An Evaluation of the Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score in Cushing’s Syndrome and Mild Autonomous Cortisol Secretion
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis of Demographic, Biochemical, and Hormonal Parameters
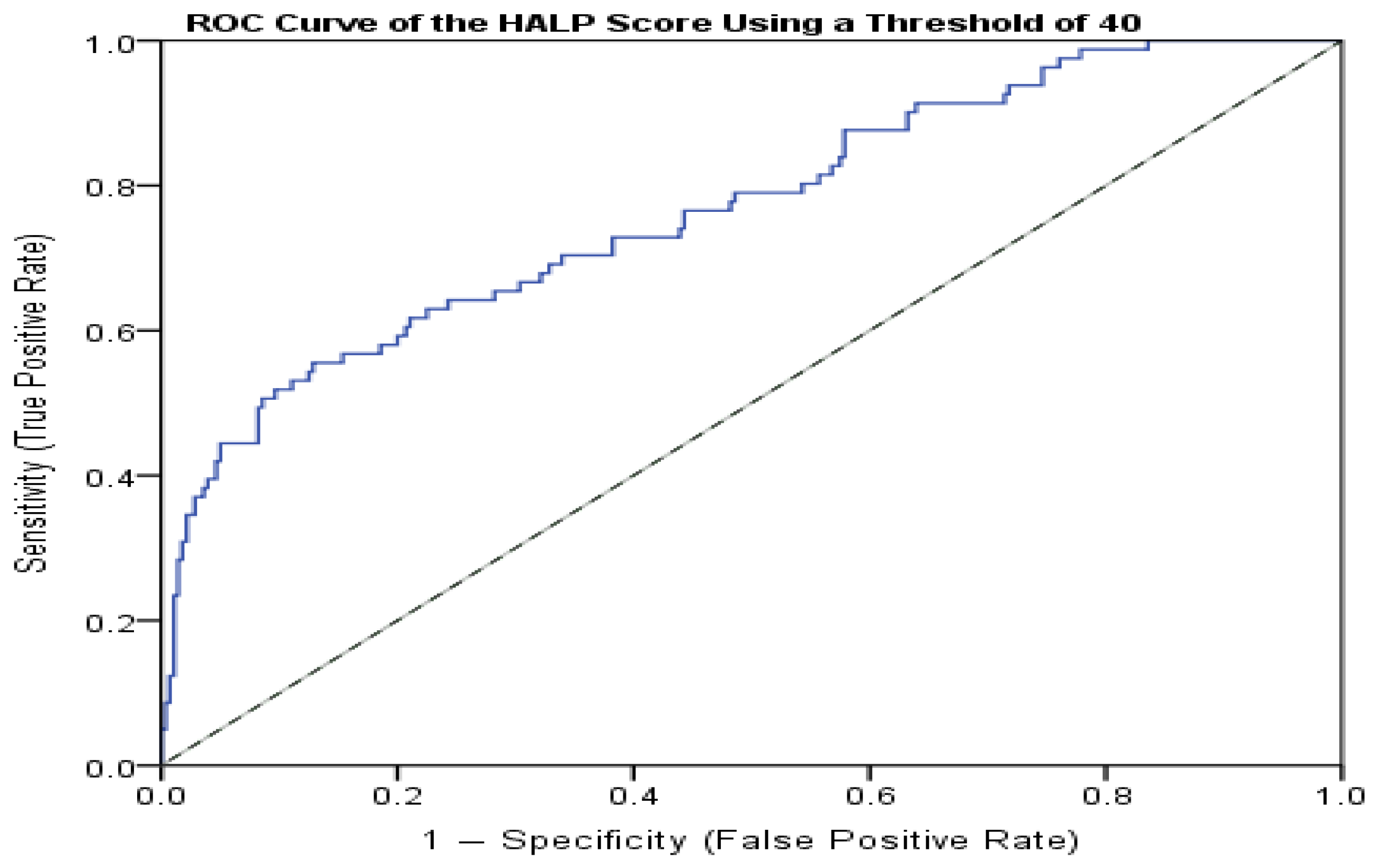
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacroix, A.; Feelders, R.A.; Stratakis, C.A.; Nieman, L.K. Cushing’s syndrome. Lancet 2015, 386, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Arnaldi, G.; Angeli, A.; Atkinson, A.B.; Bertagna, X.; Cavagnini, F.; Chrousos, G.P.; Fava, G.A.; Findling, J.W.; Gaillard, R.C.; Grossman, A.B.; et al. Diagnosis and complications of Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2003, 88, 5593–5602. [Google Scholar] [CrossRef]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenal incidentalomas. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef]
- Loriaux, D.L.; Longo, D.L. Diagnosis and differential diagnosis of Cushing’s syndrome. N. Engl. J. Med. 2017, 376, 1451–1459. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Nugent, C.A.; Warner, H.R.; Dunn, J.T.; Tyler, F.H. Probability theory in the diagnosis of Cushing’s syndrome. J. Clin. Endocrinol. Metab. 1964, 24, 621–627. [Google Scholar] [CrossRef]
- Detomas, M.; Altieri, B.; Chifu, I.; Remde, H.; Zhou, X.; Landwehr, L.-S.; Sbiera, S.; Kroiss, M.; Fassnacht, M.; Deutschbein, T. Subtype-specific pattern of white blood cell differential in endogenous hypercortisolism. Eur. J. Endocrinol. 2022, 187, 439–449. [Google Scholar] [CrossRef]
- Kawa, M.P.; Sobuś, A.; Pius-Sadowska, E.; Łuczkowska, K.; Rogińska, D.; Wnęk, S.; Paczkowska, E.; Walczak, M.; Syrenicz, A.; Machaliński, B. Apoptosis evaluation in circulating CD34+-enriched hematopoietic stem and progenitor cells in patients with abnormally increased production of endogenous glucocorticoids in Cushing’s syndrome. Int. J. Mol. Sci. 2022, 23, 15794. [Google Scholar] [CrossRef]
- Sterling, K. The effect of Cushing’s syndrome upon serum albumin metabolism. J. Clin. Investig. 1960, 39, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hiramatsu, R.; Iwaoka, T.; Fujii, Y.; Shimada, T.; Umeda, T. Changes of platelets, γ-glutamyltranspeptidase, choline esterase, creatine phosphokinase, and serum lactic dehydrogenase levels in patients with Cushing’s syndrome. Tohoku J. Exp. Med. 1984, 142, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Tronche, F.; Wessely, O.; Kellendonk, C.; Reichardt, H.M.; Steinlein, P.; Schütz, G.; Beug, H. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999, 13, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, L.; Geer, E.B.; Martelli, F.; Mazzarini, M.; Funnell, A.; Bieker, J.J.; Papayannopoulou, T.; Migliaccio, A.R. Hypercortisolemic Cushing’s patients possess a distinct class of hematopoietic progenitor cells leading to erythrocytosis. Haematologica 2022, 108, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Stellacci, E.; Di Noia, A.; Di Baldassarre, A.; Migliaccio, G.; Battistini, A.; Migliaccio, A.R. Interaction between the glucocorticoid and erythropoietin receptors in human erythroid cells. Exp. Hematol. 2009, 37, 559–572. [Google Scholar] [CrossRef]
- Penguin, D.; Zhang, C.-J.; Tang, Q.; Zhang, L.; Yang, K.-W.; Yu, X.-T.; Gong, Y.; Li, X.-S.; He, Z.-S.; Zhou, L.-Q. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. 2018, 18, 20. [Google Scholar] [CrossRef]
- Li, Q.; Chen, M.; Zhao, H.; Zeng, J. The prognostic and clinicopathological value of HALP score in non-small cell lung cancer. Front. Immunol. 2025, 16, 1576326. [Google Scholar] [CrossRef]
- Qian, C.; Liu, J.; Meng, C.; Cheng, J.; Wu, B.; Liao, J. The significant prognostic value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score in digestive system cancers: A systematic review and meta-analysis. BMC Gastroenterol. 2025, 25, 15068. [Google Scholar] [CrossRef]
- Lin, L.; Huang, H.; Wu, M.; Chen, F.; Li, C. The modified HALP score is associated with short-term mortality in critically ill patients with sepsis: A cohort study. J. Infect. Dev. Ctries. 2025, 19, 924–933. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Li, H. Hemoglobin albumin lymphocyte and platelet score and all-cause mortality in coronary heart disease: A retrospective cohort study of NHANES database. Front. Cardiovasc. Med. 2023, 10, 1241217. [Google Scholar] [CrossRef]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef]
- Mohanty, S.; Das, D.; Ghosh, A.; Maiti, R.; Nanda, P. Hematological parameters as an early marker of deep vein thrombosis in diabetes mellitus: An observational study. Cureus 2023, 15, e36813. [Google Scholar] [CrossRef]
- Eskin, F.; Köseoğlu, H.; Düzenli, T.; Ozden, M.; Bebek, B.; Kaya, M.; Sezikli, M. A new index for predicting malignant causes in patients with extrahepatic biliary obstruction: The hemoglobin, albumin, lymphocyte, and platelet (HALP) score. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2514–2521. [Google Scholar] [PubMed]
- Akbas, A.; Koyuncu, S.; Hacım, N.A.; Dasiran, M.F.; Kasap, Z.A.; Okan, I. Can HALP score differentiate between malignant and benign causes of acute mechanical intestinal obstruction? Cancer Biother. Radiopharm. 2022, 37, 199–204. [Google Scholar] [PubMed]
- Benli, S.; Tazeoğlu, D. The efficacy of HALP score in signifying acute appendicitis severity and postoperative outcomes. Updates Surg. 2023, 75, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Cay, F.; Duran, A. Predictive factors of success in sleeve gastrectomy: One-year follow-up and the significance of HALP score. J. Coll. Physicians Surg. Pak. 2021, 31, 1406–1411. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Liu, K.; Zhao, J.; Fang, H.; Tao, Y.; Pei, L.; Tian, M.; Liu, H.; Wang, X.; et al. HALP-based prognostic model for patients with cerebral venous sinus thrombosis: A retrospective cohort study. J. Atheroscler. Thromb. 2023, 30, 1742–1749. [Google Scholar] [CrossRef]
- Kamgang, V.W.; Murkwe, M.; Wankeu-Nya, M. Biological effects of cortisol. In Cortisol—Between Physiology and Pathology; IntechOpen: London, UK, 2023. [Google Scholar]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The kaleidoscope of glucocorticoid effects on immune system. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef]
- Pitzalis, C.; Pipitone, N.; Bajocchi, G.; Hall, M.; Goulding, N.; Lee, A.; Kingsley, G.; Lanchbury, J.; Panayi, G. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: An additional mechanism for their anti-inflammatory effect. J. Immunol. 1997, 158, 5007–5016. [Google Scholar] [CrossRef]
- Gursoy, A.; Dogruk Unal, A.; Ayturk, S.; Karakus, S.; Izol, A.N.; Tutuncu, N.B.; Demirag, N.G. Polycythemia as the first manifestation of Cushing’s disease. J. Endocrinol. Investig. 2006, 29, 742–744. [Google Scholar] [CrossRef]
- Dusek, T.; Kastelan, D.; Solak, M.; Kinda, S.B.; Aganovic, I.; Korsic, M. Polycythemia as the first manifestation of Cushing’s disease. J. Endocrinol. Investig. 2008, 31, 940. [Google Scholar] [CrossRef]
- Detomas, M.; Deutschbein, T.; Tamburello, M.; Chifu, I.; Kimpel, O.; Sbiera, S.; Kroiss, M.; Fassnacht, M.; Altieri, B. Erythropoiesis in Cushing syndrome: Sex-related and subtype-specific differences. Eur. J. Endocrinol. 2023, 189, K35–K45. [Google Scholar] [CrossRef]
- Ambrogio, A.G.; De Martin, M.; Ascoli, P.; Cavagnini, F.; Giraldi, F.P. Gender-dependent changes in haematological parameters in patients with Cushing’s disease before and after remission. Eur. J. Endocrinol. 2014, 170, 393–400. [Google Scholar] [CrossRef] [PubMed]
| Variable | CD (n = 44, 39.6%) | ACS (n = 37, 33.3%) | MACS (n = 30, 27.0%) | p | Compact Ordering |
|---|---|---|---|---|---|
| Age (years) * | 52 (22–70) | 53 (22–74) | 56.5 (36–74) | 0.148 | ≈ |
| Female, n (%) | 39 (88.6%) | 35 (94.6%) | 21 (70.0%) | 0.013 (χ2) | ACS ≈ CD > MACS |
| BMI (kg/m2) * | 33.0 (23.8–49.6) | 34.2 (23.4–48.4) | 28.5 (21.5–39.5) | <0.001 | CD ≈ ACS > MACS |
| Glucose (mg/dL) * | 95.0 (66–273) | 96.0 (73–264) | 86.5 (70–147) | 0.033 | ACS > MACS ≈ CD |
| Creatinine (mg/dL) * | 0.660 (0.40–0.95) | 0.700 (0.40–1.30) | 0.675 (0.40–1.00) | 0.723 | ≈ |
| ALT (U/L) * | 25 (12–156) | 22 (12–93) | 20 (8–45) | 0.009 | CD > ACS ≈ MACS |
| TKOL (mg/dL) | 201.9 ± 46.1 | 197.3 ± 39.9 | 192.4 ± 43.6 | 0.648 | ≈ |
| LDL (mg/dL) | 118.1 ± 40.8 | 118.2 ± 31.3 | 116.2 ± 38.1 | 0.971 | ≈ |
| HDL (mg/dL) * | 49.0 (24–78) | 47.0 (26–93) | 44.5 (30–72) | 0.776 | ≈ |
| TG (mg/dL) * | 149.5 (69–486) | 129.0 (66–322) | 146.0 (61–311) | 0.610 | ≈ |
| A1C (%) * | 6.0 (5.1–10.8) | 6.0 (5.1–9.4) | 5.9 (5.2–7.7) | 0.368 | ≈ |
| K (mmol/L) * | 4.50 (2.1–5.34) | 4.40 (3.6–5.1) | 4.10 (2.8–4.7) | 0.001 | CD ≈ ACS > MACS |
| Test (Unit) | CD | ACS | MACS | p (Kruskal–Wallis) | Compact Ordering |
|---|---|---|---|---|---|
| Plasma ACTH (pg/mL) * (n = 111) | 53.0 (12.2–189.0) (n = 44) | 6.2 (0.0–14.6) (n = 37) | 6.2 (5.0–14.9) (n = 30) | <0.001 | CD > ACS ≈ MACS |
| Morning serum cortisol (µg/dL) * (n = 111) | 22.45 (7.8–63.0) (n = 44) | 14.6 (6.0–30.9) (n = 37) | 12.85 (6.5–26.5) (n = 30) | <0.001 | CD > ACS ≈ MACS |
| DHEAS (µg/dL) * (n = 105) | 174.5 (3–621) (n = 40) | 22.0 (0–590) (n = 35) | 42.0 (10–214) (n = 30) | <0.001 | CD > ACS ≈ MACS |
| DST 1 mg cortisol (µg/dL) * (n = 111) | 13.5 (2–73) (n = 44) | 3.9 (1.9–30.9) (n = 37) | 3.53 (1.9–28.0) (n = 30) | <0.001 | CD > ACS ≈ MACS |
| 1.8–5 µg/dL (mild elevation) | 13 (29.5%) | 24 (64.9%) | 21 (70%) | ||
| 5–10 µg/dL (moderate) | 4 (9.1%) | 3 (8.1%) | 7 (23%) | ||
| >10 µg/dL (marked) | 27 (61.4%) | 10 (27.0%) | 2 (6.7%) | p < 0.001 | CD > ACS > MACS |
| 24-h urinary free cortisol (µg/24 h) * (n = 104) | 86.5 (17–2279) (n = 40) | 56.0 (4.4–565) (n = 34) | 52.5 (12.4–442) (n = 30) | 0.014 | CD > ACS ≈ MACS |
| ≤1× (normal) | 11 (27.5%) | 12 (35.5%) | 15 (50%) | ||
| 1–3× (mild high) | 13 (32.5%) | 15 (44.1%) | 10 (33%) | ||
| >3× (marked high) | 15 (37.5%) | 5 (14.7%) | 2 (6.7%) | p = 0.019 | CD > ACS ≈ MACS |
| Late-night serum cortisol (µg/dL) * (n = 59) | 17.1 (7.0–57.0) (n = 32) | 10.7 (2.4–49.1) (n = 14) | 5.6 (2.8–24.4) (n = 13) | <0.001 | CD > ACS > MACS |
| Late-night salivary cortisol (µg/dL) * (n = 65) | 0.66 (0.00–8.40) (n = 29) | 0.48 (0.02–4.17) (n = 23) | 0.39 (0.02–1.58) (n = 20) | 0.462 | CD ≈ ACS ≈ MACS |
| Feature | CD (n = 44) | ACS (n = 37) | MACS (n = 30) | p Value | Post Hoc |
|---|---|---|---|---|---|
| Obesity | 26 (59.1%) | 26 (70.3%) | 9 (30.0%) | <0.001 | ACS ≫ CD ≫ MACS |
| Hirsutism * | 8 (18.2%) | 4 (10.8%) | 0 (0%) | 0.033 | CD > ACS > MACS |
| Plethora | 13 (29.5%) | 7 (18.9%) | 0 (0%) | 0.005 | CD ≫ ACS ≫ MACS |
| Moon face | 20 (45.5%) | 18 (48.6%) | 0 (0%) | <0.001 | CD ≈ ACS ≫ MACS |
| Buffalo hump | 20 (45.5%) | 16 (43.2%) | 0 (0%) | <0.001 | CD ≈ ACS ≫ MACS |
| Striae * | 10 (22.7%) | 4 (10.8%) | 0 (0%) | 0.011 | CD > ACS > MACS |
| Easy bruising * | 7 (15.9%) | 4 (10.8%) | 0 (0%) | 0.051 | Trend: CD > ACS > MACS |
| Proximal muscle weakness | 12 (27.3%) | 2 (5.4%) | 1 (3.3%) | 0.003 | CD ≫ ACS ≈ MACS |
| Centripetal obesity | 13 (29.5%) | 17 (45.9%) | 0 (0%) | <0.001 | ACS ≫ CD ≫ MACS |
| Total symptom count (median [IQR]) | 5 (3–6) | 3 (2–4) | 1 (0–2) | <0.001 | CD > ACS > MACS |
| Comorbidity | CD (n = 44) | ACS (n = 37) | MACS (n = 30) | p | Significant Pattern |
|---|---|---|---|---|---|
| Type 2 Diabetes Mellitus | 19 (43.2%) | 15 (40.5%) | 9 (30.0%) | 0.501 | NS |
| Hypertension | 25 (56.8%) | 22 (59.5%) | 18 (60.0%) | 0.955 | NS |
| Dyslipidemia | 13 (29.5%) | 12 (32.4%) | 10 (33.3%) | 0.933 | NS |
| Coronary Artery Disease | 5 (11.4%) | 1 (2.7%) | 4 (13.3%) | 0.249 | NS (trend CD ≈ MACS > ACS) |
| Osteoporosis | 12 (27.3%) | 6 (16.2%) | 4 (13.3%) | 0.268 | NS (trend CD > ACS > MACS) |
| Total Comorbidity Count (Mean ± SD) | 1.68 ± 1.44 | 1.51 ± 1.22 | 1.50 ± 1.38 | 0.858 | NS (Kruskal–Wallis p > 0.05) |
| Variable | MACS | ACS | CD | NFA | p | Compact Ordering (sig. Only) |
|---|---|---|---|---|---|---|
| Sex (female %) | 21 (70.0%) | 35 (94.6%) | 39 (88.6%) | 153 (61.2%) | <0.001 | ACS ≈ CD ≈ MACS > NFA |
| Age (years) * | 56.5 (36–74) | 53.0 (22–74) | 52.0 (22–70) | 55.0 (18–82) | 0.041 | NFA > CD |
| Glucose (mg/dL) * | 86.5 (70–147) | 96.0 (73–264) | 95.0 (66–273) | 92.0 (67–381) | 0.042 | ACS ≈ CD ≈ NFA > MACS |
| Creatinine (mg/dL) * | 0.675 (0.40–1.00) | 0.700 (0.40–1.30) | 0.660 (0.40–0.95) | 0.775 (0.46–1.34) | <0.001 | NFA > ACS ≈ MACS ≈ CD |
| Albumin (g/L) | 44.70 ± 2.98 | 44.08 ± 3.10 | 42.90 ± 4.34 | 46.03 ± 2.40 | <0.001 | NFA > CD and NFA > ACS |
| ALT (U/L) * | 20 (8–45) | 22 (12–93) | 25 (12–156) | 23 (6–237) | 0.027 | CD > ACS ≈ NFA ≈ MACS |
| Hemoglobin (g/dL) | 13.91 ± 1.50 | 13.38 ± 1.12 | 13.22 ± 1.18 | 14.19 ± 1.23 | <0.001 | CD, ACS < MACS, NFA |
| Lymphocytes (×109/L) | 2.28 ± 0.70 | 2.25 ± 0.57 | 1.90 ± 0.55 | 2.25 ± 0.64 | 0.007 | CD < ACS ≈ MACS ≈ NFA |
| Platelets (×109/L) | 263.3 ± 79.6 | 290.5 ± 58.0 | 295.1 ± 87.3 | 253.3 ± 53.3 | <0.001 | ACS ≈ CD > NFA |
| DST 1 mg (µg/dL) * | 3.53 (1.90–28.00) | 3.90 (1.90–30.95) | 13.50 (2.00–73.00) | 1.07 (0.40–1.78) | <0.001 | CD > ACS ≈ MACS > NFA |
| HALP score * | 55.13 (23.47) | 47.01 (24.78) | 37.78 (22.45) | 58.36 (22.32) | <0.001 | CD < ACS < MACS ≈ NFA |
| Analysis | Group | n | OR (95% CI) | p Value |
|---|---|---|---|---|
| Multivariable regression | HALP score | 361 | 0.928 (0.907–0.950) | <0.001 |
| (Both sex and HALP score included) | Female sex | 361 | 5.71 | <0.001 |
| Sex-stratified | Males only | 113 | 0.914 (0.846–0.987) | 0.022 |
| Females only | 248 | 0.930 (0.907–0.952) | <0.001 | |
| Control group (NFA) | Males | 106 | 59.52 ± 17.23 | 0.471 a |
| HALP score comparison | Females | 174 | 58.01 ± 16.83 |
| HALP Score ≤ 40 | HALP Score > 40 | Total | |
|---|---|---|---|
| CD/ACS | 42 | 39 | 81 |
| NFA + MACS | 27 | 253 | 280 |
| Total | 69 | 292 | 361 |
| Group | n | Preop Median (IQR) | Postop Median (IQR) | p (2-Tailed) |
|---|---|---|---|---|
| MACS | 13 | 53.1 (23.5) | 57.1 (28.4) | 0.600 |
| ACS | 18 | 43.7 (24.8) | 48.6 (34.4) | 0.001 |
| CD | 18 | 37.8 (22.5) | 49.4 (22.1) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakı, S.; Tam, A.A.; Balsak, B.Ö.T.; Karaahmetli, G.; Altay, F.P.; Özdemir, D.; Topaloğlu, O.; Ersoy, R.; Çakır, B. An Evaluation of the Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score in Cushing’s Syndrome and Mild Autonomous Cortisol Secretion. J. Clin. Med. 2025, 14, 8207. https://doi.org/10.3390/jcm14228207
Fakı S, Tam AA, Balsak BÖT, Karaahmetli G, Altay FP, Özdemir D, Topaloğlu O, Ersoy R, Çakır B. An Evaluation of the Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score in Cushing’s Syndrome and Mild Autonomous Cortisol Secretion. Journal of Clinical Medicine. 2025; 14(22):8207. https://doi.org/10.3390/jcm14228207
Chicago/Turabian StyleFakı, Sevgül, Abbas Ali Tam, Belma Özlem Tural Balsak, Gülsüm Karaahmetli, Feride Pınar Altay, Didem Özdemir, Oya Topaloğlu, Reyhan Ersoy, and Bekir Çakır. 2025. "An Evaluation of the Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score in Cushing’s Syndrome and Mild Autonomous Cortisol Secretion" Journal of Clinical Medicine 14, no. 22: 8207. https://doi.org/10.3390/jcm14228207
APA StyleFakı, S., Tam, A. A., Balsak, B. Ö. T., Karaahmetli, G., Altay, F. P., Özdemir, D., Topaloğlu, O., Ersoy, R., & Çakır, B. (2025). An Evaluation of the Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score in Cushing’s Syndrome and Mild Autonomous Cortisol Secretion. Journal of Clinical Medicine, 14(22), 8207. https://doi.org/10.3390/jcm14228207







