Recurrent Ulceration and Disease at the Ileocolonic Anastomosis in Crohn’s Disease: Etiology, Prevention, and Management, a Review Article
Abstract
1. Introduction
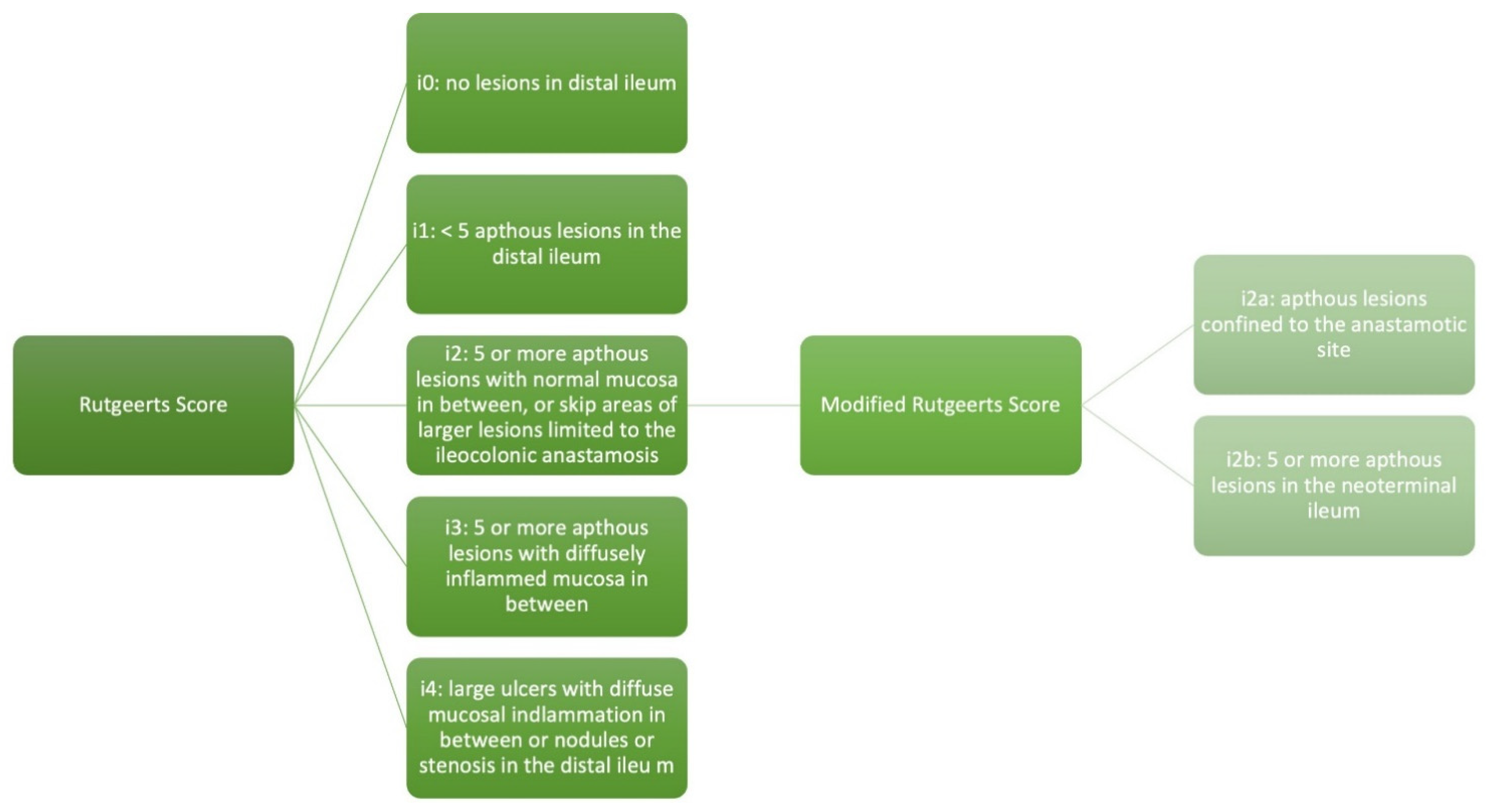
2. Rutgeerts Score and Modified Rutgeerts Score
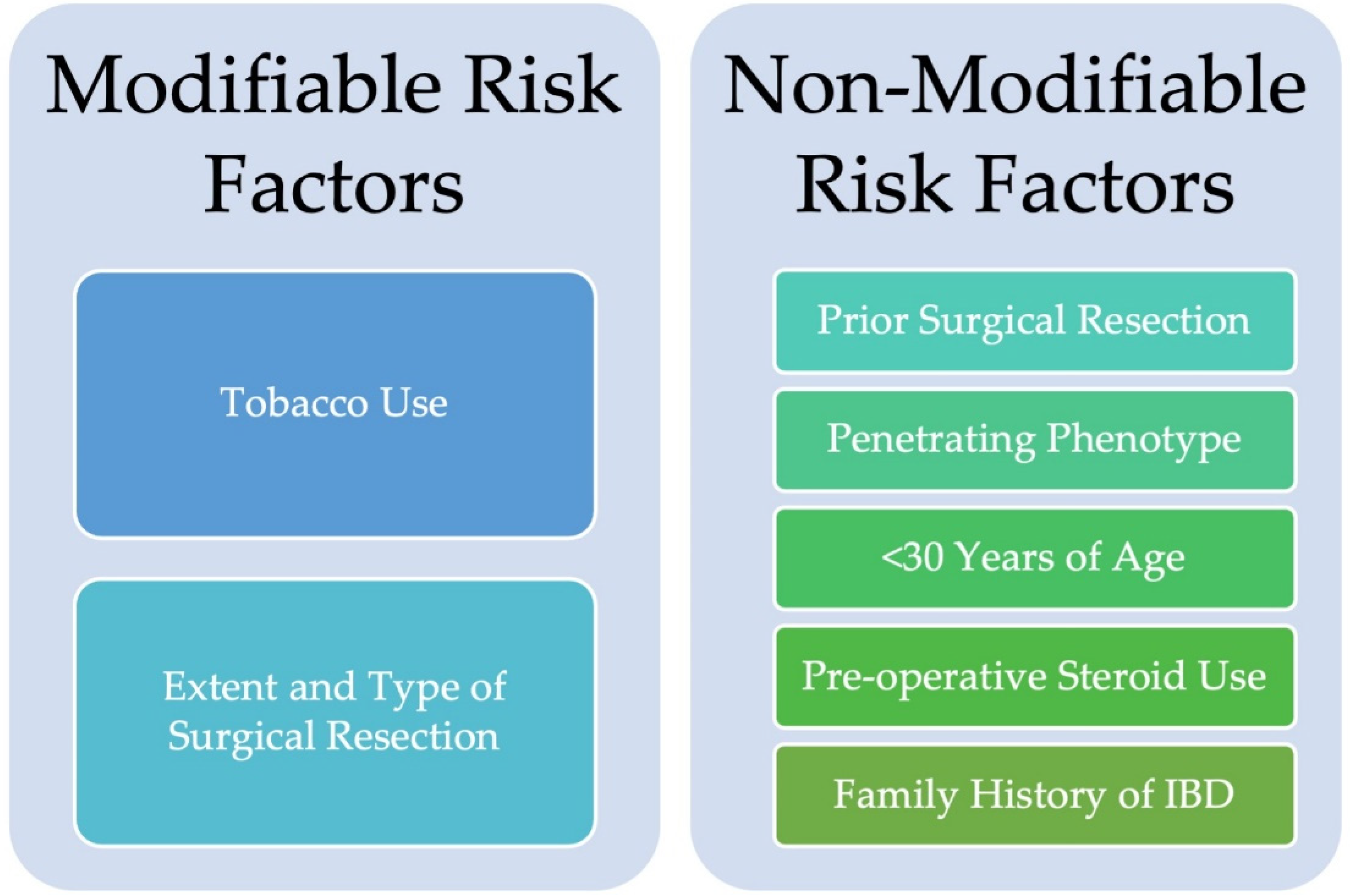
3. Risk Factors for Disease Recurrence at the Ileocolonic Anastomosis
4. Proposed Mechanisms for Disease Recurrence
5. Surveillance
6. Post-Operative Prevention of Recurrent Disease
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| IBD | Inflammatory Bowel Disease |
| RS | Rutgeerts Score |
| mRS | Modified Rutgeerts Score |
| anti-TNF | Anti-tumor necrosis factor |
References
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Vind, I.; Riis, L.; Jess, T.; Knudsen, E.; Pedersen, N.; Elkjær, M.; Andersen, I.B.; Wewer, V.; Nørregaard, P.; Moesgaard, F.; et al. Increasing Incidences of Inflammatory Bowel Disease and Decreasing Surgery Rates in Copenhagen City and County, 2003–2005: A Population-Based Study from the Danish Crohn Colitis Database. Off. J. Am. Coll. Gastroenterol. 2006, 101, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Harmsen, S.W.; Tremaine, W.J.; Zinsmeister, A.R.; Sandborn, W.J.; Loftus, E.V.J. Surgery in a Population-Based Cohort of Crohn’s Disease From Olmsted County, Minnesota (1970–2004). Off. J. Am. Coll. Gastroenterol. 2012, 107, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Diagnosis and monitoring of postoperative recurrence in Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 55–66. [Google Scholar] [CrossRef]
- Fichera, A.; Lovadina, S.; Rubin, M.; Cimino, F.; Hurst, R.D.; Michelassi, F. Patterns and operative treatment of recurrent Crohn’s disease: A prospective longitudinal study. Surgery 2006, 140, 649–654. [Google Scholar] [CrossRef]
- Chari, S.T.; Keate, R.F. Ileocolonic anastomotic ulcers: A case series and review of the literature. Am. J. Gastroenterol. 2000, 95, 1239–1243. [Google Scholar] [CrossRef]
- Ueda, T.; Koyama, F.; Sugita, A.; Ikeuchi, H.; Futami, K.; Fukushima, K.; Nezu, R.; Iijima, H.; Mizushima, T.; Itabashi, M.; et al. Endoscopic Lesions of Postoperative Anastomotic Area in Patients With Crohn’s Disease in the Biologic Era: A Japanese Multi-Centre Nationwide Cohort Study. J. Crohn’s Colitis 2023, 17, 1968–1979. [Google Scholar] [CrossRef]
- van der Does de Willebois, E.M.L.; Duijvestein, M.; Wasmann, K.A.; D’Haens, G.; van der Bilt, J.D.W.; Mundt, M.W.; Hompes, R.; van der Vlugt, M.; Buskens, C.J.; Bemelman, W.A. Endoscopic Recurrence or Anastomotic Wound Healing Phenomenon after Ileocolic Resection for Crohn’s Disease: The Challenges of Accurate Endoscopic Scoring. J. Crohn’s Colitis 2023, 17, 693–699. [Google Scholar] [CrossRef]
- Onali, S.; Petruzziello, C.; Calabrese, E.; Condino, G.; Zorzi, F.; Sica, G.S.; Pallone, F.; Biancone, L. Frequency, pattern, and risk factors of postoperative recurrence of Crohn’s disease after resection different from ileo-colonic. J. Gastrointest. Surg. 2009, 13, 246–252. [Google Scholar] [CrossRef]
- Hernández-Rocha, C.; Walshe, M.; Birch, S.; Sabic, K.; Korie, U.; Chasteau, C.; Miladinova, V.M.; Sabol, W.B.; Mengesha, E.; Hanna, M.; et al. Clinical Predictors of Early and Late Endoscopic Recurrence Following Ileocolonic Resection in Crohn’s Disease. J. Crohn’s Colitis 2023, 18, 615–627. [Google Scholar] [CrossRef]
- Unkart, J.T.; Anderson, L.; Li, E.; Miller, C.; Yan, Y.; Charles Gu, C.; Chen, J.; Stone, C.D.; Hunt, S.; Dietz, D.W. Risk Factors for Surgical Recurrence after Ileocolic Resection of Crohn’s Disease. Dis. Colon Rectum 2008, 51, 1211–1216. [Google Scholar] [CrossRef]
- He, X.; Chen, Z.; Huang, J.; Lian, L.; Rouniyar, S.; Wu, X.; Lan, P. Stapled side-to-side anastomosis might be better than handsewn end-to-end anastomosis in ileocolic resection for Crohn’s disease: A meta-analysis. Dig. Dis. Sci. 2014, 59, 1544–1551. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Zhu, W.; Gong, J.; Li, N.; Li, J. Comparing outcomes between side-to-side anastomosis and other anastomotic configurations after intestinal resection for patients with Crohn’s disease: A meta-analysis. World J. Surg. 2013, 37, 893–901. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B. ECCO guidelines on therapeutics in Crohn’s disease: Surgical treatment. J. Crohn’s Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef]
- Alshantti, A.; Hind, D.; Hancock, L.; Brown, S.R. The role of Kono-S anastomosis and mesenteric resection in reducing recurrence after surgery for Crohn’s disease: A systematic review. Colorectal Dis. 2021, 23, 7–17. [Google Scholar] [CrossRef]
- Coffey, C.J.; Kiernan, M.G.; Sahebally, S.M.; Jarrar, A.; Burke, J.P.; Kiely, P.A.; Shen, B.; Waldron, D.; Peirce, C.; Moloney, M.; et al. Inclusion of the Mesentery in Ileocolic Resection for Crohn’s Disease is Associated With Reduced Surgical Recurrence. J. Crohn’s Colitis 2018, 12, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.T.J.; ten Bokkel Huinink, S.; Erler, N.S.; Bodelier, A.G.L.; Dijkstra, G.; Romberg-Camps, M.; de Boer, N.K.H.; Hoentjen, F.; Stassen, L.P.S.; van der Meulen-de Jong, A.E.; et al. Prognostic Value of the Modified Rutgeerts Score for Long-Term Outcomes After Primary Ileocecal Resection in Crohn’s Disease. Off. J. Am. Coll. Gastroenterol. 2024, 119, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.H.; Park, J.C.; Noh, S.; Lee, J.S.; Kim, J.; Ham, N.S.; Oh, E.H.; Hwang, S.W.; Yang, D.H.; et al. The Clinical Significance of Anastomotic Ulcers After Ileocolic Resection to Predict Postoperative Recurrence of Crohn’s Disease. Dig. Dis. Sci. 2021, 66, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Ungaro, R.C.; Castaneda, D.; Lopatin, S.; Sands, B.E.; Colombel, J.F.; Cohen, B.L. Anastomotic Ulcers After Ileocolic Resection for Crohn’s Disease Are Common and Predict Recurrence. Inflamm. Bowel Dis. 2019, 26, 1050–1058. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Loftus, E.V.; Hirano, I.; Falck–Ytter, Y.; Singh, S.; Sultan, S.; Flamm, S.L.; Lim, J.K.; Rubenstein, J.H.; Smalley, W.E. American Gastroenterological Association Institute guideline on the management of Crohn’s disease after surgical resection. Gastroenterology 2017, 152, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Off. J. Am. Coll. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.; Lowenberg, M.; Bossuyt, P.; Rutgeerts, P.J.; Vermeire, S.; Stitt, L.; Vandervoort, M.K.; Sandborn, W.; Feagan, B.G.; Samaan, M.A. Agreement among experts in the endoscopic evaluation of postoperative recurrence in Crohn’s disease using the Rutgeerts score. Gastroenterology 2014, 146, S-227. [Google Scholar] [CrossRef]
- Rivière, P.; Pekow, J.; Hammoudi, N.; Wils, P.; De Cruz, P.; Wang, C.P.; Mañosa, M.; Ollech, J.; Allez, M.; Nachury, M.; et al. Comparison of the Risk of Crohn’s Disease Postoperative Recurrence Between Modified Rutgeerts Score i2a and i2b Categories: An Individual Patient Data Meta-analysis. J. Crohn’s Colitis 2022, 17, 269–276. [Google Scholar] [CrossRef]
- Rivière, P.; Vermeire, S.; Irles-Depe, M.; Van Assche, G.; Rutgeerts, P.; Denost, Q.; Wolthuis, A.; D’Hoore, A.; Laharie, D.; Ferrante, M. Rates of Postoperative Recurrence of Crohn’s Disease and Effects of Immunosuppressive and Biologic Therapies. Clin. Gastroenterol. Hepatol. 2021, 19, 713–720.e1. [Google Scholar] [CrossRef]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; de Buck van Overstraeten, A.; Burke, J.P.; Buskens, C.J.; Colombo, F. ECCO-ESCP consensus on surgery for Crohn’s disease. J. Crohn’s Colitis 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Domènech, E.; López-Sanromán, A.; Nos, P.; Vera, M.; Chaparro, M.; Esteve, M.; Gisbert, J.P.; Mañosa, M. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on the monitoring, prevention and treatment of post-operative recurrence in Crohn’s disease. Gastroenterol. Hepatol. (Engl. Ed.) 2017, 40, 472–483. [Google Scholar] [CrossRef]
- Domènech, E.; Mañosa, M.; Bernal, I.; Garcia-Planella, E.; Cabré, E.; Piñol, M.; Lorenzo-Zúñiga, V.; Boix, J.; Gassull, M.A. Impact of azathioprine on the prevention of postoperative Crohn’s disease recurrence: Results of a prospective, observational, long-term follow-up study. Inflamm. Bowel Dis. 2008, 14, 508–513. [Google Scholar] [CrossRef]
- Hammoudi, N.; Auzolle, C.; Tran Minh, M.L.; Boschetti, G.; Bezault, M.; Buisson, A.; Pariente, B.; Treton, X.; Seksik, P.; Fumery, M.; et al. Postoperative Endoscopic Recurrence on the Neoterminal Ileum But Not on the Anastomosis Is Mainly Driving Long-Term Outcomes in Crohn’s Disease. Am. J. Gastroenterol. 2020, 115, 1084–1093. [Google Scholar] [CrossRef]
- Rivière, P.; Vermeire, S.; Irles-Depe, M.; Van Assche, G.; Rutgeerts, P.; de Buck van Overstraeten, A.; Denost, Q.; Wolthuis, A.; D’Hoore, A.; Laharie, D.; et al. No Change in Determining Crohn’s Disease Recurrence or Need for Endoscopic or Surgical Intervention with Modification of the Rutgeerts’ Scoring System. Clin. Gastroenterol. Hepatol. 2019, 17, 1643–1645. [Google Scholar] [CrossRef]
- Bayart, P.; Duveau, N.; Nachury, M.; Zerbib, P.; Gerard, R.; Branche, J.; Maunoury, V.; Wils, P.; Boruchowicz, A.; Boualit, M.; et al. Ileal or Anastomotic Location of Lesions Does Not Impact Rate of Postoperative Recurrence in Crohn’s Disease Patients Classified i2 on the Rutgeerts Score. Dig. Dis. Sci. 2016, 61, 2986–2992. [Google Scholar] [CrossRef]
- van der Does de Willebois, E.M.L.; Bellato, V.; Duijvestein, M.; van Dieren, S.; Danese, S.; Sileri, P.; Buskens, C.J.; Vignali, A.; Bemelman, W.A. How Reliable Is Endoscopic Scoring of Postoperative Recurrence in Crohn Disease?: A Systematic Review and Meta-Analysis. Ann. Surg. Open 2024, 5, e397. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Pinto, J.S.L.D.; Marques da Costa, P.; Correia, L.; GEDII. Disagreement Among Gastroenterologists Using the Mayo and Rutgeerts Endoscopic Scores. Inflamm. Bowel Dis. 2018, 24, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Laharie, D.; Colombel, J.-F.; Martin, L.; Coevoet, H.; Allez, M.; Cadiot, G.; Bourreille, A.; Carbonnel, F.; Bouhnik, Y.; et al. Interobserver Variation Study of the Rutgeerts Score to Assess Endoscopic Recurrence after Surgery for Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 1001–1005. [Google Scholar] [CrossRef]
- Ma, C.; Gecse, K.B.; Duijvestein, M.; Sandborn, W.J.; Zou, G.; Shackelton, L.M.; Stitt, L.W.; Parker, C.E.; Bossuyt, P.; Löwenberg, M.; et al. Reliability of Endoscopic Evaluation of Postoperative Recurrent Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2139–2141.e2. [Google Scholar] [CrossRef] [PubMed]
- Pascua, M.; Su, C.; Lewis, J.; Brensinger, C.; Lichtenstein, G. Meta-analysis: Factors predicting post-operative recurrence with placebo therapy in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 28, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Lied, G.A.; Arebi, N.; Phillips, R.K.; Kamm, M.A. Clinical and surgical recurrence of Crohn’s disease after ileocolonic resection in a specialist unit. Eur. J. Gastroenterol. Hepatol. 2009, 21, 551–557. [Google Scholar] [CrossRef]
- McLeod, R.S.; Wolff, B.G.; Ross, S.; Parkes, R.; McKenzie, M. Recurrence of Crohn’s disease after ileocolic resection is not affected by anastomotic type: Results of a multicenter, randomized, controlled trial. Dis. Colon Rectum 2009, 52, 919–927. [Google Scholar] [CrossRef]
- Gionchetti, P.; Dignass, A.; Danese, S.; Magro Dias, F.J.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: Surgical management and special situations. J. Crohn’s Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef]
- Aeberhard, P.; Berchtold, W.; Riedtmann, H.-J.; Stadelmann, G. Surgical recurrence of perforating and nonperforating Crohn’s disease: A study of 101 surgically treated patients. Dis. Colon Rectum 1996, 39, 80–87. [Google Scholar] [CrossRef]
- Simillis, C.; Yamamoto, T.; Reese, G.E.; Umegae, S.; Matsumoto, K.; Darzi, A.W.; Tekkis, P.P. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Off. J. Am. Coll. Gastroenterol. 2008, 103, 196–205. [Google Scholar] [CrossRef]
- Blonski, W.; Buchner, A.M.; Lichtenstein, G.R. Clinical predictors of aggressive/disabling disease: Ulcerative colitis and Crohn disease. Gastroenterol. Clin. 2012, 41, 443–462. [Google Scholar] [CrossRef] [PubMed]
- De Barcelos, I.; Kotze, P.; Spinelli, A.; Suzuki, Y.; Teixeira, F.; de Albuquerque, I.; Saad-Hossne, R.; da Silva Kotze, L.; Yamamoto, T. Factors affecting the incidence of early endoscopic recurrence after ileocolonic resection for Crohn’s disease: A multicentre observational study. Color. Dis. 2017, 19, O39–O45. [Google Scholar] [CrossRef] [PubMed]
- Kurer, M.; Stamou, K.; Wilson, T.; Bradford, I.; Leveson, S. Early symptomatic recurrence after intestinal resection in Crohn’s disease is unpredictable. Color. Dis. 2007, 9, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Morar, P.; Faiz, O.; Hodgkinson, J.; Zafar, N.; Koysombat, K.; Purcell, M.; Hart, A.; Warusavitarne, J. Concomitant colonic disease (Montreal L3) and re-resectional surgery are predictors of clinical recurrence following ileocolonic resection for Crohn’s disease. Color. Dis. 2015, 17, O247–O255. [Google Scholar] [CrossRef]
- Sher, M.E.; Bank, S.; Greenberg, R.; Sardinha, T.C.; Weissman, S.; Bailey, B.; Gilliland, R.; Wexner, S.D. The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 1999, 5, 73–78. [Google Scholar] [CrossRef]
- Hatoum, O.A.; Binion, D.G.; Otterson, M.F.; Gutterman, D.D. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology 2003, 125, 58–69. [Google Scholar] [CrossRef]
- Tomasello, G.; Tralongo, P.; Damiani, P.; Sinagra, E.; Di Trapani, B.; Zeenny, M.N.; Hussein, I.H.; Jurjus, A.; Leone, A. Dismicrobism in inflammatory bowel disease and colorectal cancer: Changes in response of colocytes. World J. Gastroenterol. 2014, 20, 18121–18130. [Google Scholar] [CrossRef]
- Bringiotti, R.; Ierardi, E.; Lovero, R.; Losurdo, G.; Di Leo, A.; Principi, M. Intestinal microbiota: The explosive mixture at the origin of inflammatory bowel disease? World J. Gastrointest. Pathophysiol. 2014, 5, 550–559. [Google Scholar] [CrossRef]
- Anthony, A.; Dhillon, A.P.; Pounder, R.E.; Wakefield, A.J. Ulceration of the ileum in Crohn’s disease: Correlation with vascular anatomy. J. Clin. Pathol. 1997, 50, 1013–1017. [Google Scholar] [CrossRef]
- Okazaki, M.; Matsukuma, S.; Suto, R.; Miyazaki, K.; Hidaka, M.; Matsuo, M.; Noshima, S.; Zempo, N.; Asahara, T.; Nomoto, K. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: A prospective, randomized control trial. Nutrition 2013, 29, 1224–1230. [Google Scholar] [CrossRef]
- Le Cosquer, G.; Altwegg, R.; Rivière, P.; Bournet, B.; Boivineau, L.; Poullenot, F.; Bozon, A.; Buscail, L.; Laharie, D.; Gilletta, C. Prevention of post-operative recurrence of Crohn’s disease among patients with prior anti-TNFα failure: A retrospective multicenter study. Dig. Liver Dis. 2023, 55, 727–734. [Google Scholar] [CrossRef]
- Hernandez-Rocha, C.; Turpin, W.; Nayeri, S.; Borowski, K.; Stempak, J.M.; Schumm, L.P.; Brant, S.R.; Lazarev, M.; Rioux, J.D.; Mcgovern, D.P. 555: Mucosal microbial profiles at the neo-terminal ileum are associated with future development of postoperative endoscopic recurrence in crohn’s disease patients. Gastroenterology 2022, 162, S-132. [Google Scholar] [CrossRef]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Tabata, S.; et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. J. Parenter. Enter. Nutr. 2011, 35, 317–328. [Google Scholar] [CrossRef]
- Fazio, V.W.; Marchetti, F.; Church, J.M.; Goldblum, J.R.; Lavery, I.C.; Hull, T.L.; Milsom, J.W.; Strong, S.A.; Oakley, J.R.; Secic, M. Effect of resection margins on the recurrence of Crohn’s disease in the small bowel: A randomized controlled trial. Ann. Surg. 1996, 224, 563–573. [Google Scholar] [CrossRef]
- Ryan, J.M.; Rogers, A.C.; O’Toole, A.; Burke, J.P. Meta-analysis of histological margin positivity in the prediction of recurrence after Crohn’s resection. Dis. Colon Rectum 2019, 62, 882–892. [Google Scholar] [CrossRef]
- van der Does de Willebois, E.M.L.; Bellato, V.; Duijvestein, M.; van der Bilt, J.D.W.; van Dongen, K.; Spinelli, A.; D’Haens, G.R.; Mundt, M.W.; Furfaro, F.; Danese, S.; et al. Effect of mesenteric sparing or extended resection in primary ileocolic resection for Crohn’s disease on postoperative endoscopic recurrence (SPICY): An international, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 793–801. [Google Scholar] [CrossRef]
- Simillis, C.; Purkayastha, S.; Yamamoto, T.; Strong, S.A.; Darzi, A.W.; Tekkis, P.P. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn’s disease. Dis. Colon Rectum 2007, 50, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.S.; Li, J.Y.; Yang, Z.; Chen, X.Y.; Mo, J.J.; Li, S.H. Stapled side-to-side anastomosis might be benefit in intestinal resection for Crohn’s disease: A systematic review and network meta-analysis. Medicine 2018, 97, e0315. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Vogel, J.D.; Carmichael, J.C.; Keller, D.S.; Shah, S.A.; Mahadevan, U.; Kane, S.V.; Paquette, I.M.; Steele, S.R.; Feingold, D.L. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surgical Management of Crohn’s Disease. Dis. Colon Rectum 2020, 63, 1028–1052. [Google Scholar] [CrossRef] [PubMed]
- Luglio, G.; Rispo, A.; Imperatore, N.; Giglio, M.C.; Amendola, A.; Tropeano, F.P.; Peltrini, R.; Castiglione, F.; De Palma, G.D.; Bucci, L. Surgical Prevention of Anastomotic Recurrence by Excluding Mesentery in Crohn’s Disease: The SuPREMe-CD Study—A Randomized Clinical Trial. Ann. Surg. 2020, 272, 210–217. [Google Scholar] [CrossRef]
- Haanappel, A.E.G.; Bellato, V.; Buskens, C.J.; Armuzzi, A.; van der Bilt, J.D.W.; de Boer, N.K.H.; Danese, S.; van der Does de Willebois, E.M.L.; Duijvestein, M.; van der Horst, D.; et al. Optimising surgical anastomosis in ileocolic resection for Crohn’s disease with respect to recurrence and functionality: Two international parallel randomized controlled trials comparing handsewn (END-to-end or Kono-S) to stapled anastomosis (HAND2END and the End2End STUDIES). BMC Surg. 2024, 24, 71. [Google Scholar] [CrossRef]
- Osborne, M.J.; Hudson, M.; Piasecki, C.; Dhillon, A.P.; Lewis, A.A.M.; Pounder, R.E.; Wakefield, A.J. Crohn’s disease and anastomotic recurrence: Microvascular ischaemia and anastomotic healing in an animal model. Br. J. Surg. 1993, 80, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Mashiana, S.; Cohen, B.L.; Sands, B.E.; Colombel, J.F.; Harpaz, N. Ileocolic anastomotic inflammation after resection for Crohn’s disease indicates disease recurrence: A histopathologic study. Scand. J. Gastroenterol. 2020, 55, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Zuo, L.; Shen, B. The Role of the Mesentery in Crohn’s Disease: The Contributions of Nerves, Vessels, Lymphatics, and Fat to the Pathogenesis and Disease Course. Inflamm. Bowel Dis. 2016, 22, 1483–1495. [Google Scholar] [CrossRef]
- Ng, S.C.; Lied, G.A.; Kamm, M.A.; Sandhu, F.; Guenther, T.; Arebi, N. Predictive value and clinical significance of myenteric plexitis in Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; De Hertogh, G.; Hlavaty, T.; D’Haens, G.; Penninckx, F.; D’Hoore, A.; Vermeire, S.; Rutgeerts, P.; Geboes, K.; Van Assche, G. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology 2006, 130, 1595–1606. [Google Scholar] [CrossRef]
- Sokol, H.; Brot, L.; Stefanescu, C.; Auzolle, C.; Barnich, N.; Buisson, A.; Fumery, M.; Pariente, B.; Le Bourhis, L.; Treton, X.; et al. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut 2020, 69, 462–472. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; De Cruz, P.; Wright, E.K.; Feng, H.; Wagner, J.; Sung, J.J.Y.; Kirkwood, C.D.; Inouye, M.; Teo, S.M. Luminal microbiota related to Crohn’s disease recurrence after surgery. Gut Microbes 2020, 11, 1713–1728. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Geboes, K.; Peeters, M.; Baert, F.; Penninckx, F.; Rutgeerts, P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998, 114, 262–267. [Google Scholar] [CrossRef]
- Savarino, E.; Bodini, G.; Dulbecco, P.; Assandri, L.; Bruzzone, L.; Mazza, F.; Frigo, A.C.; Fazio, V.; Marabotto, E.; Savarino, V. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn’s disease: A randomized controlled trial. Am. J. Gastroenterol. 2013, 108, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, M.; Schraut, W.; Baidoo, L.; Kip, K.E.; Sepulveda, A.R.; Pesci, M.; Harrison, J.; Plevy, S.E. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009, 136, 441–450.e1, quiz 716. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Fukunaga, K.; Ikeuchi, H.; Kamikozuru, K.; Hida, N.; Ohda, Y.; Yokoyama, Y.; Iimuro, M.; Takeda, N.; Kato, K.; et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: A 3-year prospective randomized open trial. Inflamm. Bowel Dis. 2012, 18, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, M.; Feagan, B.G.; Zou, B.; Johanns, J.; Blank, M.A.; Chevrier, M.; Plevy, S.; Popp, J.; Cornillie, F.J.; Lukas, M.; et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology 2016, 150, 1568–1578. [Google Scholar] [CrossRef]
- Armuzzi, A.; Felice, C.; Papa, A.; Marzo, M.; Pugliese, D.; Andrisani, G.; Federico, F.; De Vitis, I.; Rapaccini, G.L.; Guidi, L. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn’s disease: An open-label pilot study. J. Crohn’s Colitis 2013, 7, e623–e629. [Google Scholar] [CrossRef]
- Beelen, E.M.J.; Nieboer, D.; Arkenbosch, J.H.C.; Regueiro, M.D.; Satsangi, J.; Ardizzone, S.; López-Sanromán, A.; Savarino, E.; Armuzzi, A.; Janneke van der Woude, C.; et al. Risk Prediction and Comparative Efficacy of Anti-TNF vs Thiopurines, for Preventing Postoperative Recurrence in Crohn’s Disease: A Pooled Analysis of 6 Trials. Clin. Gastroenterol. Hepatol. 2022, 20, 2741–2752.e6. [Google Scholar] [CrossRef]
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Crohn’s disease management after intestinal resection: A randomised trial. Lancet 2015, 385, 1406–1417. [Google Scholar] [CrossRef]
- López-Sanromán, A.; Vera-Mendoza, I.; Domènech, E.; Taxonera, C.; Vega Ruiz, V.; Marín-Jiménez, I.; Guardiola, J.; Castro, L.; Esteve, M.; Iglesias, E.; et al. Adalimumab vs. Azathioprine in the Prevention of Postoperative Crohn’s Disease Recurrence. A GETECCU Randomised Trial. J. Crohn’s Colitis 2017, 11, 1293–1301. [Google Scholar] [CrossRef]
- Ardizzone, S.; Maconi, G.; Sampietro, G.M.; Russo, A.; Radice, E.; Colombo, E.; Imbesi, V.; Molteni, M.; Danelli, P.G.; Taschieri, A.M.; et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn’s disease. Gastroenterology 2004, 127, 730–740. [Google Scholar] [CrossRef]
- Mowat, C.; Arnott, I.; Cahill, A.; Smith, M.; Ahmad, T.; Subramanian, S.; Travis, S.; Morris, J.; Hamlin, J.; Dhar, A.; et al. Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection (TOPPIC): A multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2016, 1, 273–282. [Google Scholar] [CrossRef]
- Dragoni, G.; Ding, N.; Gecse, K.B.; Mansfield, J.C.; Kopylov, U.; Beaugerie, L.; Bossuyt, P.; Sebastian, S.; Milla, M.; Bagnoli, S.; et al. The prevention and management of Crohn’s disease postoperative recurrence: Results from the Y-ECCO/ClinCom 2019 Survey. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1062–1066. [Google Scholar] [CrossRef]
- Yamada, A.; Komaki, Y.; Patel, N.; Komaki, F.; Pekow, J.; Dalal, S.; Cohen, R.D.; Cannon, L.; Umanskiy, K.; Smith, R. The use of vedolizumab in preventing postoperative recurrence of Crohn’s disease. Inflamm. Bowel Dis. 2018, 24, 502–509. [Google Scholar] [CrossRef]
- Mañosa, M.; Fernández-Clotet, A.; Nos, P.; Martín-Arranz, M.D.; Manceñido, N.; Carbajo, A.; Hinojosa, E.; Hernández-Camba, A.; Muñoz-Pérez, R.; Boscá-Watts, M.; et al. Ustekinumab and vedolizumab for the prevention of postoperative recurrence of Crohn’s disease: Results from the ENEIDA registry. Dig. Liver Dis. 2023, 55, 46–52. [Google Scholar] [CrossRef]
- Walshe, M.; Nayeri, S.; Ji, J.; Hernandez-Rocha, C.; Sabic, K.; Hu, L.; Giri, M.; Nayar, S.; Brant, S.; McGovern, D.P.B.; et al. A Role for CXCR3 Ligands as Biomarkers of Post-Operative Crohn’s Disease Recurrence. J. Crohns Colitis 2022, 16, 900–910. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubach, A.; Sultan, K.; Swaminath, A. Recurrent Ulceration and Disease at the Ileocolonic Anastomosis in Crohn’s Disease: Etiology, Prevention, and Management, a Review Article. J. Clin. Med. 2025, 14, 8158. https://doi.org/10.3390/jcm14228158
Schubach A, Sultan K, Swaminath A. Recurrent Ulceration and Disease at the Ileocolonic Anastomosis in Crohn’s Disease: Etiology, Prevention, and Management, a Review Article. Journal of Clinical Medicine. 2025; 14(22):8158. https://doi.org/10.3390/jcm14228158
Chicago/Turabian StyleSchubach, Abigail, Keith Sultan, and Arun Swaminath. 2025. "Recurrent Ulceration and Disease at the Ileocolonic Anastomosis in Crohn’s Disease: Etiology, Prevention, and Management, a Review Article" Journal of Clinical Medicine 14, no. 22: 8158. https://doi.org/10.3390/jcm14228158
APA StyleSchubach, A., Sultan, K., & Swaminath, A. (2025). Recurrent Ulceration and Disease at the Ileocolonic Anastomosis in Crohn’s Disease: Etiology, Prevention, and Management, a Review Article. Journal of Clinical Medicine, 14(22), 8158. https://doi.org/10.3390/jcm14228158





