Profile of Patients with Primary Biliary Cholangitis and Evaluation of Response to Ursodeoxycholic Acid in a Romanian Center—Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
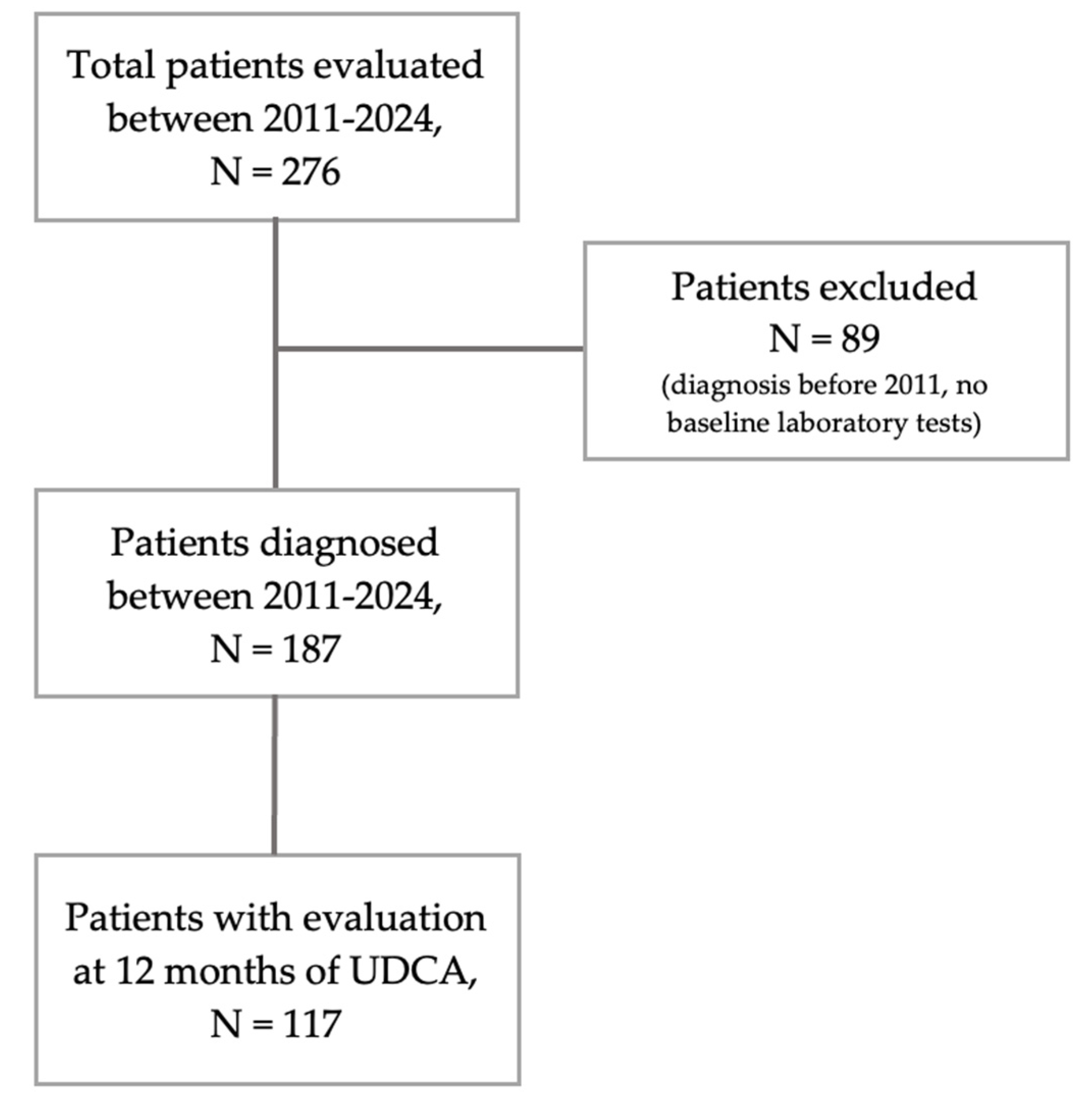
2.1. Study Design and Period of Inclusion
2.2. Inclusion and Exclusion Criteria
2.3. Variables and Endpoints
2.4. Quantitative Prognostic Index Scores Used
- GLOBE score = 0.044378 × age at start of UDCA therapy + 0.93982 × ln (TB times the upper limit of normal [ULN] at 1 year follow-up) + 0.335648 × ln (ALP × ULN at 1 year follow-up) − 2.266708 × ALB level × the lower limit of normal (LLN) at 1 year follow-up − 0.002581 × PLT count per 109/L at 1 year follow-up + 1.216865 [26,27];
- UK-PBC risk score = 1 − baseline survival function ∧exp(0.0287854 × [ALP baseline and after 12 months of therapy × ULN − 1.722136304] − 0.0422873 × [{(ALT where this was available, otherwise AST, baseline and after 12 months of therapy × ULN/10)−1} − 8.675729006] + 1.4199 × [ln{TB after 12 months of therapy × ULN/10} + 2.709607778] − 1.960303 × [ALB at baseline × LLN − 1.17673001] − 0.4161954 × [PL count at baseline × LLN − 1.873564875]) [26];
- UDCA Response Score (URS) = 0.77 + 0.60 × (√total bilirubin at diagnosis [× ULN]) − 1 − 2.73 × ln (ALP at diagnosis [×ULN]) + 0.35 × ln (ALT at diagnosis [×ULN]) + 0.03 × age [yr] − 0.15 × (time from diagnosis to the start of treatment [yr]) − 0.56 × (change in ALP concentration from diagnosis to the start of treatment [×ULN]) [28];
- AST-to-Platelet Ratio Index (APRI) = (AST × ULN)/PLT × 100 [24];
- Fibrosis-4 index (FIB-4 index) = (Age × AST)/(PLT × √(ALT)) [29];
2.5. Statistical Analysis
3. Results
3.1. Population
3.2. Special Populations
3.3. Response to Treatment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBC | Primary Biliary Cholangitis |
| ALP | Alkaline Phosphatase |
| GGT | Gamma-Glutamyl-Transpeptidase |
| AMA-M2 | Antimitochondrial Antibody M2 |
| UDCA | Ursodeoxycholic Acid |
| ULN | Upper Limit of Normal |
| Paris II | Paris II Response Criteria |
| POISE | POISE Response Criteria/Trial |
| GLOBE | Global Assessment of Liver Outcomes Score for PBC |
| UK-PBC | United Kingdom Primary Biliary Cholangitis Score |
| URS | UDCA Response Score |
| APRI | AST to PLT Ratio Index |
| FIB-4 | Fibrosis-4 index |
| ROC | Receiver Operating Characteristic |
| AUROC | Area Under the Receiver Operating Characteristic Curve |
| CI | Confidence Interval |
| EASL | European Association for the Study of the Liver |
| AMA | Antimitochondrial Antibody |
| ANAs | Antinuclear Autoantibodies |
| gp210 | Glycoprotein 210 |
| sp100 | Nuclear Body Protein sp100 |
| US | United States |
| PSC | Primary Sclerosing Cholangitis |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| AIH | Autoimmune Hepatitis |
| IgG | Immunoglobulin G |
| AST | Aspartate Aminotransferase |
| OCA | Obeticholic Acid |
| FXR | Farnesoid X Receptor |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| TB | Total Bilirubin |
| ALT | Alanine Aminotransferase |
| ALB | Albumin |
| PLT | Platelet Count |
| LLN | Lower Limit of Normal |
| OR | Odds Ratio |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| LRO | Liver-Related Outcomes |
References
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The Diagnosis and Management of Patients with Primary Biliary Cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Bernstein, D.; Shiffman, M.L.; Kwo, P.; Kim, W.R.; Kowdley, K.V.; Jacobson, I.M. Diagnosis and Management of Primary Biliary Cholangitis. Am. J. Gastroenterol. 2019, 114, 48–63. [Google Scholar] [CrossRef]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019, 69, 394–419. [Google Scholar] [CrossRef]
- Faisal, M.S.; Gonzalez, H.C.; Gordon, S.C. Primary Biliary Cholangitis: Epidemiology, Diagnosis, and Presentation. Clin. Liver Dis. 2024, 28, 63–77. [Google Scholar] [CrossRef]
- Tanaka, A.; Ma, X.; Takahashi, A.; Vierling, J.M. Primary Biliary Cholangitis. Lancet 2024, 404, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, F.; Bertazzoni, A.; Lleo, A. Contemporary Epidemiology of Primary Biliary Cholangitis. Clin. Liver Dis. 2022, 26, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Mansour, N.; John, B.V. Primary Biliary Cholangitis in Males: Pathogenesis, Clinical Presentation, and Prognosis. Clin. Liver Dis. 2022, 26, 643–655. [Google Scholar] [CrossRef]
- Gazda, J.; Drazilova, S.; Janicko, M.; Jarcuska, P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9151525. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Buchanan-Peart, K.A.; Macewan, J.P.; Levine, A.; Nair, R.; Wheeler, D.; Bessonova, L.; Goel, A.; Gish, R.G.; Bonder, A. A Nationwide Study of Primary Biliary Cholangitis Prevalence, Geographic Distribution, and Health Care Providers. Hepatol. Commun. 2025, 9, e0677. [Google Scholar] [CrossRef]
- Parés, A.; Albillos, A.; Andrade, R.J.; Berenguer, M.; Crespo, J.; Romero-Gómez, M.; Vergara, M.; Vendrell, B.; Gil, A. Primary Biliary Cholangitis in Spain. Results of a Delphi Study of Epidemiology, Diagnosis, Follow-up and Treatment. Rev. Esp. Enferm. Dig. 2018, 110, 641–649. [Google Scholar] [CrossRef]
- Martini, F.; Balducci, D.; Mancinelli, M.; Buzzanca, V.; Fracchia, E.; Tarantino, G.; Benedetti, A.; Marzioni, M.; Maroni, L. Risk Stratification in Primary Biliary Cholangitis. J. Clin. Med. 2023, 12, 5713. [Google Scholar] [CrossRef]
- Sohal, A.; Nikzad, N.; Kowdley, K.V. Overlap Syndromes in Autoimmune Liver Disease: A Review. Transl. Gastroenterol. Hepatol. 2025, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Sohal, A.; Kowdley, K.V. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis Therapy Landscape. Am. J. Gastroenterol. 2025, 120, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Trivella, J.; John, B.V.; Levy, C. Primary Biliary Cholangitis: Epidemiology, Prognosis, and Treatment. Hepatol. Commun. 2023, 7, e0179. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, D.; Tomas, K.; Marian, M.; Martin, J.; Dagmar, S.; Peter, J. The Treatment of Primary Biliary Cholangitis: From Shadow to Light. Ther. Adv. Gastroenterol. 2024, 17, 17562848241265782. [Google Scholar]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.H.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Hirschfield, G.M.; Coombs, C.; Malecha, E.S.; Bessonova, L.; Li, J.; Rathnayaka, N.; Mells, G.; Jones, D.E.; Trivedi, P.J.; et al. COBALT: A Confirmatory Trial of Obeticholic Acid in Primary Biliary Cholangitis With Placebo and External Controls. Am. J. Gastroenterol. 2024, 120, 390–400. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Bowlus, C.L.; Mayo, M.J.; Kremer, A.E.; Vierling, J.M.; Kowdley, K.V.; Levy, C.; Villamil, A.; Ladrón de Guevara Cetina, A.L.; Janczewska, E.; et al. A Phase 3 Trial of Seladelpar in Primary Biliary Cholangitis. N. Engl. J. Med. 2024, 390, 783–794. [Google Scholar] [CrossRef]
- Saeedian, B.; Babajani, N.; Bagheri, T.; Shirmard, F.O.; Pourfaraji, S.M. Efficacy and Safety of PPAR Agonists in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Gastroenterol. 2025, 25, 230. [Google Scholar] [CrossRef]
- Romanian National Agency for Drugs and Medical Devices. Evaluation Report of Medicai Technologies: SELADELPARUM; Romanian National Agency for Drugs and Medical Devices: Bucharest, Romania, 2025. [Google Scholar]
- Romanian National Agency for Drugs and Medical Devices. Evaluation Report of Medicai Technologies: ELAFIBRANORUM; Romanian National Agency for Drugs and Medical Devices: Bucharest, Romania, 2025. [Google Scholar]
- Corpechot, C.; Heurgue, A.; Tanne, F.; Potier, P.; Hanslik, B.; Decraecker, M.; de Lédinghen, V.; Ganne-Carrié, N.; Bureau, C.; Bourlière, M. Non-Invasive Diagnosis and Follow-up of Primary Biliary Cholangitis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101770. [Google Scholar] [CrossRef]
- Scaravaglio, M.; Carbone, M. Prognostic Scoring Systems in Primary Biliary Cholangitis: An Update. Clin. Liver Dis. 2022, 26, 629–642. [Google Scholar] [CrossRef]
- Tababi, R.; Mrabet, S.; Akkari, I.; Harbi, R.; Jazia, E. Ben Prognostic Scores in Primary Biliary Cholangitis. Future Sci. OA 2020, 10, FSO975. [Google Scholar] [CrossRef]
- Chang, J.I.; Kim, J.H.; Sinn, D.H.; Cho, J.Y.; Kim, K.M.; Oh, J.H.; Park, Y.; Sohn, W.; Goh, M.J.; Kang, W.; et al. Clinical Outcomes and Validation of Ursodeoxycholic Acid Response Scores in Patients with Korean Primary Biliary Cholangitis: A Multicenter Cohort Study. Gut Liver 2023, 17, 620–628. [Google Scholar] [CrossRef]
- Marenco-Flores, A.; Rojas Amaris, N.; Kahan, T.; Sierra, L.; Barba Bernal, R.; Medina-Morales, E.; Goyes, D.; Patwardhan, V.; Bonder, A. The External Validation of GLOBE and UK-PBC Risk Scores for Predicting Ursodeoxycholic Acid Treatment Response in a Large U.S. Cohort of Primary Biliary Cholangitis Patients. J. Clin. Med. 2024, 13, 4497. [Google Scholar] [CrossRef]
- De Veer, R.C.; Van Hooff, M.C.; Corpechot, C.; Thorburn, D.; Invernizzi, P.; Lammers, W.J.; Janssen, H.L.A.; Battezzati, P.M.; Nevens, F.; Lindor, K.D.; et al. Ursodeoxycholic Acid Treatment-Induced GLOBE Score Changes Are Associated With Liver Transplantation-Free Survival in Patients With Primary Biliary Cholangitis. Am. J. Gastroenterol. 2023, 118, 1196–1203. [Google Scholar] [CrossRef]
- Carbone, M.; Nardi, A.; Flack, S.; Carpino, G.; Varvaropoulou, N.; Gavrila, C.; Spicer, A.; Badrock, J.; Bernuzzi, F.; Cardinale, V.; et al. Pretreatment Prediction of Response to Ursodeoxycholic Acid in Primary Biliary Cholangitis: Development and Validation of the UDCA Response Score. Lancet Gastroenterol. Hepatol. 2018, 3, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic Accuracy of FIB-4, NAFLD Fibrosis Score and APRI for NAFLD-Related Events: A Systematic Review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Eruzun, H.; Bossen, L.; Turan Gökçe, D.; Ergenç, İ.; Harput, Z.N.; Aydemir, N.G.; Akdoğan Kayhan, M.; Yapıcı, H.B.; Doğan, T.; Holland-Fischer, P.; et al. Clinical and Biochemical Characteristics of a Danish and Turkish Cohort of Incident and Prevalent Patients with Primary Biliary Cholangitis. Turk. J. Gastroenterol. 2025, 36, 241–246. [Google Scholar] [CrossRef]
- Graf, M.; Lange, C.M.; Langer, M.M.; Schattenberg, J.M.; Seessle, J.; Dietz, J.; Vermehren, A.; Michael, F.A.; Mondorf, A.; Zeuzem, S.; et al. Primary Biliary Cholangitis (PBC)-Autoimmune Hepatitis (AIH) Variant Syndrome: Clinical Features, Response to Therapy and Long-Term Outcome. J. Clin. Med. 2023, 12, 7047. [Google Scholar] [CrossRef]
- Freedman, B.L.; Danford, C.J.; Patwardhan, V.; Bonder, A. Treatment of Overlap Syndromes in Autoimmune Liver Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1449. [Google Scholar] [CrossRef]
- Bernal, R.B.; Ferrigno, B.; Morales, E.M.; Castro, C.M.; Goyes, D.; Trivedi, H.; Patwardhan, V.R.; Bonder, A. Management of Primary Biliary Cholangitis: Current Treatment and Future Perspectives. Turk. J. Gastroenterol. 2023, 34, 89–100. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Victor, D.W.; MacEwan, J.P.; Nair, R.; Levine, A.; Hernandez, J.; Bessonova, L.; Li, J.; Wheeler, D.; Hirschfield, G. Longitudinal Relationship Between Elevated Liver Biochemical Tests and Negative Clinical Outcomes in Primary Biliary Cholangitis: A Population-Based Study. Aliment. Pharmacol. Ther. 2025, 61, 1775–1784. [Google Scholar] [CrossRef]
- Gazda, J.; Drazilova, S.; Gazda, M.; Janicko, M.; Koky, T.; Macej, M.; Carbone, M.; Jarcuska, P. Treatment Response to Ursodeoxycholic Acid in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2023, 55, 1318–1327. [Google Scholar] [CrossRef]
- D’Amato, D.; Carbone, M. Prognostic Models and Autoimmune Liver Diseases. Best Pract. Res. Clin. Gastroenterol. 2023, 67, 101878. [Google Scholar] [CrossRef]
- Corpechot, C.; Chazouillères, O.; Rousseau, A.; Le Gruyer, A.; Habersetzer, F.; Mathurin, P.; Goria, O.; Potier, P.; Minello, A.; Silvain, C.; et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N. Engl. J. Med. 2018, 378, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Shiffman, M.L.; Gulamhusein, A.; Kowdley, K.V.; Vierling, J.M.; Levy, C.; Kremer, A.E.; Zigmond, E.; Andreone, P.; Gordon, S.C.; et al. Seladelpar Efficacy and Safety at 3 Months in Patients with Primary Biliary Cholangitis: ENHANCE, a Phase 3, Randomized, Placebo-Controlled Study. Hepatology 2023, 78, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.H.; Haider, Z.; Sadia, F.N.U.; Tayyab, M.; Tariq, M.N.; Ans, H.H.; Javaid, M.B.; Khan, A.; Ahmad, M.H.; Rasheed, R.; et al. Peroxisome Proliferator-Activated Receptor (PPAR) Agonists for Patients With Primary Biliary Cholangitis With Inadequate Response to Ursodeoxycholic Acid (UDCA): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JGH Open 2025, 9, e70196. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Pinto, H.; Liberal, R.; Lopes, S.; Machado, M.V.; Carvalho, J.; Dias, T.; Santos, A.; Agostinho, C.; Figueiredo, P.; Loureiro, R.; et al. Predictors for Incomplete Response to Ursodeoxycholic Acid in Primary Biliary Cholangitis. Data from a National Registry of Liver Disease. United Eur. Gastroenterol. J. 2021, 9, 699–706. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, M.; He, H.; Lei, H.; Tai, W.; Yang, J.; Song, Z. Development and External Validation of an Early Prediction Model to Identify Irresponsive Patients and Prognosis of UDCA Treatment in Primary Biliary Cholangitis. Sci. Rep. 2024, 14, 31369. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Chazouillères, O.; Cortez-Pinto, H.; Macedo, G.; de Lédinghen, V.; Adekunle, F.; Carbone, M. A Consensus Integrated Care Pathway for Patients with Primary Biliary Cholangitis: A Guideline-Based Approach to Clinical Care of Patients. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 929–939. [Google Scholar] [CrossRef]
- Cheung, K.S.; Seto, W.K.; Fung, J.; Lai, C.L.; Yuen, M.F. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin. Transl. Gastroenterol. 2017, 8, e116. [Google Scholar] [CrossRef]
- Lam, L.; Soret, P.-A.; Lemoinne, S.; Hansen, B.; Hirschfield, G.; Gulamhusein, A.; Montano-Loza, A.J.; Lytvyak, E.; Parés, A.; Olivas, I.; et al. Dynamics of Liver Stiffness Measurement and Clinical Course of Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2024, 22, 2432–2441.e2. [Google Scholar] [CrossRef] [PubMed]
| Total PBC Patients (N = 276) | PBC Diagnosed Between 2011–2024 (N = 187) | p Value | |
|---|---|---|---|
| PBC-AIH, % | 25% | 23.22% | 0.219 |
| PBC-PSC, % | 3.26% | 3.26% | 0.086 |
| Age at diagnosis, mean (CI95%) | 53.89 (52.43–55.34) | 55.54 (53.43–56.58) | 0.001 |
| Follow-up period, months (CI95%) | 96.54 (87.37–105.7) | 65.6 (59.96–72.56) | <0.001 |
| Female patients, % | 95.28% | 94.11% | 0.167 |
| Bone disease, % | 13.77% | 12.8% | 0.399 |
| Thyroid disease, % | 19.57% | 18.96% | 0.646 |
| Sjogren’s syndrome, % | 6.88% | 7.11% | 0.790 |
| Type 2 DM, % | 5.8% | 6.16% | 0.641 |
| Liver transplant, % | 4.35% | 3.79% | 0.414 |
| AMA pos, % | 85.51% | 87.68% | 0.134 |
| gp210 pos, % | 7.25% | 8.53% | 0.138 |
| sp100 pos, % | 7.61% | 9% | 0.115 |
| Liver cirrhosis, % | 40.58% | 36.02% | 0.001 |
| Decompensated cirrhosis, % | 18.48% | 17.54% | 0.414 |
| Ascites, % | 24.11% | 27.63% | 0.164 |
| Variceal bleeding, % | 15.18% | 19.73% | 0.790 |
| Encephalopathy, % | 8.04% | 9.21% | 0.924 |
| Jaundice, % | 28.57% | 32.89% | 0.668 |
| Liver-related death, % | 4.35% | 4.74% | 0.566 |
| Variable | Value |
|---|---|
| PLT, ×109/L (IQR) | 246.6 (171–299) |
| AST, U/L (IQR) | 69.85 (34–83) |
| ALT, U/L (IQR) | 93.64 (38–99) |
| GGT, U/L (IQR) | 270.2 (68–330) |
| ALP, U/L (IQR) | 284.01 (130–362) |
| Total bilirubin, mg/dL (IQR) | 1.37 (0.5–1.2) |
| Total Cholesterol, mg/dL (IQR) | 222.1 (169–256) |
| Total Triglycerides, mg/dL (IQR) | 115.1 (74–131) |
| Albumin, mg/dL (IQR) | 4.23 (4.15–4.4) |
| GGT/ULN > ALP/ULN, % | 34.75% |
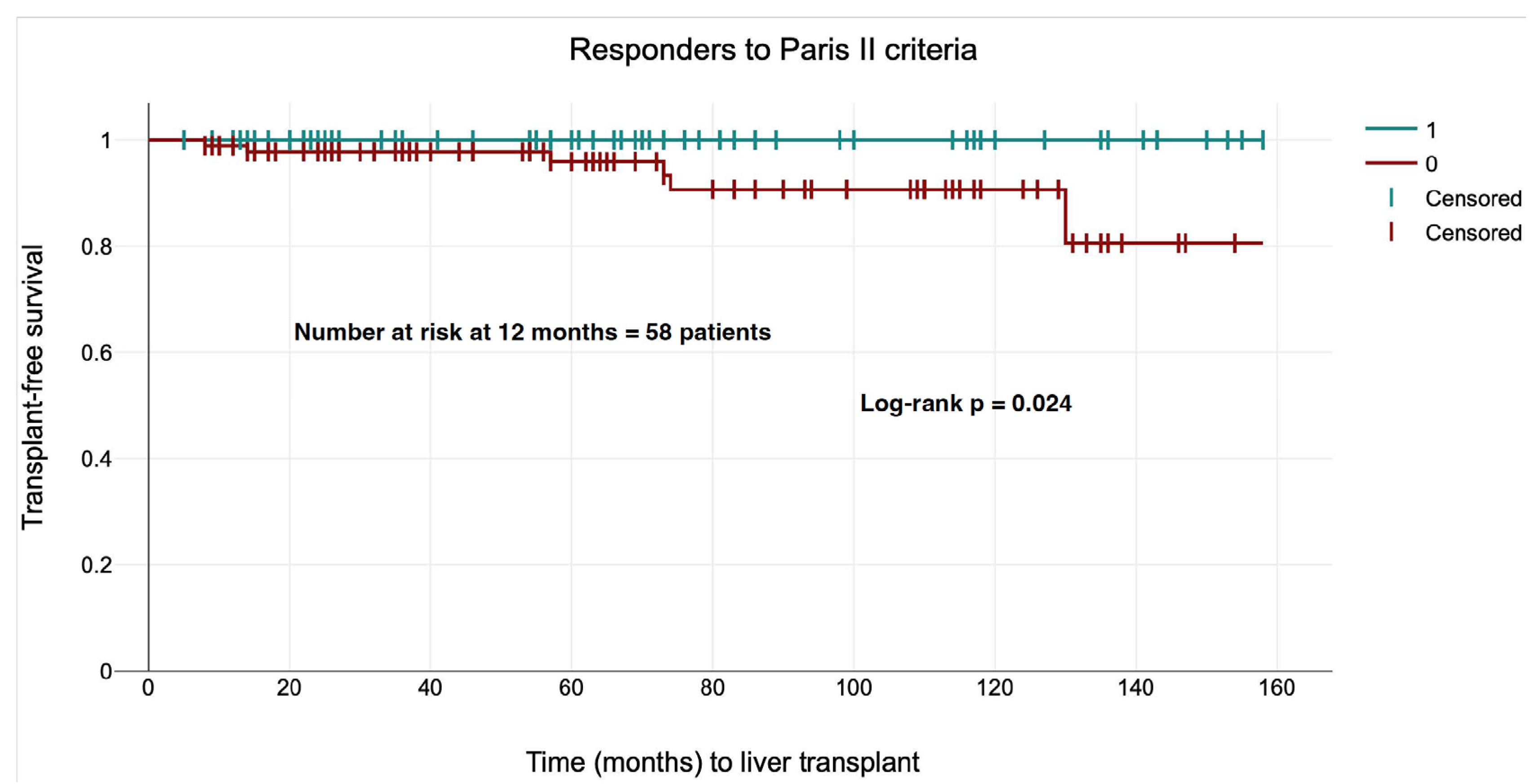
| Criteria/Score | Patients | Responders to Paris II at 1 year (N = 52) | p Value |
|---|---|---|---|
| Paris II, N (%) | 52 (44.44) | - | - |
| POISE, N (%) | 49 (41.88) | 52 (78.85) | <0.001 |
| Barcelona, N (%) | 61 (52.14) | 37 (71.15) | <0.001 |
| GGT decrease <1.5 × ULN, N (%) | 66 (56.41) | 41 (78.84) | <0.001 |
| ALP decrease <1.5 × ULN, N (%) | 64 (54.70) | 52 (100) | <0.001 |
| GGT decrease <1.67 × ULN, N (%) | 70 (59.82) | 44 (84.61) | <0.001 |
| ALP decrease <1.67 × ULN, N (%) | 73 (62.39) | 52 (100) | <0.001 |
| >50% ALP decrease, N (%) | 31 (26.49) | 16 (30.76) | <0.001 |
| >40% ALP decrease, N (%) | 47 (40.17) | 24 (46.15) | 0.391 |
| GLOBE score < 0.3, N (%) | 62 (52.99) | 40 (76.92) | <0.001 |
| URS ≥ 1.41, N (%) | 79 (67.52) | 52 (100) | <0.001 |
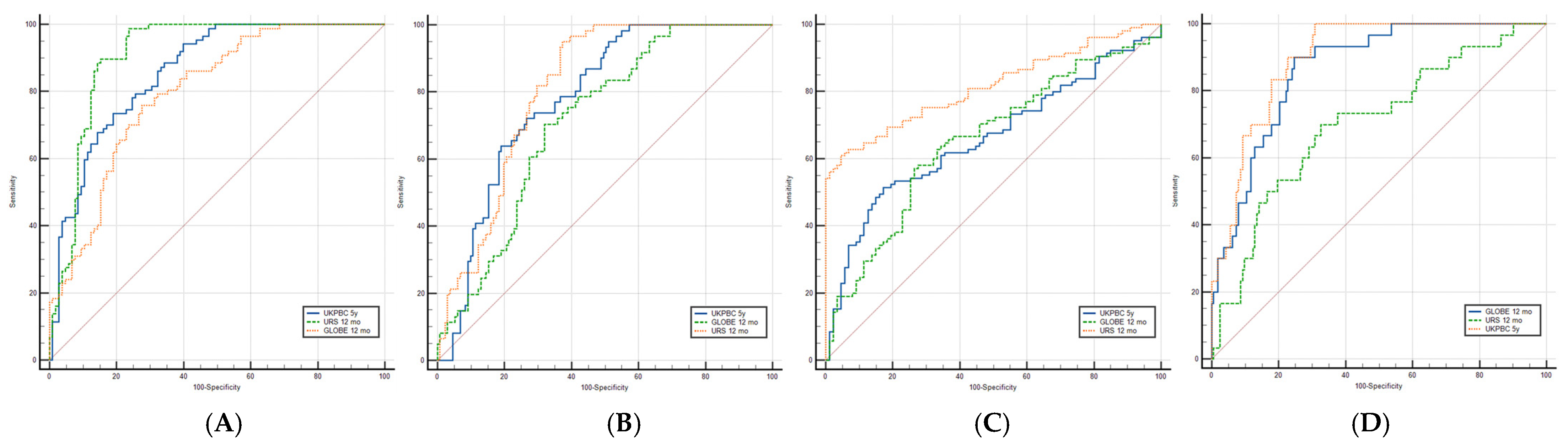
| Score/Criteria | Paris II (Responders = 52) | POISE (Responders = 49) | Barcelona (Responders = 61) | Liver-Related Outcomes (N = 30) |
|---|---|---|---|---|
| UK-PBC 5 years, AUROC (CI 95%) | 0.859 (Ss =9 9%; Sp = 98.9%) | 0.782 (Ss = 95.4%; Sp = 98.4%) | 0.662 (Ss = 98.9%; Sp = 99%) | 0.899 (Ss = 96.7%; Sp = 30.9%) |
| GLOBE score at follow-up, AUROC (CI 95%) | 0.797 (Ss = 99%; Sp = 82.8%) | 0.715 (Ss = 99.2%; Sp = 95.1%) | 0.655 (Ss = 98.9%; Sp = 99%) | 0.867 (Ss = 96.7%; Sp = 53.7%) |
| URS at follow-up, AUROC (CI 95%) | 0.910 (Ss = 98.9%; Sp = 29.5%) | 0.811 (Ss = 98.4%; Sp = 46.6%) | 0.816 (Ss = 99%; Sp = 94.3%) | 0.702 (Ss = 99.4%; Sp = 96.7%) |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Variable | Mean ± SD | p Value | OR | CI 95% | p Value |
| Age | 58.52 ± 11.88 | 0.032 | 1.02 | 0.97–1.07 | 0.406 |
| GGT | 226.29 ± 246.64 | 0.090 | - | - | - |
| ALP | 231.81 ± 177.88 | <0.001 | 1.00 | 0.99–1.00 | <0.001 |
| TB | 0.78 ± 0.56 | <0.001 | 0.53 | 0.25–1.10 | 0.089 |
| ALT | 78.23 ± 64 | 0.032 | 0.99 | 0.98–1.01 | 0.324 |
| AST | 59.38 ± 50.26 | 0.014 | 1.01 | 0.99–1.04 | 0.258 |
| ALB | 4.26 ± 0.56 | 0.003 | 1.96 | 0.70–5.52 | 0.200 |
| PLT | 255.13 ± 92.76 | 0.136 | - | - | - |
| Cholesterol | 201.82 ± 57.65 | 0.120 | - | - | - |
| Triglycerides | 114.19 ± 47.9 | 0.549 | - | - | - |
| AMA-M2 presence | 0.23(X2) | 0.630 | - | - | - |
| sp100 presence | 3.93(X2) | 0.047 | 2.40 | 0.46–12.38 | 0.297 |
| gp210 presence | 1.11(X2) | 0.293 | - | - | - |
| FIB4 index | 1.95 ± 2.03 | 0.013 | 1.11 | 0.51–2.41 | 0.795 |
| APRI score | 0.79 ± 0.9 | 0.004 | 0.49 | 0.05–4.95 | 0.548 |
| Patients Evaluated at 0–12–24 Months, N = 98 (A) | Patients Evaluated at Baseline and 24 months, N = 140 (B) | p Value | |
|---|---|---|---|
| Paris II, % | 55.10% | 56.42% | 0.427 |
| Barcelona, % | 66.33% | 70% | 0.096 |
| POISE, % | 47.96% | 45.71% | 0.441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandea, M.; Iacob, S.M.; Ghioca, M.C.; Gheorghe, C.; Gheorghe, L.S. Profile of Patients with Primary Biliary Cholangitis and Evaluation of Response to Ursodeoxycholic Acid in a Romanian Center—Retrospective Study. J. Clin. Med. 2025, 14, 8240. https://doi.org/10.3390/jcm14228240
Mandea M, Iacob SM, Ghioca MC, Gheorghe C, Gheorghe LS. Profile of Patients with Primary Biliary Cholangitis and Evaluation of Response to Ursodeoxycholic Acid in a Romanian Center—Retrospective Study. Journal of Clinical Medicine. 2025; 14(22):8240. https://doi.org/10.3390/jcm14228240
Chicago/Turabian StyleMandea, Matei, Speranta M. Iacob, Mihaela C. Ghioca, Cristian Gheorghe, and Liliana S. Gheorghe. 2025. "Profile of Patients with Primary Biliary Cholangitis and Evaluation of Response to Ursodeoxycholic Acid in a Romanian Center—Retrospective Study" Journal of Clinical Medicine 14, no. 22: 8240. https://doi.org/10.3390/jcm14228240
APA StyleMandea, M., Iacob, S. M., Ghioca, M. C., Gheorghe, C., & Gheorghe, L. S. (2025). Profile of Patients with Primary Biliary Cholangitis and Evaluation of Response to Ursodeoxycholic Acid in a Romanian Center—Retrospective Study. Journal of Clinical Medicine, 14(22), 8240. https://doi.org/10.3390/jcm14228240







