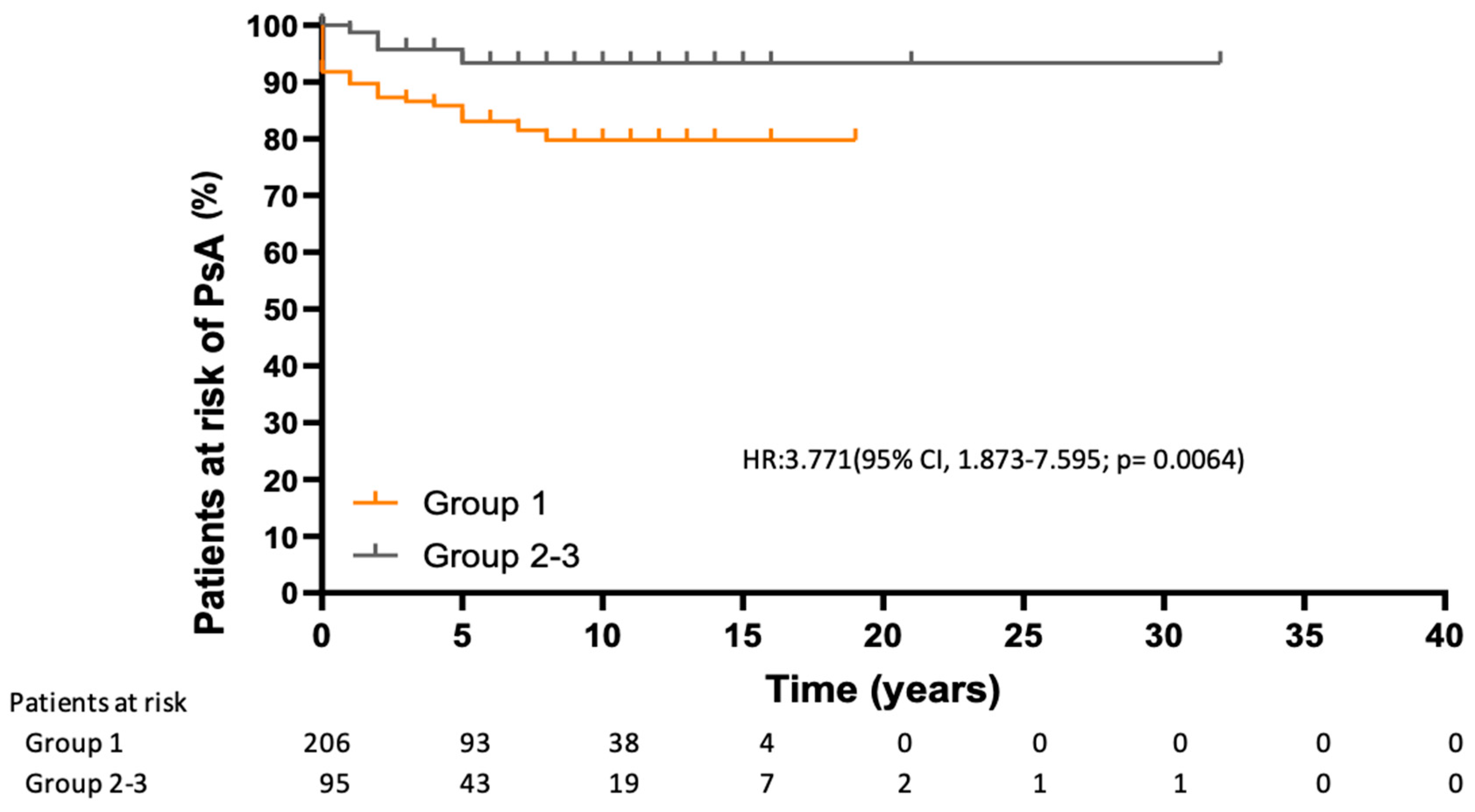
3.4. Risk of Developing PsA
3.5. Factors Related to the Development of PsA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PsA | Psoriatic arthritis |
| csDMARD | Conventional synthetic disease-modifying anti-rheumatic drugs |
| OSA | Obstructive sleep apnea |
| IMID | immune-mediated inflammatory disease |
| PASI | Psoriasis Area and Severity Index |
| CRP | C-reactive protein |
| bDMARDs | Biologic disease-modifying anti-rheumatic drugs |
References
- Ferrándiz, C.; Carrascosa, J.M.; Toro, M. Prevalence of psoriasis in Spain in the age of biologics. Actas Dermo-Sifiliogr. 2014, 105, 504–509, (In English, Spanish). [Google Scholar] [CrossRef]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Helliwell, P.S. Psoriatic arthritis: State of the art review. Clin. Med. 2017, 17, 65–70. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutr. Diabetes 2012, 2, e54. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Harskamp, C.T.; Armstrong, A.W. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J. Am. Heart Assoc. 2013, 2, e000062. [Google Scholar] [CrossRef]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.E.; Cohen, J.M.; Ho, R.S. Psoriasis and suicidality: A review of the literature. Dermatol. Ther. 2019, 32, e12771. [Google Scholar] [CrossRef]
- Zabotti, A.; De Lucia, O.; Sakellariou, G.; Batticciotto, A.; Cincinelli, G.; Giovannini, I.; Idolazzi, L.; Maioli, G.; Tinazzi, I.; Aletaha, D.; et al. Predictors, risk factors, and incidence rates of psoriatic arthritis development in psoriasis patients: A systematic literature review and meta-analysis. Rheumatol. Ther. 2021, 8, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Zabotti, A.; De Marco, G.; Gossec, L.; Baraliakos, X.; Aletaha, D.; Iagnocco, A.; Gisondi, P.; Balint, P.V.; Bertheussen, H.; Boehncke, W.H.; et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann. Rheum. Dis. 2023, 82, 1162–1170. [Google Scholar] [CrossRef]
- Gisondi, P.; Bellinato, F.; Maurelli, M.; Geat, D.; Zabotti, A.; McGonagle, D.; Girolomoni, G. Reducing the risk of developing psoriatic arthritis in patients with psoriasis. Psoriasis 2022, 12, 213–220. [Google Scholar] [CrossRef]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of pathogenesis in psoriatic arthritis: Genotype determines clinical phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef]
- Scher, J.U.; Ogdie, A.; Merola, J.F.; Ritchlin, C. Preventing psoriatic arthritis: Focusing on patients with psoriasis at increased risk of transition. Nat. Rev. Rheumatol. 2019, 15, 153–166. [Google Scholar] [CrossRef]
- Monteleone, G.; Moscardelli, A.; Colella, A.; Marafini, I.; Salvatori, S. Immune-mediated inflammatory diseases: Common and different pathogenic and clinical features. Autoimmun. Rev. 2023, 22, 103337. [Google Scholar] [CrossRef]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 induces spondyloarthropathy by acting on RORγt+CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Oak, A.S.W.; Elewski, B.E. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: A comprehensive review. Am. J. Clin. Dermatol. 2021, 22, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Bellinato, F.; Chiricozzi, A.; Piaserico, S.; Targher, G.; Gisondi, P. Could targeted pharmacotherapies exert a “disease modification effect” in patients with chronic plaque psoriasis? Int. J. Mol. Sci. 2022, 23, 12849. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Bellinato, F.; Targher, G.; Idolazzi, L.; Girolomoni, G. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann. Rheum. Dis. 2022, 81, 68–73. [Google Scholar] [CrossRef]
- Rosenthal, Y.S.; Schwartz, N.; Sagy, I.; Pavlovsky, L. Incidence of psoriatic arthritis among patients receiving biologic treatments for psoriasis: A nested case-control study. Arthritis Rheumatol. 2022, 74, 237–243. [Google Scholar] [CrossRef]
- Haberman, R.H.; MacFarlane, K.A.; Catron, S.; Samuels, J.; Blank, R.B.; Toprover, M.; Uddin, Z.; Hu, J.; Castillo, R.; Gong, C.; et al. Efficacy of guselkumab, a selective IL-23 inhibitor, in preventing arthritis in a multicentre psoriasis at-risk cohort (PAMPA): Protocol of a randomized, double-blind, placebo-controlled multicentre trial. BMJ Open 2022, 12, e063650. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Fredriksson, T.; Pettersson, U. Severe psoriasis—Oral therapy with a new retinoid. Dermatology 1978, 157, 238–244. [Google Scholar] [CrossRef]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, X.; Du, Y.; Dai, S.M. Global and regional epidemiology of psoriatic arthritis in patients with psoriasis: A comprehensive systematic analysis and modelling study. J. Autoimmun. 2024, 145, 103202. [Google Scholar] [CrossRef] [PubMed]
- Floris, A.; Mugheddu, C.; Sichi, L.; Anedda, J.; Frau, A.; Sorgia, J.; Li Volsi, L.; Paladino, M.T.; Congia, M.; Chessa, E.; et al. Treatment of psoriasis with different classes of biologics reduces the likelihood of peripheral and axial psoriatic arthritis development. Rheumatology 2025, 64, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- López Estebaranz, J.L.; Zarco-Montejo, P.; Samaniego, M.L.; García-Calvo, C. Prevalence and clinical features of psoriatic arthritis in psoriasis patients in Spain. Eur. J. Dermatol. 2015, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.M.; Puig, L.; Belinchón Romero, I.; Salgado-Boquete, L.; Del Alcázar, E.; Andrés Lencina, J.J.; Moreno, D.; de la Cueva, P. Actualización práctica de las recomendaciones del Grupo de Psoriasis de la Academia Española de Dermatología y Venereología (GPS) para el tratamiento de la psoriasis con terapia biológica. Parte 1. «Conceptos y manejo general de la psoriasis con terapia biológica». Actas Dermo-Sifiliogr. 2022, 113, 261–277. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgo, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, S.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: Treatment and monitoring recommendations. Br. J. Dermatol. 2021, 184, 550–567. [Google Scholar] [CrossRef]
- Watad, A.; Zabotti, A.; Patt, Y.S.; Gendelman, O.; Dotan, A.; Ben-Shabat, N.; Fisher, L.; McGonagle, D.; Amital, H. From Psoriasis to Psoriatic Arthritis: Decoding the Impact of Treatment Modalities on the Prevention of Psoriatic Arthritis. Rheumatol. Ther. 2024, 11, 963–976. [Google Scholar] [CrossRef]
- Felquer, M.L.A.; LoGiudice, L.; Galimberti, M.L.; Rosa, J.; Mazzuoccolo, L.; Soriano, E.R. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 74–79. [Google Scholar] [CrossRef]
- Meer, E.; Merola, J.F.; Fitzsimmons, R.; Love, T.J.; Wang, S.; Shin, D.; Chen, Y.; Xie, S.; Choi, H.; Zhang, Y.; et al. Does biologic therapy impact the development of PsA among patients with psoriasis? Ann. Rheum. Dis. 2022, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Jackson, C.L.; Li, T.Y.; Wu, S.; Qureshi, A.A. Sleep disordered breathing and the risk of psoriasis among US women. Arch. Dermatol. Res. 2015, 307, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Kang, J.H.; Lin, H.C. Increased risk of psoriasis following obstructive sleep apnea: A longitudinal population-based study. Sleep Med. 2012, 13, 285–289. [Google Scholar] [CrossRef]
- Ger, T.Y.; Fu, Y.; Chi, C.C. Bidirectional Association Between Psoriasis and Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 5931. [Google Scholar] [CrossRef] [PubMed]
| Total n = 308 | Group 1 n = 207 (67.2%) | Group 2 n = 70 (22.7%) | Group 3 n = 31 (10.1%) | p Value | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age | 55.7 ± 14.75 | 56.5 ± 14.4 | 55.9 ± 16.1 | 49.8 ± 13.1 | NS |
| Female sex | 152 (49.4) | 100 (48.3) | 37 (52.9) | 15 (48.4) | NS |
| Variables related to psoriasis | |||||
| Type of psoriasis | |||||
| Plaque | 141(45.8) | 79 (38.2) | 42 (60) | 20 (64.5) | NA |
| Scalp | 54 (17.5) | 48 (23.2) | 3 (4.3) | 3 (9.7) | NA |
| Inverse | 12 (3.9) | 12 (5.8) | 0 | 0 | NA |
| Pustular | 35 (11.4) | 24 (11.6) | 9 (12.9) | 2 (6.5) | NA |
| Nail involvement | 22 (7.1) | 20 (9.7) | 0 | 2 (6.5) | NA |
| Cutaneous and nail | 44 (14.3) | 24 (11.6) | 16 (22.9) | 4 (12.9) | NA |
| PASI | 7.8 [4.2–11] | 8.9 [5–12] | 6.6 [4.1–10.9] | 3.6 [1–6.6] | 0.003 |
| Psoriasis family history | 115 (37.3) | 77 (45.8) | 27 (40.9) | 11 (37.9) | NS |
| PsA family history | 23 (7.5) | 16 (10.7) | 7 (11.7) | 0 | NS |
| Time (months) between onset of symptoms and diagnosis of psoriasis | 6 [0–24] | 6 [0.3–26.5] | 3 [0–18.5] | 16 [1.5–48.8] | NS |
| Comorbidities | |||||
| Smoker/former smoker | 195 (64.1) | 142 (69.6) | 37 (52.9) | 16 (53.3) | 0.018 |
| Alcohol consumption | 19 (6.2) | 17 (8.2) | 1 (1.4) | 1 (3.2) | NA |
| Overweight/obesity | 112 (36.4) | 95 (45.9) | 14 (20) | 3 (9.7) | <0.001 |
| Hypertension | 79 (25.6) | 57 (27.5) | 18 (25.7) | 4 (12.9) | NS |
| Dyslipidemia | 135 (43.8) | 102 (49.3) | 22 (31.4) | 11 (35.5) | 0.021 |
| Diabetes | 30 (9.7) | 21 (10.1) | 6 (8.6) | 3 (9.7) | NS |
| Depression | 23 (7.5) | 18 (8.7) | 4 (5.7) | 1 (3.2) | NS |
| Hepatic steatosis | 45 (14.6) | 35 (16.9) | 9 (12.9) | 1 (3.2) | NS |
| COPD | 12 (3.9) | 7 (3.4) | 3 (4.3) | 2 (6.5) | NA |
| Hyperuricemia | 26 (8.4) | 19 (9.2) | 5 (7.1) | 2 (6.5) | NS |
| OSA | 10 (3.2) | 6 (2.9) | 0 | 4 (12.9) | NA |
| Extracutaneous variables | |||||
| Musculoskeletal | |||||
| Symptoms | 92 (29.9) | 54 (26.1) | 27 (38.6) | 11 (35.5) | NS |
| Uveitis | 2 (0.6) | 1 (0.5) | 1 (1.4) | 0 | NA |
| IBD | 2 (0.6) | 2 (1) | 0 | 0 | NA |
| CRP | 2.4 (0.8–4.9) | 3.1 (0.9–4.9) | 2.2 (0.6–4.2) | 4.1 (0.4–10) | NS |
| HLA B27-positive | 13 (8.5) | 12 (8.6%) | 0 | 1 (25) | NA |
| Treatments | |||||
| TNF inhibitors | 127 (41.2) | 127 (61.4) | 0 | 0 | <0.001 |
| Anti-IL17 | 38 (12.3) | 38 (18.4) | 0 | 0 | <0.001 |
| Anti-IL12/23 | 67 (21.8) | 67 (32.4) | 0 | 0 | <0.001 |
| Anti-IL23 | 48 (15.6) | 48 (23.2) | 0 | 0 | <0.001 |
| Apremilast | 67 (21.8) | 67 (32.4) | 0 | 0 | <0.001 |
| Jak inhibitor | 1 (0.3) | 1 (0.5) | 0 | 0 | NA |
| Methotrexate | 202 (65.6) | 156 (75.4) | 46 (65.7) | 0 | <0.001 |
| Cyclosporine | 103 (33.4) | 85 (41.4) | 18 (25.7) | 0 | <0.001 |
| Acitretin | 156 (50.6) | 110 (53.1) | 46 (65.7) | 0 | <0.001 |
| Phototherapy | 301 (97.7) | 200 (96.6) | 70 (100) | 31 (100) | NA |
| n = 36 (%) | |
|---|---|
| Demographic data | |
| Age | 55.7 ± 15.53 |
| Female sex | 19 (52.8) |
| Variables related to psoriasis | |
| Type of psoriasis | |
| Plaque | 10 (27.8) |
| Scalp | 6 (16.7) |
| Inverse | 3 (8.3) |
| Pustular | 2 (5.5) |
| Nail involvement | 1 (2.78) |
| Cutaneous and nail | 14 (38.9) |
| PASI | 8 + 4.9 |
| Psoriasis family history | 14 (38.9) |
| PsA family history | 5 (13.9) |
| Time (months) between onset of symptoms and diagnosis of psoriasis | 7 (0–12) |
| Variables related to PsA | |
| Type of PsA | |
| Peripheral | 22 (66.1) |
| Axial | 6 (16.7) |
| Peripheral and axial | 8 (22.2) |
| Time (months) between onset of symptoms and diagnosis of psoriasis | 11 [0–12] |
| Comorbidities | |
| Smoker/former smoker | 20 (55.5) |
| Alcohol consumption | 3 (8.3) |
| Overweight/obesity | 14 (38.9) |
| Hypertension | 12 (33) |
| Dyslipidemia | 18 (50) |
| Diabetes | 6 (16.7) |
| Depression | 5 (13.9) |
| Hepatic steatosis | 7 (19.4) |
| COPD | 1 (2.8) |
| Hyperuricemia | 3 (8.3) |
| OSA | 5 (13.9) |
| Extracutaneous and extramusculoskeletal variables | |
| Uveitis | 1 (2.8) |
| IBD | 0 |
| CRP | 3.1 [0.7–11] |
| HLA B27-positive | 6 (17.6) |
| Psoriasis treatment at diagnosis of PsA | |
| csDMARDs | 16 (44.4) |
| Methotrexate | 11 (30.5) |
| Cyclosporine | 4 (11.1) |
| Acitretin | 1 (2.7) |
| Biologics | 11 (30.5) |
| TNF inhibitors | 6 (16.6) |
| Anti-IL17 | 1 (2.7) |
| Anti-IL12/23 | 2 (5.5) |
| Anti-IL23 | 2 (5.5) |
| Treatments | |
| TNF inhibitors | 23 (63.9) |
| Anti-IL17 | 7 (19.44) |
| Anti-IL12/23 | 12 (33.3) |
| Anti-IL23 | 11 (30.6) |
| Apremilast | 11 (30.6) |
| Jak inhibitor | 0 |
| Methotrexate | 30 (83.3) |
| Cyclosporine | 10 (27.8) |
| Acitretin | 17 (47.2) |
| Phototherapy | 36 (100) |
| Number of biologics or small molecules (group 1) | |
| 1 | 11 (34.4) |
| 2 | 14 (43.7) |
| 3 | 4 (12.5) |
| >3 | 3 (8.35) |
| Time (years) from psoriasis and PsA diagnosis | 7.5 (2–20) |
| Group 1 | 7.5 (2–20) |
| Group 2 | 21 (4–22.5) |
| Group 3 | 2 [2–2] |
| Time (months) from musculoskeletal symptoms to PsA | 15 [5.5–34.7] |
| Group 1 | 11 [4–34.5] |
| Group 2 | 25 [19.5–46] |
| Group 3 | NA |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age at diagnosis | 1 (0.9–1) | NS | ||
| Female sex | 1.1 (0.5–2.3) | NS | ||
| Time (years) from onset of psoriasis symptoms and diagnosis of PsA | 0.9 (0.9–1) | NS | ||
| Time (years) from diagnosis of psoriasis until initiation of psoriasis treatment | 1 (0.9–1) | NS | ||
| Disease duration | 0.9 (0.9–0.9) | 0.043 | 0.9 (0.9–1) | 0.051 |
| PASI | 0.9 (0.9–1) | NS | ||
| Smoker/former smoker | 0.6 (0.3–1.3) | NS | ||
| Alcohol consumption | 1.4 (0.4–5.2) | NS | ||
| OSA | 8.6 (2.3–31.4) | 0.001 | 19.5 (3.5–108.9) | <0.001 |
| Hepatic steatosis | 1.4 (0.6–3.6) | NS | ||
| Depression | 2.2 (0.7–6.5) | NS | ||
| Overweigh and obesity | 1.1 (0.5–2.3) | NS | ||
| Family history of psoriasis | 0.9 (0.4–2.1) | NS | ||
| Family history of PsA | 0.4 (0.1–1.2) | NS | ||
| Treatment group | ||||
| Group 1 | ||||
| Group 2 | 0.2 (0.07–0.8) | 0.023 | 0.262 (0.07–0.9) | 0.034 |
| Group 3 | 0.1 (0.02–1.3) | NS | 0.067 (0.006–7.55) | 0.029 |
| Type of psoriasis | ||||
| Nail involvement | 0.3 (0.04–2.6) | NS | ||
| Nail only or nail and skin | 3.09 (1.4–6.4) | 0.002 | 3.605 (1.66–7.83) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara-Dangond, C.; Cobo-Ibáñez, T.; Cueva-Nájera, G.; Valverde-Garrido, R.; García-Yubero, C.; Trives-Folguera, L.; Paredes-Romero, B.; Esteban-Vázquez, A.V.; Romero-Bogado, L.; De La Cámara-Fernández, I.; et al. Prevalence of Psoriatic Arthritis in Patients with Moderate-to-Severe Psoriasis in the Era of Biologics and Small Molecule Therapies. J. Clin. Med. 2025, 14, 8359. https://doi.org/10.3390/jcm14238359
Vergara-Dangond C, Cobo-Ibáñez T, Cueva-Nájera G, Valverde-Garrido R, García-Yubero C, Trives-Folguera L, Paredes-Romero B, Esteban-Vázquez AV, Romero-Bogado L, De La Cámara-Fernández I, et al. Prevalence of Psoriatic Arthritis in Patients with Moderate-to-Severe Psoriasis in the Era of Biologics and Small Molecule Therapies. Journal of Clinical Medicine. 2025; 14(23):8359. https://doi.org/10.3390/jcm14238359
Chicago/Turabian StyleVergara-Dangond, Cristina, Tatiana Cobo-Ibáñez, Gabriela Cueva-Nájera, Ricardo Valverde-Garrido, Cristina García-Yubero, Laura Trives-Folguera, Beatriz Paredes-Romero, Ana Victoria Esteban-Vázquez, Liz Romero-Bogado, Isabel De La Cámara-Fernández, and et al. 2025. "Prevalence of Psoriatic Arthritis in Patients with Moderate-to-Severe Psoriasis in the Era of Biologics and Small Molecule Therapies" Journal of Clinical Medicine 14, no. 23: 8359. https://doi.org/10.3390/jcm14238359
APA StyleVergara-Dangond, C., Cobo-Ibáñez, T., Cueva-Nájera, G., Valverde-Garrido, R., García-Yubero, C., Trives-Folguera, L., Paredes-Romero, B., Esteban-Vázquez, A. V., Romero-Bogado, L., De La Cámara-Fernández, I., Steiner, M., Richi-Alberti, P., Acosta-Alfaro, A. V., Prats, I., & Muñoz-Fernández, S. (2025). Prevalence of Psoriatic Arthritis in Patients with Moderate-to-Severe Psoriasis in the Era of Biologics and Small Molecule Therapies. Journal of Clinical Medicine, 14(23), 8359. https://doi.org/10.3390/jcm14238359








