Conformational Reconstruction in Head and Neck Bone Cancer: Could Fibula Free Flap Become the Gold Standard Flap?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics (Table 1 and Table 2)
| Case No. | Age/ Gender | Diagnosis/ Staging | Classification (Brown) | Adjuvant-Treatment | FFF Segments | Complications/ Implants Placed |
|---|---|---|---|---|---|---|
| 1 | 67/M | Squamous cell carcinoma/St. IVa |  Class II | 1 | 2 implants | |
| 2 | 59/F | Squamous cell carcinoma/St. III |  Class IV | 2 | 7 implants | |
| 3 | 70/F | Squamous cell carcinoma/St. IVa |  Class II | RT | 2 | 3 implants |
| 4 | 50/M | Squamous cell carcinoma/St. IVa |  Class IV c | RT | 4 | Volume deficit on right cheek treated by lipofilling\6 implants |
| 5 | 45/F | Squamous cell carcinoma/St. IVa |  Class II | RT | 2 | 2 implants |
| 6 | 53/M | Squamous cell carcinoma/St. IVa |  Class IV | 2 | 6 implants | |
| 7 | 43/M | Squamous cell carcinoma/St. III | 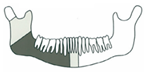 Class II | 2 | 3 implants | |
| 8 | 63/F | Squamous cell carcinoma/St. IVb |  Class IV | RT | 3 | Dead for cancer spreading metastasis |
| 9 | 48/M | Squamous cell carcinoma/St. II |  Class IV | 2 | 5 implants | |
| 10 | 62/M | Squamous cell carcinoma/St. IVa | 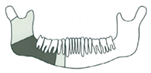 Class I | 1 | No implant rehabilitation | |
| 11 | 52/M | Squamous cell carcinoma/St. IVb |  Class II | RT | 2 | Dead for cancer spreading metastasis |
| 12 | 59/M | Squamous cell carcinoma/St. IVa |  Class II | 2 | 2 implants | |
| 13 | 42/M | Squamous cell carcinoma/St. IVa | 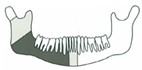 Class II | RT | 2 | No implant rehabilitation |
| 14 | 64/M | Squamous cell carcinoma/St. IVa | 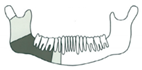 Class I | 2 | No implant rehabilitation/loss of FFF skin | |
| 15 | 38/M | Squamous cell carcinoma/St. IVa |  Class II | RT | 2 | left side cervical hematoma after 1 month\2 implants |
| 16 | 47/M | Squamous cell carcinoma/St. III |  Class II | 2 | Osteordionecrosis and screws removal plus bone curettage/2 implants | |
| 17 | 66/M | Squamous cell carcinoma/St. IVa |  Class IV | RT | 3 | 8 implants |
| 18 | 39/M | Squamous cell carcinoma/St. II |  Class IV | 2 | 6 implants | |
| 19 | 28/F | Osteosarcoma |  Class II d | CHT | 2 | Left nostril defect treated by ear cartilage graft and lipofilling\5 implants |
| 20 | 57/M | Squamous cell carcinoma/St. IVa |  Class II | 2 | Volume deficit on left cheek treated by lipofilling\2 implants | |
| 21 | 65/M | Squamous cell carcinoma/St. IVa |  Class IIc | RT | 2 | No implant rehabilitation/partial loss of FFF skin |
| 22 | 77/M | Osteosarcoma |  Class II b | CHT | 2 | 3 implants |
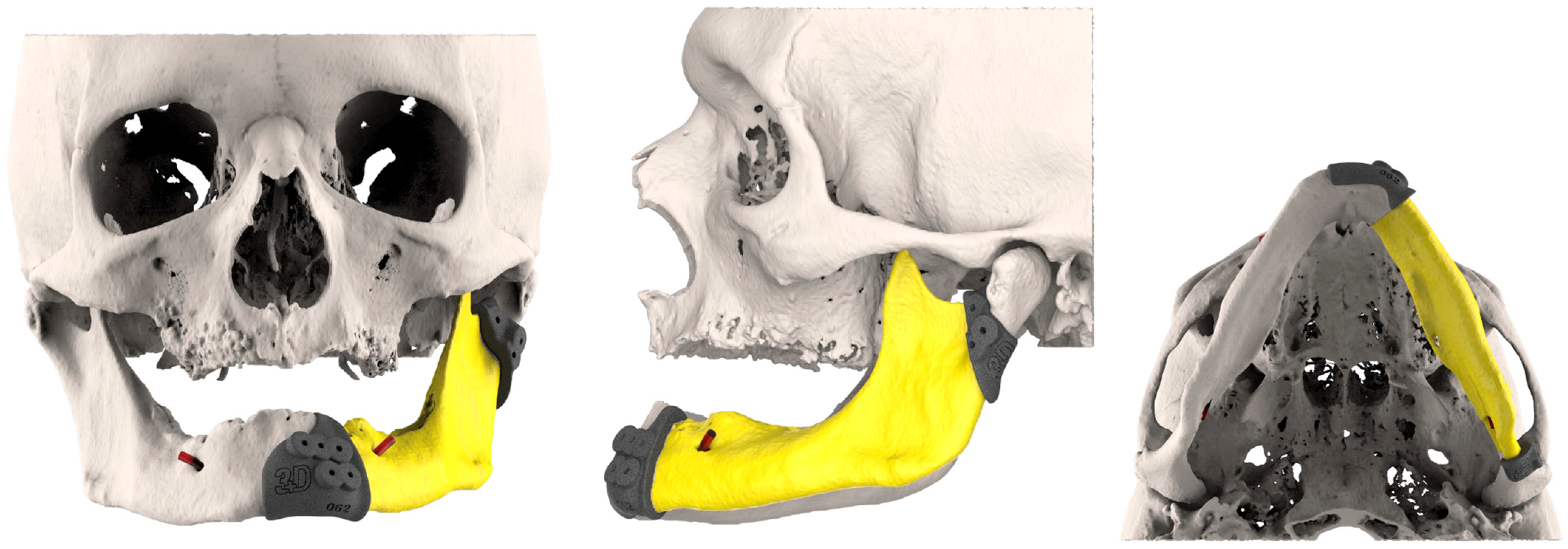
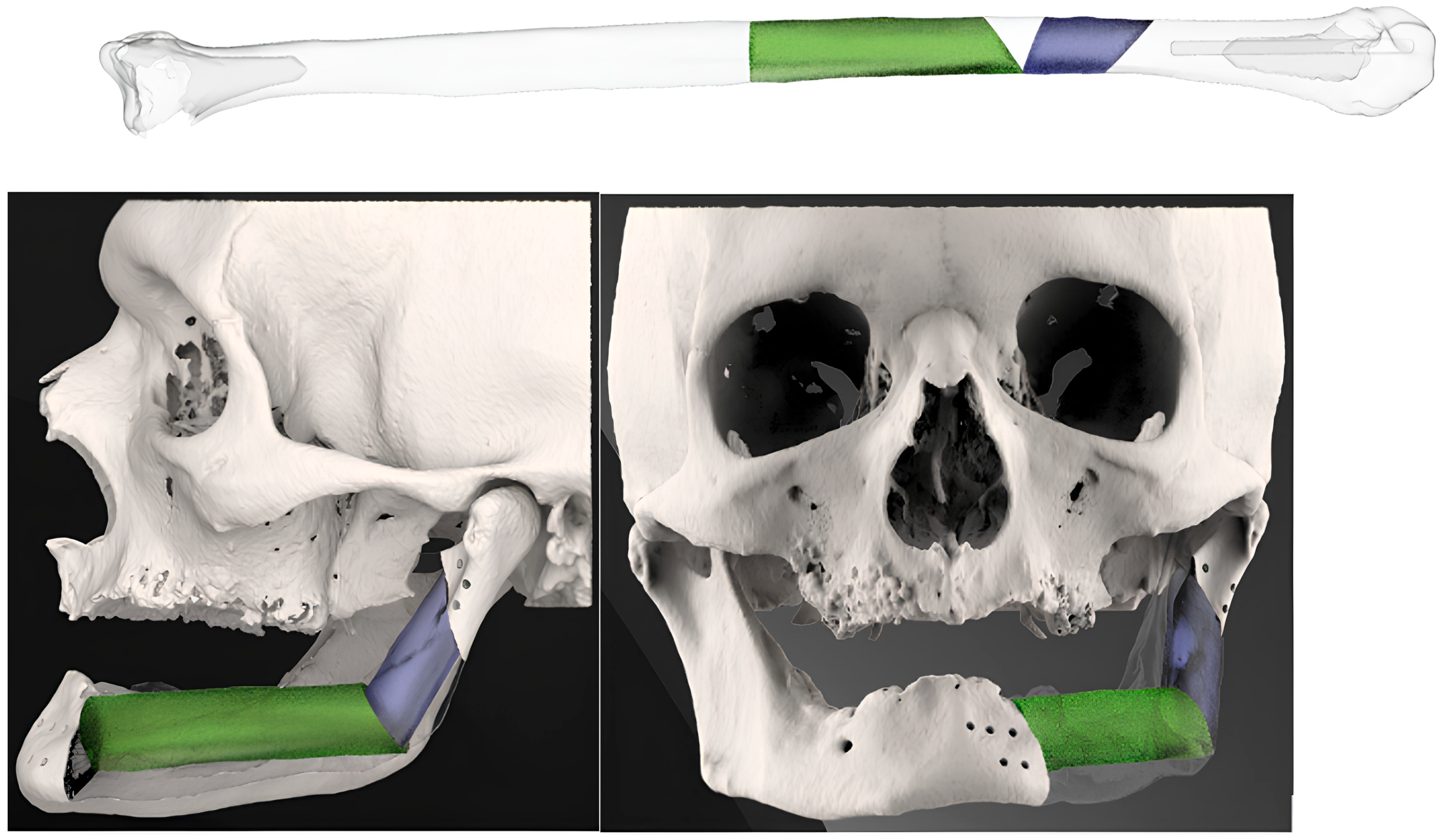
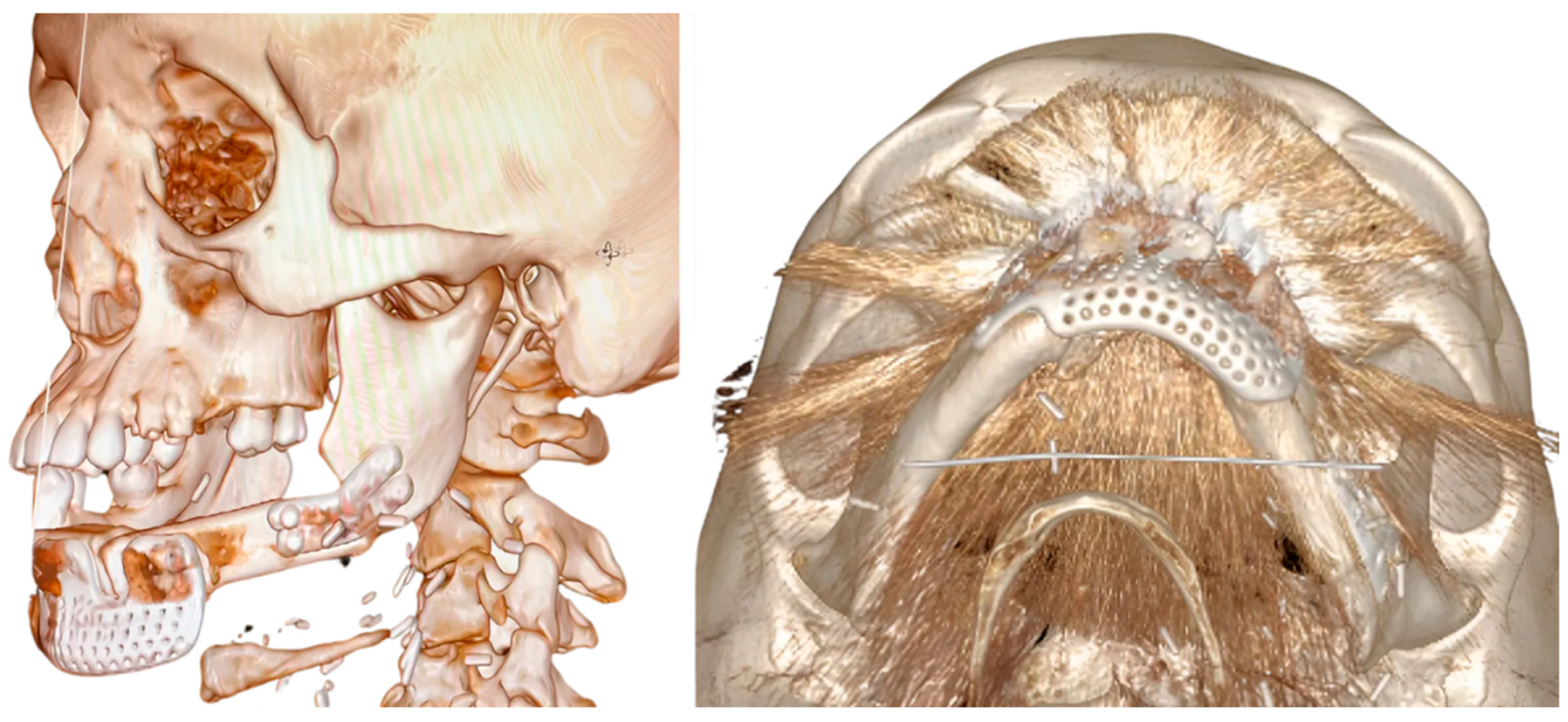
3.2. Fibula Reconstruction (Table 1)
3.3. Complications
3.3.1. Complications and Follow-Up
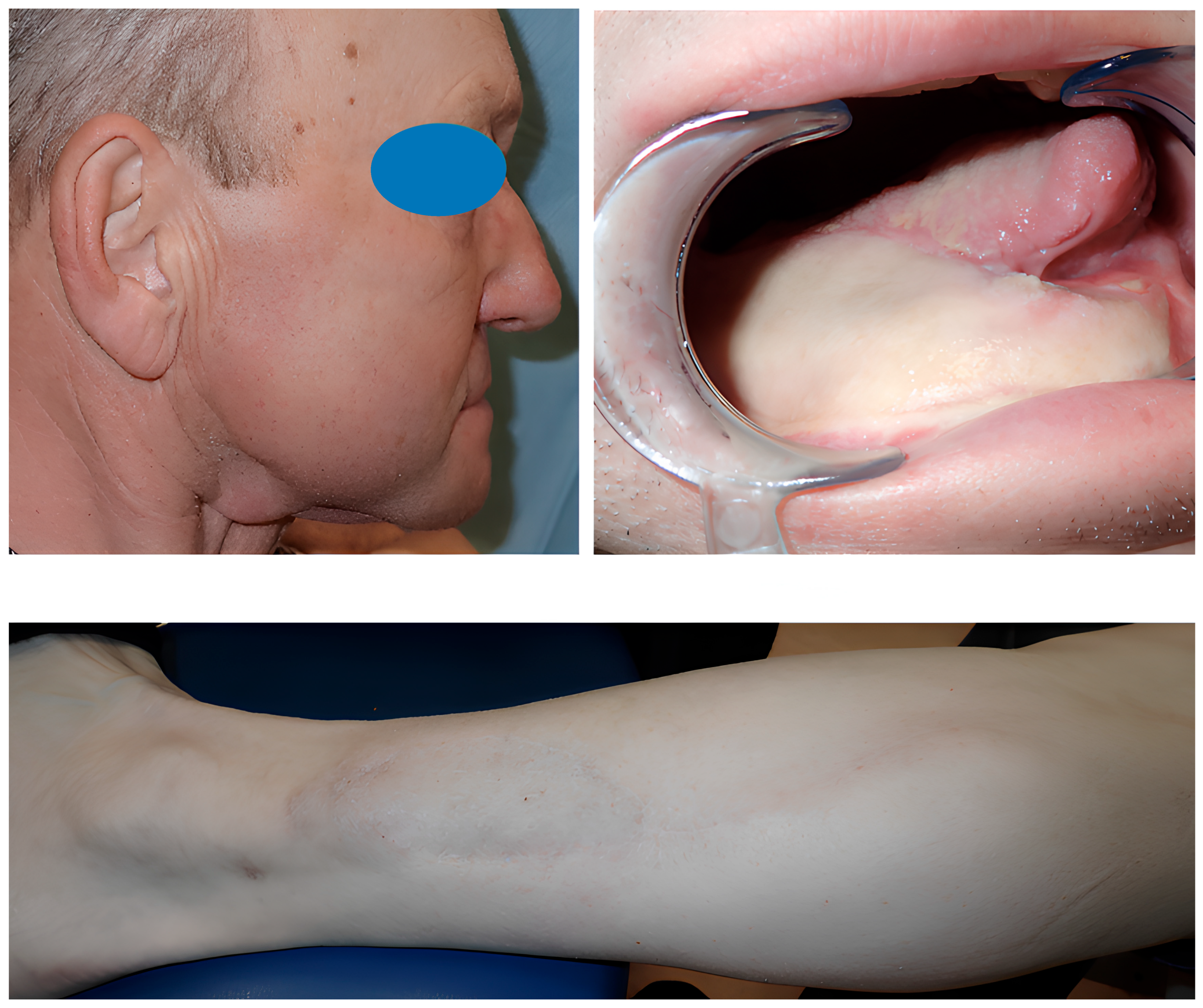
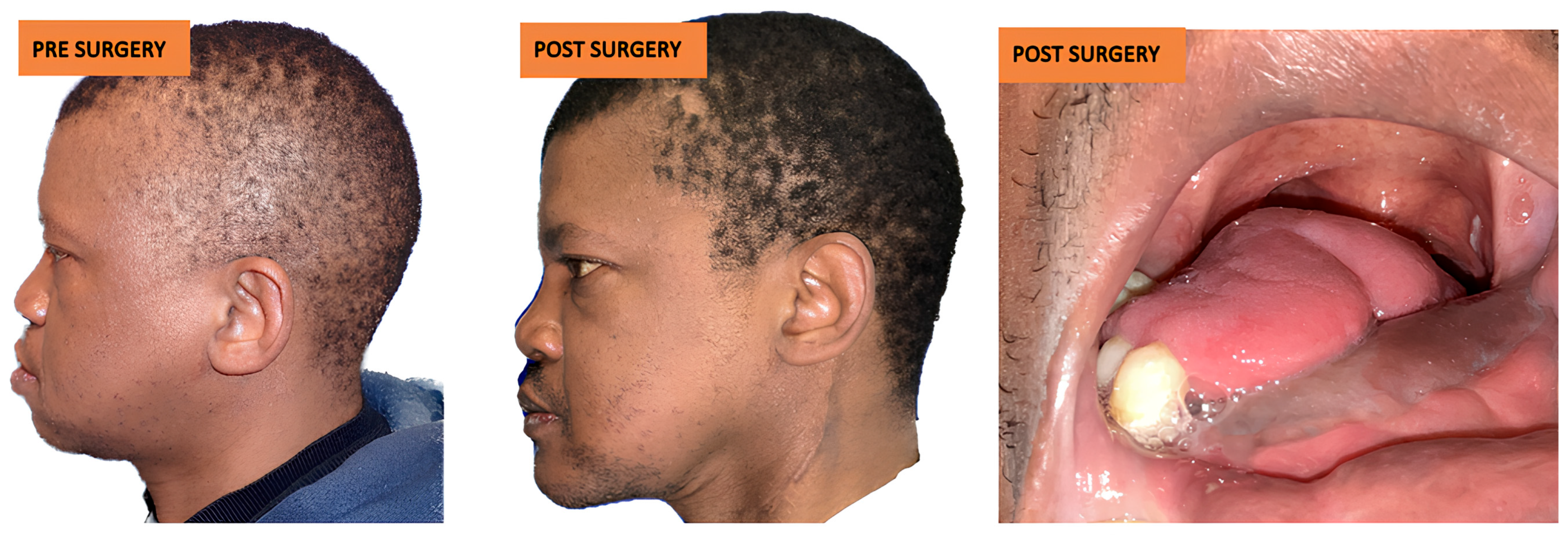
3.3.2. Aesthetic Complications
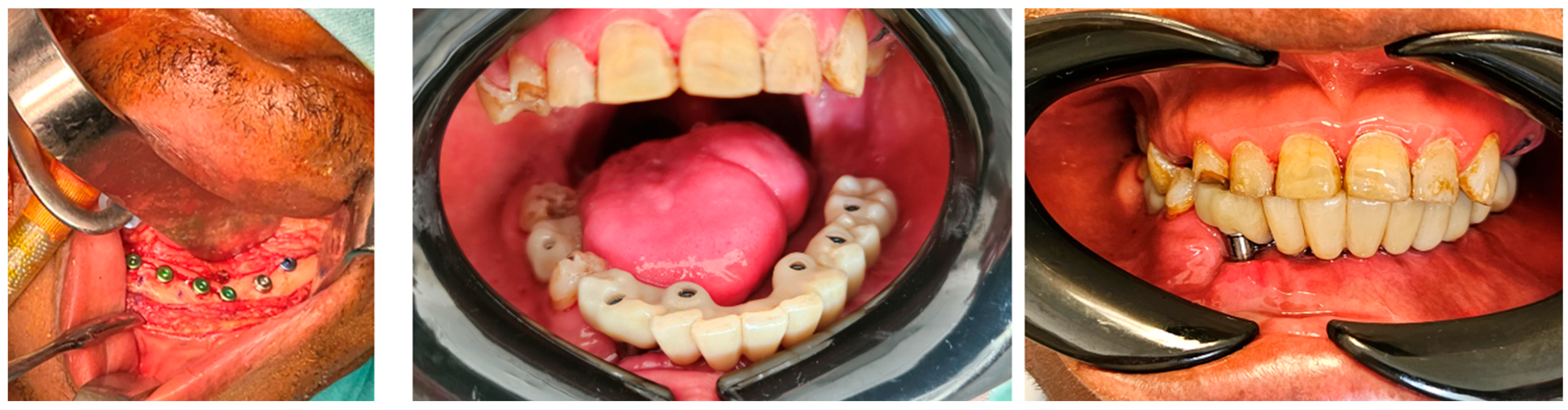
3.4. Dental Rehabilitation
3.5. Bioengineering Analysis
3.5.1. Statistical Analysis
| Case NO. | Standard Deviation Between 3D Project Models and Post Surgery Models (mm) | Cutting Guide Segments | Project Size of FFF Segment (mm) | FFF Segment Size After Surgery (mm) | Main Difference (mm) |
|---|---|---|---|---|---|
| 1 | 1.735 | I | 46 | 41 | 0 |
| 2 | 1.760 | I | 33 | 34 | +1 |
| II | 43 | 40 | −3 | ||
| 3 | 2.034 | I | 29 | 32 | +3 |
| II | 44 | 42 | −2 | ||
| 4 | 1.942 | I | 32 | 29 | −3 |
| II | 46 | 44 | −2 | ||
| III | 43 | 41 | −2 | ||
| IV | 42 | 41 | −1 | ||
| 5 | 2.330 | I | 34 | 38 | +4 |
| II | 45 | 47 | +2 | ||
| 6 | 2.132 | I | 51 | 49 | −2 |
| II | 37 | 37 | 0 | ||
| 7 | 1.156 | I | 52 | 52 | 0 |
| II | 34 | 33 | −1 | ||
| 8 | 2.020 | I | 28 | 26 | −2 |
| II | 44 | 42 | −2 | ||
| III | 39 | 35 | −4 | ||
| 9 | 2.372 | I | 33 | 27 | −6 |
| II | 53 | 47 | −6 | ||
| 10 | 1.986 | I | 61 | 60 | −1 |
| 11 | 2.221 | I | 52 | 49 | −3 |
| II | 28 | 24 | −4 | ||
| 12 | 1.975 | I | 40 | 37 | −3 |
| II | 35 | 31 | −4 | ||
| 13 | 2.127 | I | 56 | 59 | +3 |
| II | 32 | 27 | −5 | ||
| 14 | 1.845 | I | 51 | 50 | −1 |
| II | 29 | 28 | −1 | ||
| 15 | 2.097 | I | 48 | 53 | +5 |
| II | 29 | 25 | −4 | ||
| 16 | 2.541 | I | 50 | 44 | −6 |
| II | 53 | 46 | −7 | ||
| 17 | 2.313 | I | 27 | 23 | −4 |
| II | 44 | 42 | −2 | ||
| III | 39 | 36 | −3 | ||
| 18 | 2.344 | I | 24 | 21 | −3 |
| II | 51 | 48 | −3 | ||
| 19 | 1.937 | I | 26 | 22 | −4 |
| II | 25 | 24 | −1 | ||
| 20 | 1.910 | I | 27 | 31 | +4 |
| II | 72 | 72 | 0 | ||
| 21 | 1.429 | I | 54 | 52 | −2 |
| II | 60 | 60 | 0 | ||
| 22 | 1.826 | I | 26 | 27 | +1 |
| II | 29 | 28 | −1 |
| FFF Number of Sgments | Number of Patients | Total FFF Sgments for Each Type |
|---|---|---|
| 1 | 2 (9.1%) | 2 (4.3%) |
| 2 | 17 (77.3%) | 34 (74%) |
| 3 | 2 (9.1%) | 6 (13%) |
| 4 | 1 (4.5%) | 4 (8.7%) |
| total | 22 | 46 |
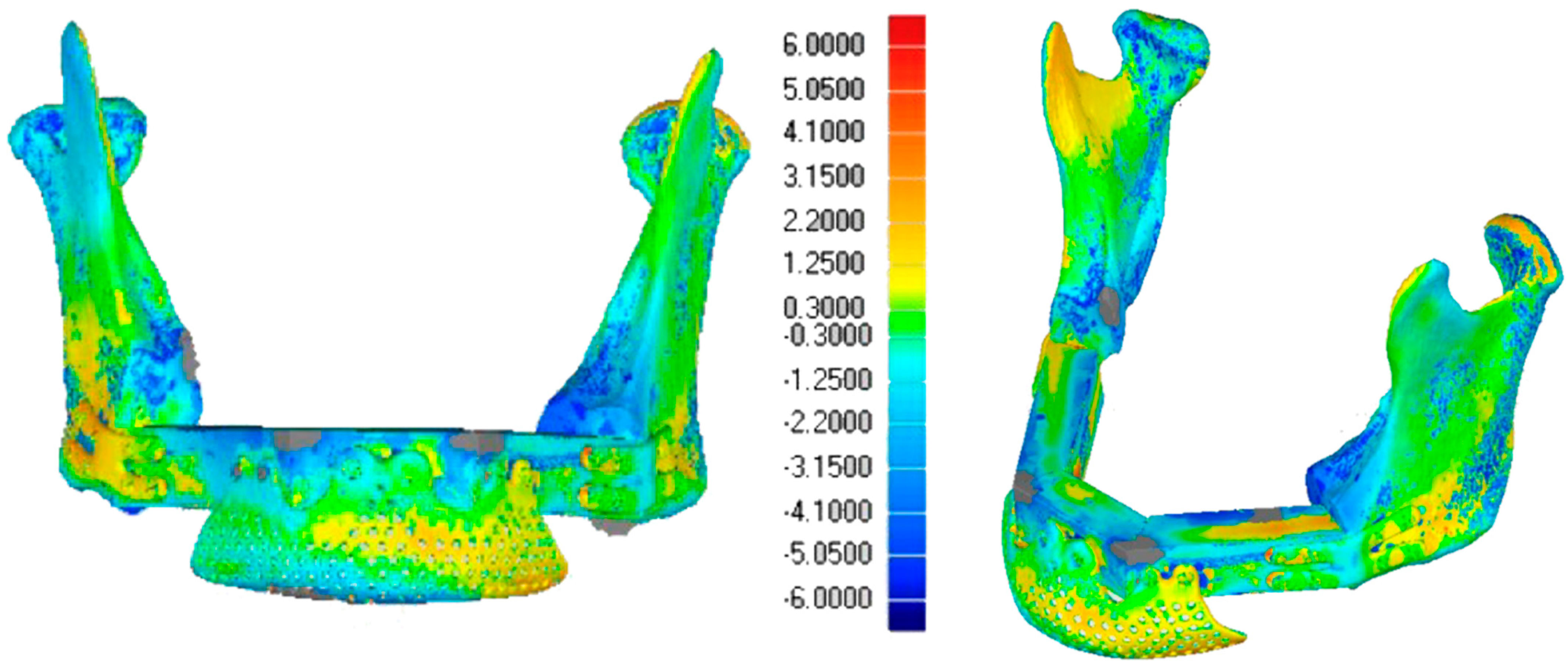
3.5.2. Alignment Precision
3.5.3. Morphological Restoration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D-CMP | Three-dimensional conformational miniplates |
| CAD/CAM | Computer-Aided Design/Computer-Aided Manufacturing |
| FFF | Fibula Free Flap |
| SD | Standard deviation |
References
- Disa, J.J.; Cordeiro, P.G. Mandible reconstruction with microvascular surgery. Semin. Surg. Oncol. 2000, 19, 226–234. [Google Scholar] [CrossRef]
- Valentini, V.; Gennaro, P.; Torroni, A.; Longo, G.; Aboh, I.V.; Cassoni, A.; Battisti, A.; Anelli, A. Scapula free flap for complex maxillofacial reconstruction. J. Craniofac. Surg. 2009, 20, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Soutar, D.S.; Widdowson, W.P. Immediate reconstruction of the mandible using a vascularized segment of radius. Head Neck Surg. 1986, 8, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Urken, M.L.; Vickery, C.; Weinberg, H.; Buchbinder, D.; Biller, H.F. The internal oblique-iliac crest osseomyocutaneous microvascular free flap in head and neck reconstruction. J. Reconstr. Microsurg. 1989, 5, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I.; Miller, G.D.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef]
- Chen, Z.W.; Yan, W. The study and clinical application of the osteocutaneous flap of fibula. Microsurgery 1983, 4, 11–16. [Google Scholar] [CrossRef]
- Jones, N.F.; Swartz, W.M.; Mears, D.C.; Jupiter, J.B.; Grossman, A. The “double barrel” free vascularized fibular bone graft. Plast. Reconstr. Surg. 1988, 81, 378–385. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Fibula free flap: A new method of mandible reconstruction. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef]
- Weyh, A.M.; Fernandes, R.P. Narrative review: Fibula free flap, indications, tips, and pitfalls. Front. Oral Maxillofac. Med. 2021, 3, 4. [Google Scholar] [CrossRef]
- Kumar, V.V.; Ebenezer, S.; Kämmerer, P.W.; Jacob, P.C.; Kuriakose, M.A.; Hedne, N.; Al-Nawas, B. Implants in free fibula flap supporting dental rehabilitation. J. Craniomaxillofac. Surg. 2016, 44, 1849–1858. [Google Scholar] [CrossRef]
- Gangwani, P.; Almana, M.; Barmak, B.; Kolokythas, A. Success of implants placed in fibula flap: A systematic review. J. Oral Maxillofac. Res. 2022, 13, e3. [Google Scholar] [CrossRef]
- Attia, S.; Wiltfang, J.; Pons-Kühnemann, J.; Wilbrand, J.F.; Streckbein, P.; Kähling, C.; Schaaf, H. Survival of dental implants in vascularised fibula free flaps. J. Craniomaxillofac. Surg. 2018, 46, 1205–1210. [Google Scholar] [CrossRef]
- Kääriäinen, M.; Kuuskeri, M.; Gremoutis, G.; Kuokkanen, H.; Miettinen, A.; Laranne, J. Utilization of 3D CAD in maxillary and mandibular reconstruction. J. Reconstr. Microsurg. 2016, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, A.; Del Corso, G.; Ciocca, L. Mandibular reconstructions using CAD/CAM. J. Craniomaxillofac. Surg. 2015, 43, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Zavattero, E.; Bolzoni, A.; Dell’Aversana, G.; Santagata, M.; Massarelli, O.; Ferri, A.; Della Monaca, M.; Copelli, C.; Gessaroli, M.; Valsecchi, S.; et al. Accuracy of fibula reconstruction using CAD/CAM plates. Laryngoscope 2021, 131, E2169–E2175. [Google Scholar] [CrossRef] [PubMed]
- Sass, T.; Piffklo, J.; Oberna, F. Vertical mandibular bone augmentation by osteodistraction. J. Craniomaxillofac. Surg. 2021, 49, 1044–1053. [Google Scholar] [CrossRef]
- Khayat, S.; Sada Urmeneta, Á.; González Moure, B.; Fernández Acosta, D.; Benito Anguita, M.; López López, A.; Navarro Cuéllar, C. Reconstruction of mandibular defects with double-barrel fibula flap. J. Clin. Med. 2024, 13, 3547. [Google Scholar] [CrossRef]
- Bähr, W.; Stoll, P.; Wächter, R. Use of the “double barrel” free vascularized fibula in mandibular reconstruction. J. Oral Maxillofac. Surg. 1998, 56, 38–44. [Google Scholar] [CrossRef]
- Klesper, B.; Lazar, F.; Siessegger, M.; Hidding, J.; Zöller, J.E. Vertical distraction osteogenesis of fibula transplants for mandibular reconstruction—A preliminary study. J. Craniomaxillofac. Surg. 2002, 30, 280–285. [Google Scholar] [CrossRef]
- Saito, N.; Funayama, A.; Arai, Y.; Suda, D.; Takata, Y.; Kobayashi, T. Vertical distraction osteogenesis of a reconstructed mandible with a free vascularized fibula flap: A report of two cases. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 32. [Google Scholar] [CrossRef]
- Trilles, J.; Chaya, B.F.; Daar, D.A.; Anzai, L.; Boczar, D.; Rodriguez Colon, R.; Levine, J.P. Double-barrel versus single-barrel fibula flaps for mandibular reconstruction: Safety and outcomes. Laryngoscope 2021, 132, 1576–1581. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, X.H.; Sun, J.; Li, J.; Shi, J.; Huang, W.; Ow, A. Double-barrel vascularised fibula graft in mandibular reconstruction: A 10-year experience with an algorithm. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Rogers, S.N.; McNally, D.N.; Boyle, M. A modified classification for the maxillectomy defect. Head Neck 2000, 22, 17–26. [Google Scholar] [CrossRef]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, S.; Marchetti, C.; Sgarzani, R.; Cipriani, R.; Scotti, R.; Ciocca, L. Prosthetically guided maxillofacial surgery: Evaluation of the accuracy of a surgical guide and custom-made bone plate in oncology patients after mandibular reconstruction. Plast. Reconstr. Surg. 2013, 131, 1376–1385. [Google Scholar] [CrossRef]
- Avraham, T.; Franco, P.; Brecht, L.E.; Ceradini, D.J.; Saadeh, P.B.; Hirsch, D.L.; Levine, J.P. Functional outcomes of virtually planned free fibula flap reconstruction of the mandible. Plast. Reconstr. Surg. 2014, 134, 628e–634e. [Google Scholar] [CrossRef]
- Wilde, F.; Hanken, H.; Probst, F.; Schramm, A.; Heiland, M.; Cornelius, C.P. Multicenter study on the use of patient-specific CAD/CAM reconstruction plates for mandibular reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 2035–2051. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Lai, Y.-S.; Lin, C.-Y.; Wang, J.-D.; Pan, S.-C.; Shieh, S.-J.; Lee, J.-W.; Lee, Y.-C. Plate-related complication and health-related quality of life after mandibular reconstruction by fibula flap with reconstruction plate or miniplate versus anterolateral thigh flap with reconstruction plate. Microsurgery 2023, 43, 131–141. [Google Scholar] [CrossRef]
- Liu, S.P.; Cai, Z.G.; Zhang, J.; Zhang, J.G.; Zhang, Y. Stability and complications of miniplates for mandibular reconstruction with a fibular graft: Outcomes for 544 patients. Br. J. Oral Maxillofac. Surg. 2016, 54, 496–500. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H. Biological mechanism of surgery-mediated acceleration of orthodontic tooth movement: A narrative review. J. Int. Med. Res. 2022, 50, 3000605221123904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prucher, G.M.; Gaggio, L.; Neri, F.; Astarita, F.; Sani, L.; Desiderio, C.; Allegri, D.; Pauro, N.; Sandi, A.; Baietti, A.M. Conformational Reconstruction in Head and Neck Bone Cancer: Could Fibula Free Flap Become the Gold Standard Flap? J. Clin. Med. 2025, 14, 8159. https://doi.org/10.3390/jcm14228159
Prucher GM, Gaggio L, Neri F, Astarita F, Sani L, Desiderio C, Allegri D, Pauro N, Sandi A, Baietti AM. Conformational Reconstruction in Head and Neck Bone Cancer: Could Fibula Free Flap Become the Gold Standard Flap? Journal of Clinical Medicine. 2025; 14(22):8159. https://doi.org/10.3390/jcm14228159
Chicago/Turabian StylePrucher, Gian Marco, Leonardo Gaggio, Fabrizio Neri, Fabio Astarita, Lorenzo Sani, Cristina Desiderio, Davide Allegri, Nicholas Pauro, Andrea Sandi, and Anna Maria Baietti. 2025. "Conformational Reconstruction in Head and Neck Bone Cancer: Could Fibula Free Flap Become the Gold Standard Flap?" Journal of Clinical Medicine 14, no. 22: 8159. https://doi.org/10.3390/jcm14228159
APA StylePrucher, G. M., Gaggio, L., Neri, F., Astarita, F., Sani, L., Desiderio, C., Allegri, D., Pauro, N., Sandi, A., & Baietti, A. M. (2025). Conformational Reconstruction in Head and Neck Bone Cancer: Could Fibula Free Flap Become the Gold Standard Flap? Journal of Clinical Medicine, 14(22), 8159. https://doi.org/10.3390/jcm14228159






