1. Introduction
Diabetes mellitus (DM) is the term used for a metabolic disorder group in which carbohydrate metabolism is altered, resulting in a chronic hyperglycemic status [
1]. This group includes type 1 DM (T1DM), type 2 DM (T2DM), specific forms of DM due to specific causes (i.e., exocrine pancreas diseases, monogenic syndromes, chemical or drug-induced DM) and gestational DM (GDM) [
1,
2]. DM represents an important health issue, as its incidence has an increasing trend worldwide due to the obesity pandemic. It affects more and more young adults, including females of childbearing age [
3].
In conditions of poor glycemic control, pregestational DM and GDM lead to well-known vascular complications, the most frequent being cardiovascular (CV) disease, ocular, vascular, renal, and neurologic pathologies. Also, they are associated with short-term and long-term morbidities that affect both the mother and the fetus. Among short-term morbidities, we encounter preterm birth, gestational hypertension, preeclampsia [
4], large for gestational age fetuses and macrosomia [
5], polyhydramnios [
6], intrauterine growth restriction [
7], neonatal complications (respiratory distress syndrome, cardiomyopathy, hypoglycemia, hypomagnesemia, hypocalcemia or hyperbilirubinemia) [
8] or even stillbirth [
9]. Furthermore, due to hyperglycemia in the first trimester and periconceptional period, pregestational DM is associated with a two-to-four-fold higher risk of congenital malformations: congenital heart disease (transposition of the great arteries, tetralogy of Fallot, anomalous venous return or septal defects) [
10], central nervous malformations (spina bifida, anencephaly, hydrocephaly, microtia or anotia, encephalocel) [
11] and sacral or caudal dysplasia [
12].
During the peripartum period, newborns of diabetic mothers are at a higher risk of shoulder dystocia, brachial plexus palsy or clavicular fracture, independently of the birth weight [
13]. After childbirth, mothers with GDM present a higher risk of developing metabolic syndrome in the first three months [
14], as well as a nearly tenfold higher risk of developing T2DM in the course of time [
15]. Additionally, they have a twofold increased risk of developing CV disease compared to healthy females, independently of the presence of T2DM [
16]. Long-term complications for the offspring include a future risk for developing obesity [
17], T1DM or T2DM [
18]. Regarding long-term complications, infants from diabetic mothers are at high risk of becoming obese [
19], as well as developing metabolic syndrome, T1DM or T2DM in the future [
20].
Due to the constantly expanding incidence of DM, it is difficult to quantify the exact number of cases. A study published by Jovanovič et al. [
21] in 2015 concluded that the incidence of DM in pregnancy in the United States was 7.9% in their study period (2005–2011). Of those patients, 0.1% had T1DM, 1.2% had T2DM, 6.3% had GDM, and 0.2% had progressing GDM. Another study reported that, in 2020, the prevalence of GDM varied from 2% to 38% in other countries [
22], while in the United States, it was 7.8% [
23].
Considering the alarmingly rising number of DM cases during pregnancy as well as the burden that DM during pregnancy places on the healthcare system, mothers and infants, this article aims to report DM in pregnancy incidence numbers and analyze the dynamics in Romania, as well as propose prevention strategies for pregnancies complicated by GDM or T2DM.
4. Discussion
This study aimed to analyze the data reported by the Romanian National Public Health Institute in order to provide a comprehensive overview of the incidence of DM in Romanian pregnancies between 2014 and 2024. This analysis was undertaken due to the alarming rise in GDM incidence, which has placed a significant strain on the healthcare system. Two databases were obtained for this analysis. Database 1 was reported by medical units from the public and private sectors associated with the National Health Insurance House (according to the Health Minister Order no 1782/2006). Database 2, reported by the general practitioner to the Public Health Department. However, these databases cannot be merged due to the risk of overreporting. Combining these databases provides a clear view of the situation, enabling analysis of its dynamics and the development of prevention strategies for pregnancies complicated by GDM, T1DM, or T2DM. This is especially important because the data includes information on patients’ place of residence and risk category. It is important to note that, in light of national regulatory frameworks, Database 2 is the most likely to report more accurate incidence rates.
An initial observation of the data reported in Database 1, which relates to the annual incidence of reported pregnancies in Romania, reveals a worrying downward trend in the number of pregnant patients over the period in question. This phenomenon aligns with the findings of the 2021 Global Burden of Disease Study [
26], which provides an interpretation of the data. The study indicates a global decline in fertility characterized by varying degrees of steepness in the downward trend of livebirths commencing in 2000. The study’s global forecast is an enigmatic one, encompassing persistently declining fertility rates. Additionally, an analysis of the distribution of DM in pregnancy shows that reported rates were lower from 2014 to 2024 than those published in the literature.
Data from Database 1 reveal that the prevalence of DM in pregnancy ranged from approximately 1.01 to 3.08 per 1000 (95% CI spanning roughly from 1.00 to 3.20) between 2014 and 2024. Meanwhile, data from Database 2 reveal a prevalence of DM in pregnancy ranging from 0.84‰ to 5.88‰ (95% CI spanning from 0.82‰ to 5.95‰) over the same period, indicating a consistently increasing trend. The prevalence of pregestational DM in Romania between 2014 and 2024 is approximately 0.45‰ (95% CI 0.44‰–0.46‰). A systematic review and meta-analysis of the existing literature [
27] reports an overall prevalence of pregestational DM between 2011 and 2020 of 1.0% (95% CI 0.6–1.5), while the pooled prevalence in Europe was 0.5% (95% CI 0.4–0.7,
p < 0.01). A systematic review and meta-analysis conducted by Paulo et al. [
28] concerning the prevalence of GDM in Europe between 2014 and 2019, concluded that the mean prevalence of GDM in Europe is approximately 11%, reaching a higher prevalence of around 31.5% in Eastern European countries. The overall prevalence of pregnancies complicated by GDM in Romania from 2014 to 2024 is approximately 1.86‰ (95% CI 1.84–1.89), with the highest prevalence being reported in 2019 at 2.56‰ (95% CI 2.53–2.58). This difference can be explained by a deficient reporting system from the medical units based on the incomplete codification of the diagnosis.
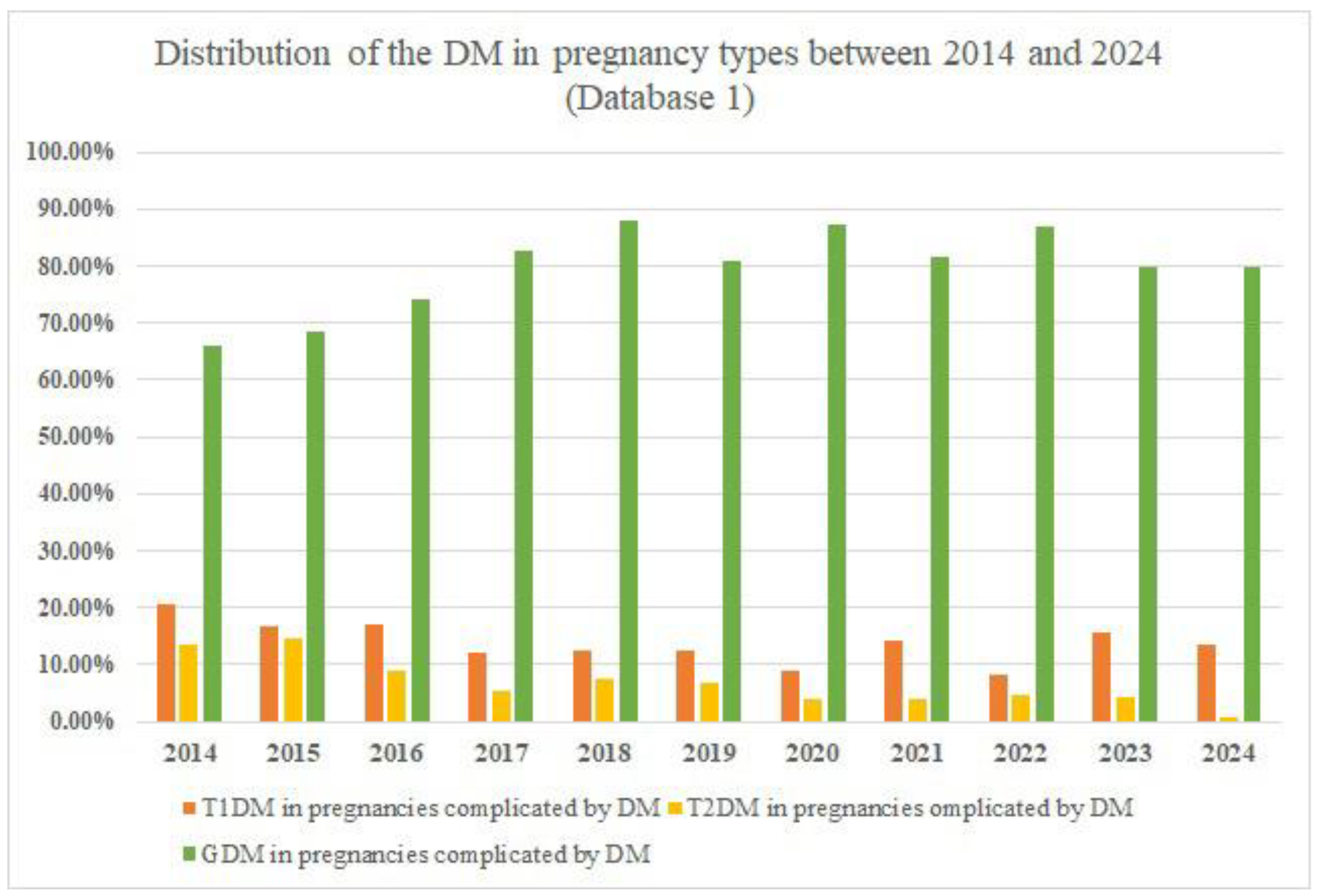
The rate of pregnancies complicated by DM peaked in 2018 with 353 reported cases. Another observation is represented by the fact that GDM accounts for at least two-thirds of all cases of DM in pregnancy. A study published in 2022 [
29] also reported a division between pregestational DM and GDM, with GDM accounting for the majority of cases. The study examined the population of Belgrade, Serbia, between 2010 and 2020 and found that the prevalence of DM in pregnancy was 3.4%, with GDM accounting for 2.7% of cases and pregestational DM accounting for 0.7%. Additionally, a study conducted on the German population between 2013 and 2019 and published in 2023 [
30] reported a GDM prevalence of 5.7%, with a much lower pregestational DM prevalence of 0.93%. Another important fact is that many cases could be misdiagnosed as GDM instead of pregestational DM due to the lack of medical history, preconceptional screening for DM, or the homogeneity of the GDM diagnosis method. The correct diagnosis becomes clear after birth. This issue is persistent worldwide, as there is no consensus on the GDM diagnosis method. For example, ADA presents two methods of GDM diagnosis: the one-step and two-step methods [
2]. In contrast, the Australasian Diabetes in Pregnancy Society recommends using only the 75 g oral glucose tolerance test [
31].
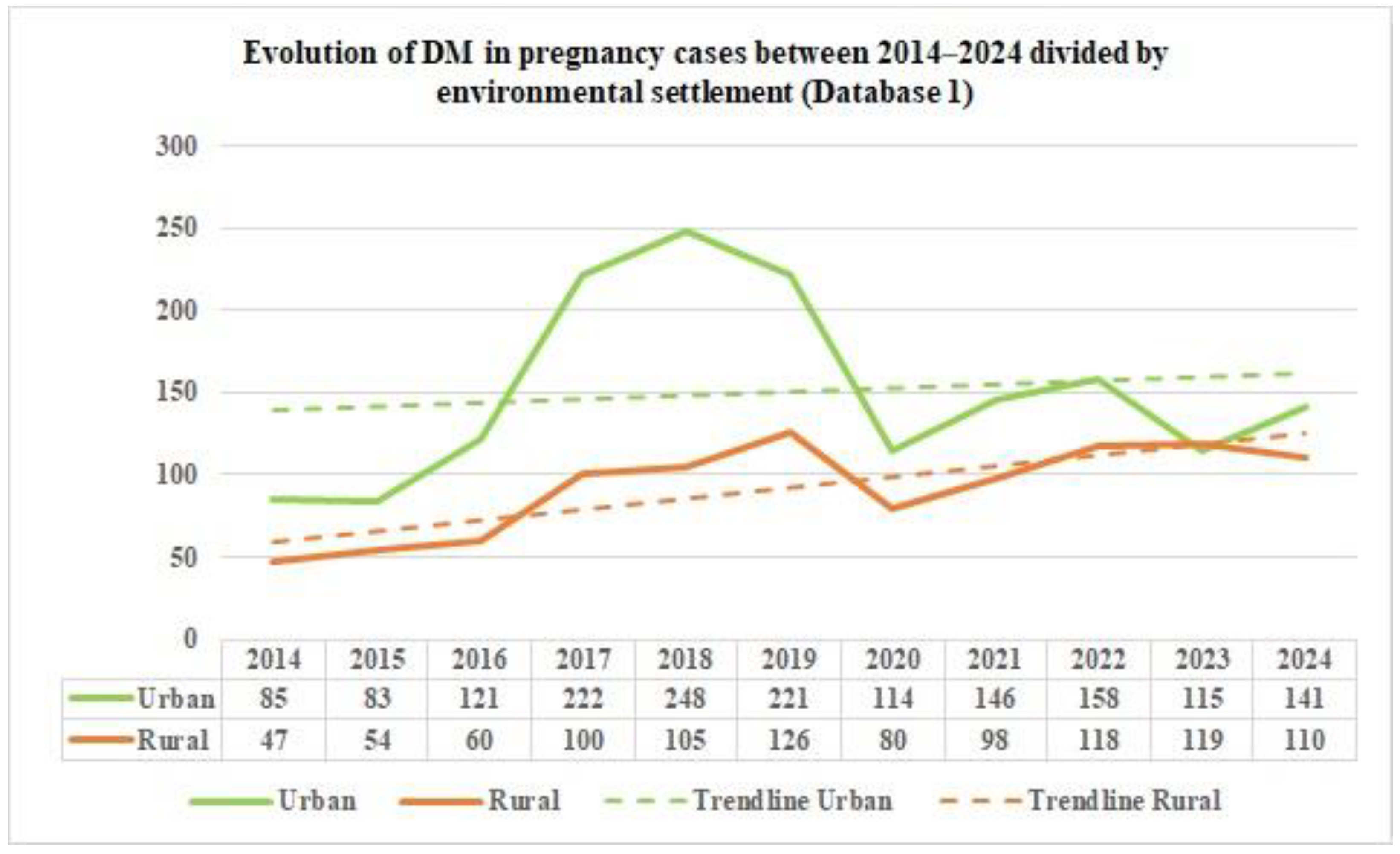
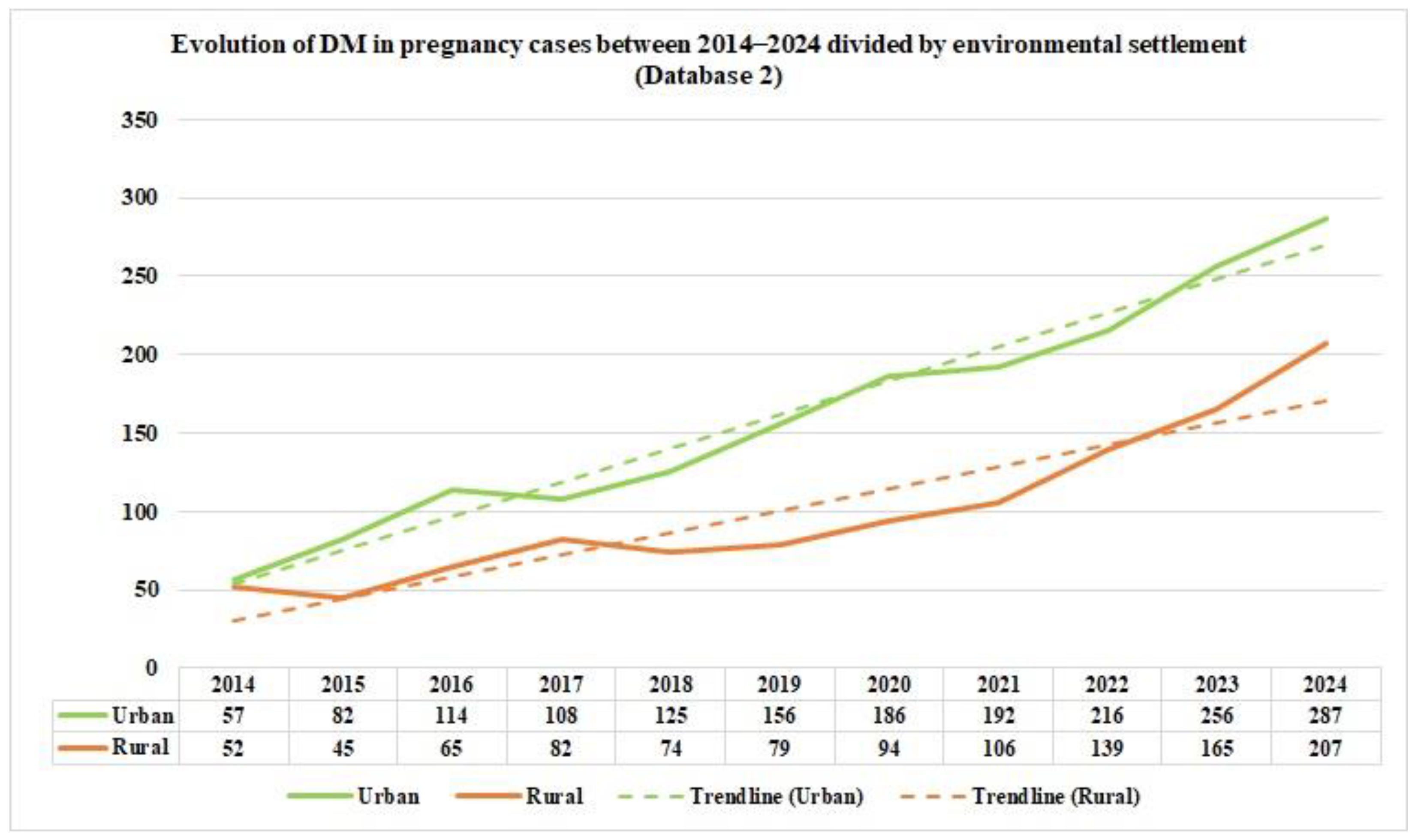
From the data on the distribution of DM in pregnancy cases in Romania by age group, as reported by Database 2, in the study period, we can notice that the majority of DM in pregnancy cases occurred in the 20–39 age group during the study period. Furthermore, based on the data from the two distinct databases, we can emphasize the fact that there is a significant difference in the reported data starting in 2017. This is because the number of cases in the Database has almost doubled; this can be explained by the exclusion of general practitioners from pregnancy monitoring. From 2020 onwards, the number of cases in Database 1 is lower, suggesting either fewer hospitalizations of pregnant patients with DM or deficient reporting by medical units. Regarding environmental settlement, analyzing the number of cases of DM in pregnancy from Romania between 2014 and 2024, as reported in Database 1, reveals a higher prevalence in the urban areas in 2014, at around two-thirds, suggesting urban–rural disparities. After eleven years, the rates in the rural and urban areas equalized.
This homogenization between areas could be due to many factors, such as better accessibility to health services and diagnosis in the rural areas, decreased fear or increased awareness of the need for medical appointments and pregnancy monitoring, or, in the worst-case scenario, a decrease in food quality intake and an increase in unhealthy diets and lifestyles in the rural areas. In contrast, the data concerning the environmental settlement of the DM in pregnancy cases from Romania between 2014 and 2024, as reported in Database 2, shows an almost equalization of the cases of DM in pregnancy in rural and urban areas. From 2017 to 2024, there was a higher prevalence of approximately two-thirds in the urban areas. A study conducted on Moroccan pregnant patients with GDM, conducted between October 2018 and February 2019 [
32], found no statistically significant difference in environmental settlement (
p = 0.171) and reported 52.9% cases from the rural areas and 47.1% cases belonging to the urban areas. In contrast, a study published in 2023 [
33], conducted between 2011 and 2019 in the United States, analyzing 12,401,888 singleton live births from nulliparous females, concluded that patients living in the rural areas present an increased overall risk of developing pregestational DM, with an age-adjusted rate ratio of 1.48 (95% CI 1.45–1.51), as well as a higher overall risk of GDM, with an age adjusted rate ratio of 1.17 (95% CI 1.16–1.18)
p < 0.01, compared to females living in the urban areas. There was an increasing trend over time.
The data from Database 1 regarding all cases of DM in pregnancy were divided into three age groups, each presenting certain peculiarities. The first category comprises adolescents and very young adults. This category is at high risk from a social point of view with regard to the education of the mother and the infant, as well as from a medical point of view with regard to adherence to treatment and recommendations. Although there are not many reported cases, it is important to bear in mind that this is a vulnerable group requiring special attention from social services as well as the medical system. Specific medical recommendations and follow-ups are necessary to ensure the best possible pregnancy outcome and postnatal care.
The second group, aged between 20 and 39 years, represents the majority of cases; the females from this group are of childbearing age. The last group, aged 40–49 years, is another high-risk category in which GDM is prevalent and where there are frequent pregnancy complications, including spontaneous abortions, GDM, gestational hypertension, preeclampsia and so on. This group also has a higher risk of adverse pregnancy outcomes, including premature birth, neonatal intensive care unit admissions and lower Apgar scores [
34]. This group also has a higher risk of presenting comorbidities such as being overweight and obese, and it is the group in which artificial reproductive techniques are most commonly used. This group is also at higher risk of postpartum depression and requires a multidisciplinary approach to treatment and postpartum care [
35]. Patients in the 10–14 age group in the two high-risk categories who received insufficient prenatal care, as well as socially vulnerable patients, although few were reported in this eleven-year period, still exist in real-life practice, indicating and signalling the fact that there are adolescents who could benefit from improved prenatal care. The highest rate of pregnant patients who received insufficient prenatal care was recorded in the 20–39 years age group (prevalence ranging from 0‰ to 0.50‰), followed by the 15–19 years age group. The numbers in these categories reached a peak of prevalence in 2017, followed by a 50% decrease in the numbers in 2024. Also, these age subgroups have reached the majority in the socially vulnerable group, leading to unsuitable prenatal care.
Regarding the urban–rural distribution of these high-risk pregnancies, the pregnant patients in the rural areas have a higher rate of insufficient prenatal care that ranges from 81.81% in 2014 to a slight decrease to 74.19% in 2024. The rates of socially vulnerable pregnancies also have a predominant rural distribution: 75% in 2014, an inverted ratio in favour of the urban area in 2016 and 2017, an equalized ratio in 2019 between rural and urban areas, and a similar distribution in 2024. By analyzing the particularities of these patients, pregnant patients who received insufficient prenatal care and socially vulnerable patients, the most vulnerable categories are adolescents and young adults, respectively, living in rural areas. We can conclude that there are measures that need to be taken in order to improve the care of defenceless females: improving the healthcare (preconceptionally, prenatally and also in the postpartum period), enhancing the medical knowledge, especially of the high-risk population, revising health policies and involving more and more social services. In addition, there is a critical need to emphasize the implications of underreporting, such as masking the true burden of DM in pregnancy, weakening health surveillance and policy planning, and altering preventive measures. Moreover, underreporting jeopardizes public safety, patients’ outcomes, resource planning, legal compliance and professional integrity.
4.1. Preventive Strategies
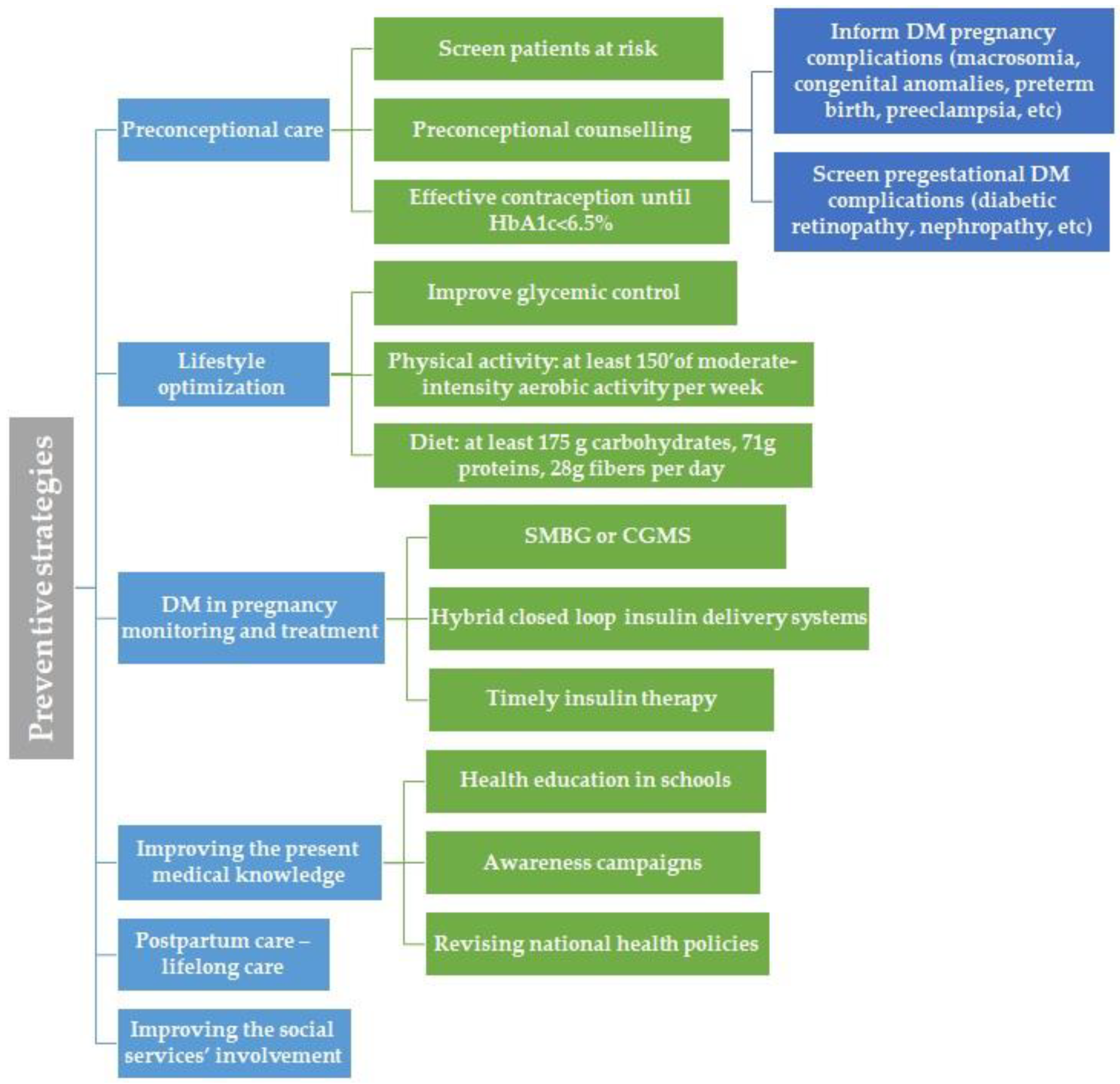
Following these observations, we would like to point out several aspects that require improvement. Firstly, primary care should include T2DM and GDM prevention and preconceptional care for pregestational DM via general practitioners and obstetricians, aligning with the ADA recommendations.
4.2. Preconceptional Care and DM in Pregnancy Complications Prevention
Preconceptional counselling should begin at puberty and continue for all people of childbearing age and DM, alongside with effective contraception and family planning focusing on educating the people about the importance of a proper glycemic control, reaching to an ideal a HbA1c value of <6.5%, in order to prevent the major DM complications in pregnancy, such as macrosomia, congenital anomalies, preterm birth or preeclampsia [
35]. Patients with a history of GDM should be screened preconceptionally for DM and should receive preconception care in order to diagnose and treat hyperglycemia, therefore reducing the risk of congenital malformations [
35]. The attention should focus on physical activity, nutrition, DM self-care education, as well as screening for DM comorbidities and complications, such as diabetic retinopathy or nephropathy. Patients with pregestational diabetes should be informed regarding the risk of developing and/or progression of diabetic retinopathy and should undergo dilated eye examinations ideally before pregnancy, every trimester and 1 year postpartum [
36].
4.3. Lifestyle Optimization
If diagnosed, the first step in managing GDM is represented by medical nutrition therapy. To prevent GDM complications, adequate glycemic control is desired. Prenatal care should include healthy lifestyle counselling; more specifically, it should include information about an adequate diet that promotes healthy fetal development and optimal glycemic control, and it should advocate for physical activity, that is, at least 150 min of aerobic activity of moderate intensity weekly [
37]. Concerning nutrition, the recommendation is not to exclude carbohydrates. The proposed diet should include at least 175 g of carbohydrates, followed by at least 71 g of protein and 28 g of fibre per day [
38]. Restricting carbohydrate intake is often accompanied by increased fat intake, which aggravates maternal insulin resistance, amplifies lipolysis, increases free fatty acid levels [
39], and is responsible for fetal overgrowth [
40].
4.4. DM in Pregnancy Monitoring and Treatment
Monitoring pregnancies with DM should focus on a multidisciplinary approach, respectively, an active collaboration between obstetricians, diabetologists and general practitioners. Obstetrical monitoring consists of fetal monitoring, including fetal growth curve, the presence or absence of polyhydramnios and fetal wellbeing, as well as maternal wellbeing. It also involves identifying possible maternal complications such as gestational hypertension [
41]. Glycemic control can be achieved using the classical method, respectively, self-monitoring blood glucose (SMBG), which involves measuring fasting blood glucose concentrations daily, as well as before each meal and one or two hours after eating [
41]. Alternatively, continuous glucose monitoring systems (CGMS) can be used [
42]. The latter have already proven their efficacy in monitoring T1DM during pregnancy in the CONCEPTT trial [
43]. Unfortunately, CGMS are not used on a large scale for pregnancies with T2DM or GDM, despite their potential to obtain a more detailed picture of the glycemic variability. It should be noted that CGMS offer information on nocturnal glycemic values and could facilitate the timely initiation of insulin therapy for GDM, thereby avoiding pregnancy complications caused by exposure to hyperglycemia in utero [
44,
45,
46]. A study by Alfadhli et al. [
47] found that pregnancies with GDM monitored by CGMS required smaller insulin doses than those monitored by SMBG.
In addition to medical nutrition therapy, insulin therapy is the second-line treatment for GDM and the first-line treatment for pregestational DM [
48,
49]. Optimal insulin therapy could prevent hyperglycemia-related complications from the first trimester, when hyperglycemia can negatively affect the normal development of the embryo, resulting in congenital malformations, to the last trimester of pregnancy, when it can prevent macrosomia, neonatal complications and even stillbirth. In this regard, hybrid closed-loop insulin delivery systems, the newest technology, could improve pregnancy outcomes in women with T1DM. This system, consisting of CGMS and insulin pumps, can be started preconceptionally to improve glycemic values and avoid changing therapy during the first trimester [
50].
4.5. Improving the Present Medical Knowledge
This should be achieved by introducing health education in schools and running nutrition awareness campaigns. National health policies should also be revised. Patients at risk of developing T2DM should be systematically screened according to the guidelines, using fasting blood glucose levels and HbA1c. This category includes overweight or obese adults with at least one of the following risk factors: a history of CV disease; belonging to a high-risk race, ancestry or ethnicity; having a first-degree relative diagnosed with DM; hypertension; physical inactivity; polycystic ovary syndrome; or other pathology associated with insulin resistance, such as metabolic dysfunction or acanthosis nigricans. It also includes patients with high-density lipoprotein (HDL) cholesterol levels of less than 35 mg/dL, patients with a history of GDM, or who are at least 35 years old. Patients in high-risk groups, such as those with human immunodeficiency virus infection or a history of pancreatitis, are also included [
2]. Screening should also not exclude children or adolescents at risk of developing prediabetes or T2DM, beginning with the onset of puberty or at the age of 10. This category includes overweight or obese children and adolescents whose mothers presented GDM or pregestational DM, or who have a first- or second-degree family member with T2DM, or who belong to a high-risk ethnic group, or who present signs of insulin resistance or insulin resistance-associated pathologies (e.g., polycystic ovary syndrome, acanthosis nigricans, dyslipidemia, hypertension), or who had a birth weight that was large or small for gestational age [
2]. Adults and children/adolescents at high-risk of developing T2DM could certainly benefit from prediabetes and DM screening and timely intervention involving lifestyle adjustments, nutritional education and the initiation of physical activity.
4.6. Postpartum Care
In our study, the majority of DM cases in pregnancy were represented by GDM. Therefore, postpartum care should include lifelong care for these patients, as well as screening every 1–3 years for the early detection of prediabetes and T2DM. This screening should be carried out at 4–12 weeks with a 75 g oral glucose tolerance test. Patients with a history of GDM who are overweight or obese should be counselled and educated to embrace a healthy lifestyle and lose weight. If prediabetes is diagnosed in these patients, thorough lifestyle interventions should be implemented and metformin administered, if necessary, to prevent the development of DM [
37].
4.7. Improving the Social Services’ Involvement
Social services should monitor high-risk pregnancies involving women at risk due to social issues or inequalities, and those who could receive inadequate prenatal care. This would ensure that these women have access to medical care and adhere to recommendations and treatment. The social services department should be involved in caring for vulnerable women and should be an institution that is easy to approach. It should offer an empathetic and safe environment for women at risk in order to achieve the best possible outcomes for pregnancies and for the future development of their children.
Finally, we created an illustration that summarizes the main proposed preventive strategies for improving care of pregnant women with DM in Romania (
Figure 4).
4.8. Future Directions
Firstly, clear and homogeneous criteria for GDM diagnosis should be established worldwide, along with universally accepted cut-offs for exact differential diagnosis between pregestational DM and GDM. Secondly, to gather all the data on DM in pregnancy in Romania and to gain a more complete picture, the reporting system requires substantial and continuous improvement. There is a gap in the reporting system from the medical units associated with the National Insurance House that needs to be addressed. The National Public Health Department would also benefit from data reported from the private medical sector. Furthermore, future efforts should aim to harmonize data collection between the two national databases to avoid duplication and underestimation. Improved digitalization and standardized ICD coding across all reporting levels could significantly increase accuracy and comparability with international statistics. Collaboration between general practitioners and public and private medical units must also be strengthened to ensure accurate reporting of cases. Another area for future research could be the study of different social, economic or demographic factors that could influence the occurrence of DM in pregnancy among Romanian patients.
Another important area for development is the integration of socio-economic and environmental factors into surveillance systems. Current findings show that adolescents, women of advanced maternal age, and those from rural areas or with insufficient prenatal care remain highly vulnerable. Personalized interventions for these groups, supported by both healthcare and social services, should be prioritized.
Moreover, prospective cohort studies and multicenter collaborations at national and regional levels could provide deeper insights into the long-term maternal and offspring outcomes of DM during pregnancy. This would enable more effective prevention strategies, timely interventions and evidence-based health policies that address both medical and social inequities.
Finally, improving the healthcare system involves the need for changing the law; healthcare providers and legislators should be focusing on:
Solving the underreporting issue by establishing a functional system or registry for reporting the DM in pregnancy cases;
Achieving DM subtype diagnosis, monitoring, treatment, peripartum and postpartum care standardization by integrating the existing international guidelines into the local and, respectively, national context;
Improving the medical knowledge of the Romanian population by introducing age-adapted healthcare classes from primary school to high school, running awareness campaigns, nutritional workshops, organizing or supporting local or regional physical activity events;
Ensuring a better availability of medical and social support for vulnerable categories (adolescents, young adults, patients from rural areas, socio-economically unstable families) in order to increase the addressability of these categories, regulating a multidisciplinary approach of such cases: general practitioner, diabetologist, obstetrician, psychologist and social services worker.
4.9. Strengths and Limitations
The main strength of the present study lies in the data regarding DM in pregnancy, as well as in pregnant females who received insufficient prenatal care or who presented social issues in Romania in the last decade. The central limitation is the national reporting system, which the data highlights as having significant gaps. This system likely underestimates the true burden of DM in pregnancy compared to international numbers. Some public institutions might have failed to classify the disease according to the ICD system, and some patients were monitored and gave birth exclusively in private medical units that do not report to the National Public Health Department. Another limitation of the present study is represented by the lack of data on socio-economic and demographic risk factors that could characterize Romanian patients.