Microbial Biofilms as Barriers to Chronic Wound Healing: Diagnostic Challenges and Therapeutic Advances
Abstract
1. Introduction
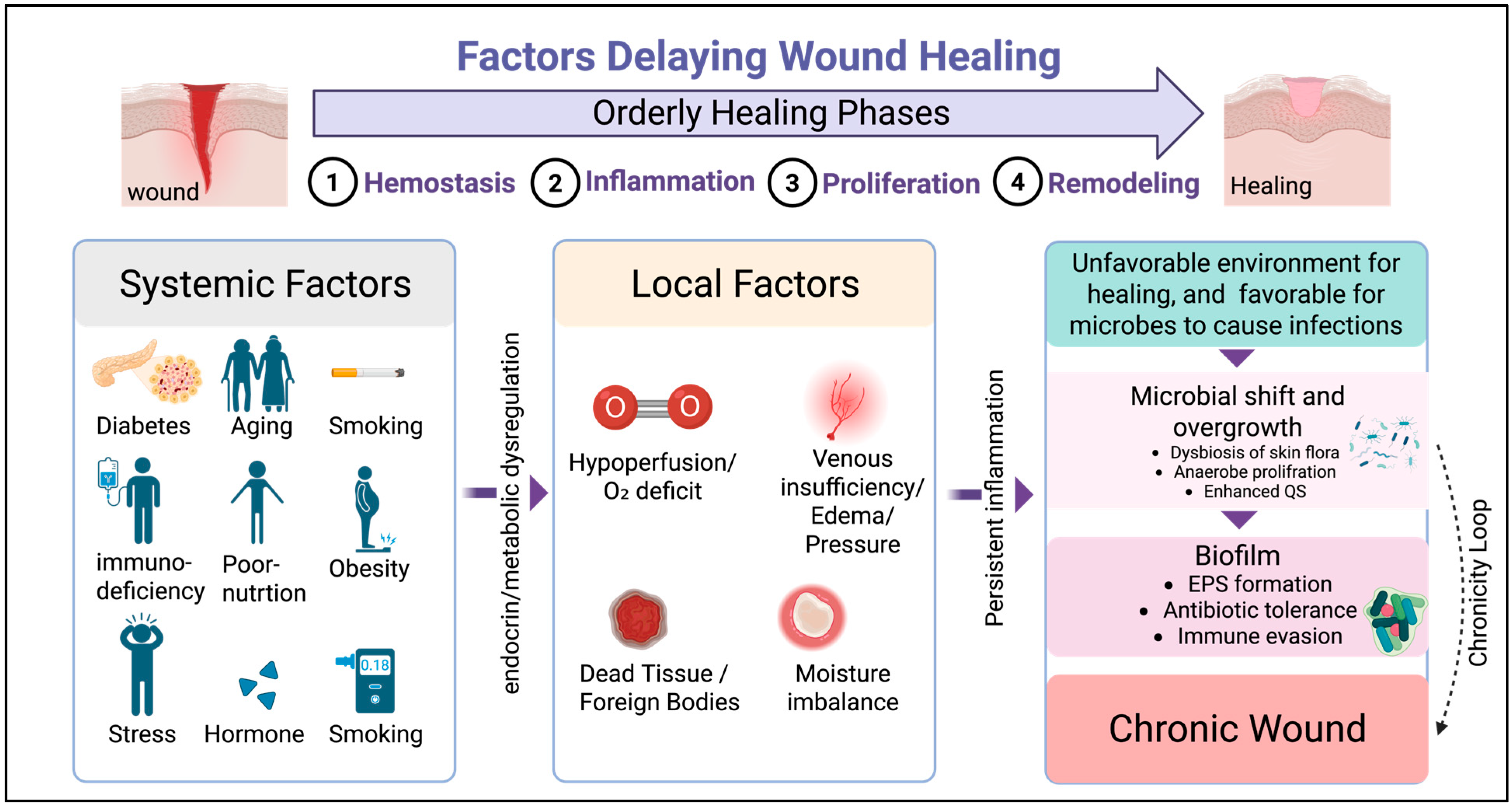
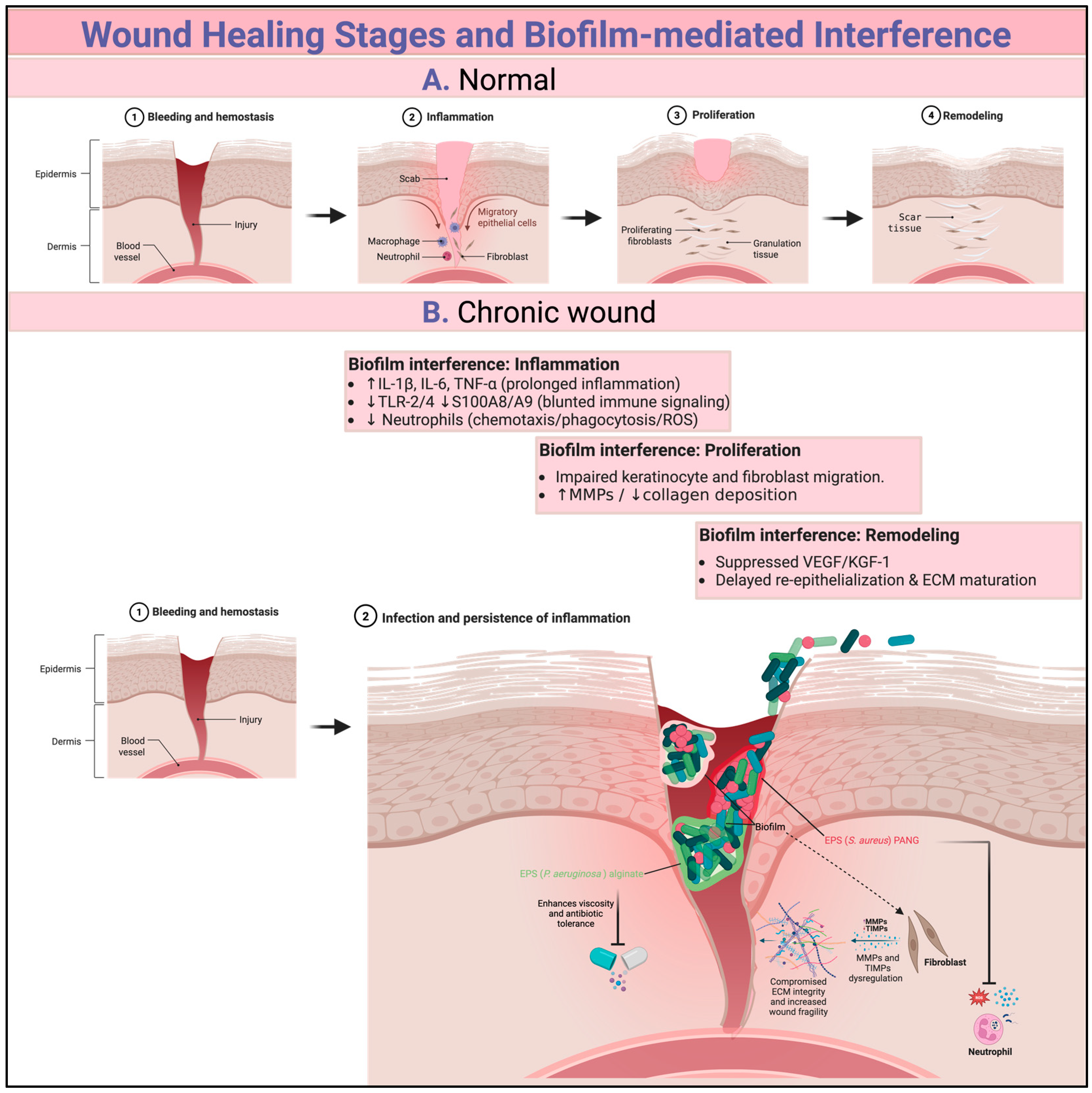
1.1. Wound Healing Stages
1.2. Factors Delaying Wound Healing
2. Biofilms in the Context of Chronic Wounds
3. Understanding Biofilms
4. Stages of Biofilm Formation on Wounds and Commonly Involved Microbial Species
5. How Biofilms Hinder the Natural Process of Wound Healing
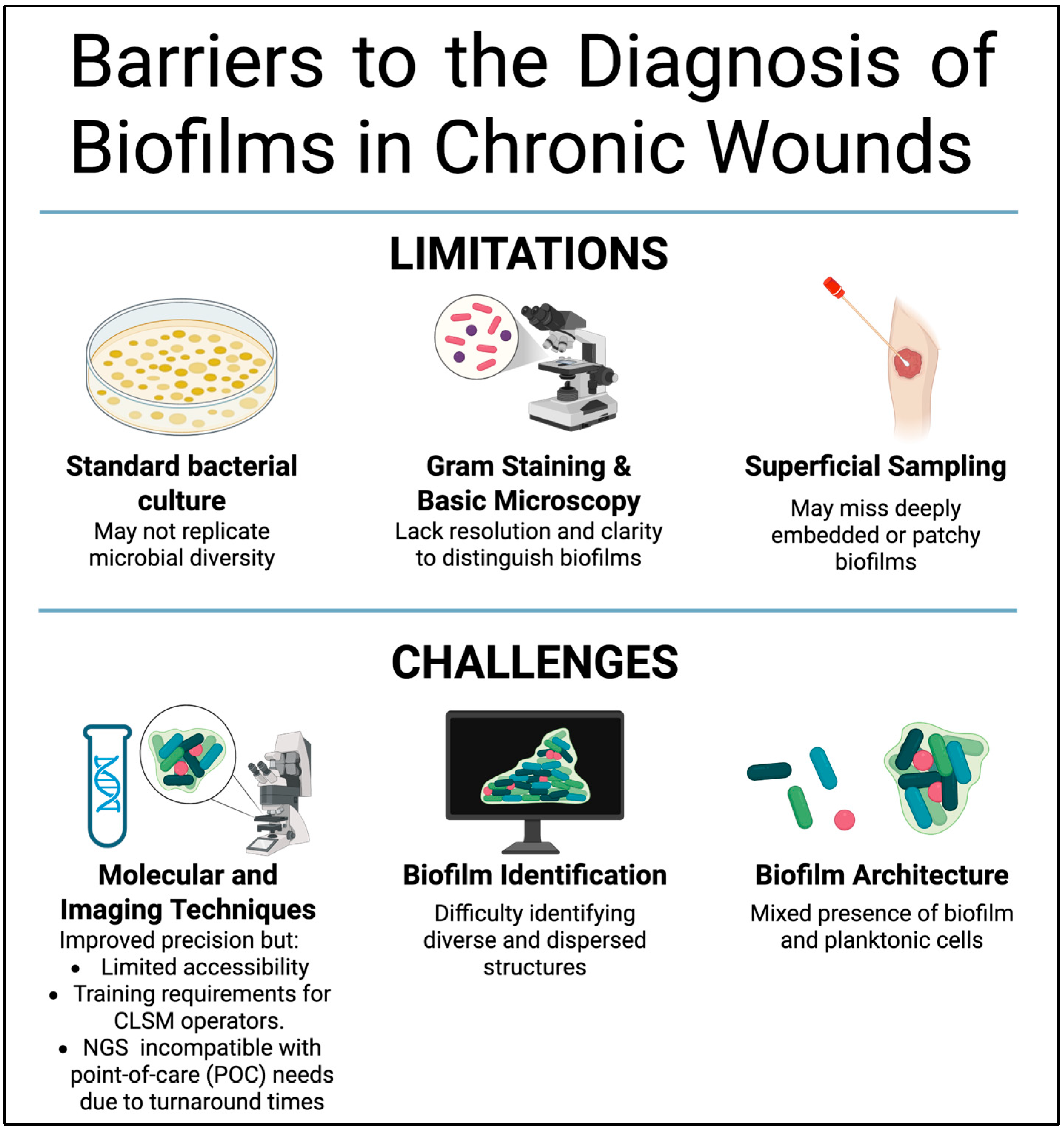
6. Barriers to the Diagnosis of Biofilms in Chronic Wounds
7. Current Treatment Strategies
8. Potential Innovative Approaches for Managing Wound Biofilms
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| EPS | Extracellular polymeric substance |
References
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Trøstrup, H.; Laulund, A.S.B.; Moser, C. Insights into Host-Pathogen Interactions in Biofilm-Infected Wounds Reveal Possibilities for New Treatment Strategies. Antibiotics 2020, 9, 396. [Google Scholar] [CrossRef]
- Luo, H.; Yu, X.; Sun, T.; Sun, Z.; Zhao, T.; Li, C.; Angelova Volponi, A.; Flores-Borja, F.; Sun, H.; An, Z. Macrophage-derived IL-1β directs fibroblast progenitor cell fate via metabolic reprogramming in wound healing. Commun. Biol. 2025, 8, 1291. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Faizan, M.; Porwal, M.; Sharma, H.; Sachan, N. An Overview and Review of Growth Factors in Wound Healing: Emerging Trends and Innovations. Curr. Diabetes Rev. 2025, 22, e15733998332692. [Google Scholar] [CrossRef]
- Boleti, A.P.d.A.; Jacobowski, A.C.; Frihling, B.E.F.; Cruz, M.V.; Santos, K.F.D.P.; Migliolo, L.; de Andrade, L.R.M.; Macedo, M.L.R. Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine. Pharmaceuticals 2025, 18, 1525. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Lu, Z.; Nie, F.; Chong, Y. Integrins regulation of wound healing processes: Insights for chronic skin wound therapeutics. Front. Cell. Infect. Microbiol. 2024, 14, 1324441. [Google Scholar] [CrossRef]
- Shi, Z.; Yao, C.; Shui, Y.; Li, S.; Yan, H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol. 2023, 14, 1284981. [Google Scholar] [CrossRef]
- Fernandez-Guarino, M.; Naharro-Rodriguez, J.; Bacci, S. Aberrances of the Wound Healing Process: A Review. Cosmetics 2024, 11, 209. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Li, H.; Zhou, L.; Shi, H.; Feng, S.; Chen, J.; Mei, Z. Effect of natural-based biological hydrogels combined with growth factors on skin wound healing. Nanotechnol. Rev. 2022, 11, 2493–2512. [Google Scholar] [CrossRef]
- Atkin, L.; Bućko, Z.; Montero, E.C.; Cutting, K.; Moffatt, C.; Probst, A.; Romanelli, M.; Schultz, G.S.; Tettelbach, W. Implementing TIMERS: The race against hard-to-heal wounds. J. Wound Care 2019, 28, S1–S50. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-enhanced immunoregulation hydrogel for oxygenation and ROS neutralization in diabetic foot ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Xiang, Y.; Pan, Z.; Qi, X.; Ge, X.; Xiang, J.; Xu, H.; Cai, E.; Lan, Y.; Chen, X.; Li, Y.; et al. A cuttlefish ink nanoparticle-reinforced biopolymer hydrogel with robust adhesive and immunomodulatory features for treating oral ulcers in diabetes. Bioact. Mater. 2024, 39, 562–581. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Boutet-Dubois, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.-P.; Loubet, P. Alternative Approaches for the Management of Diabetic Foot Ulcers. Front. Microbiol. 2021, 12, 747618. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Eberlein, T.; Malone, M.; Schultz, G. Management of wound biofilm made easy. Wounds Int. 2017, 8, 1–6. [Google Scholar]
- Shen, A.Z.; Taha, M.; Ghannoum, M.; Tyring, S.K. Biofilms and Chronic Wounds: Pathogenesis and Treatment Options. J. Clin. Med. 2025, 14, 7784. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, M.A.; McNamara, S.A.; Sanchez, D.; Hirt, P.A.; Kirsner, R.S. Evidence-based review of antibiofilm agents for wound care. Adv. Wound Care 2021, 10, 13–23. [Google Scholar] [CrossRef]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Kirketerp-Møller, K.; Kvich, L.A.; Christensen, M.H.; Fritz, B.; Jakobsen, T.H.; Bjarnsholt, T. Single cells and bacterial biofilm populations in chronic wound infections. Apmis 2024, 132, 1071–1077. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three faces of biofilms: A microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. NPJ Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Jeckel, H.; Díaz-Pascual, F.; Skinner, D.J.; Song, B.; Jimenez-Siebert, E.; Strenger, K.; Jelli, E.; Vaidya, S.; Dunkel, J.; Drescher, K. Shared biophysical mechanisms determine early biofilm architecture development across different bacterial species. PLoS Biol. 2022, 20, e3001846. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Yu, C.; Li, J.; Zhou, X. Biofilm formation: Mechanistic insights and therapeutic targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef]
- Kundukad, B.; Rice, S.A.; Doyle, P.S.; Kjelleberg, S. Alginate exopolymer significantly modulates the viscoelastic properties and resilience of bacterial biofilms. NPJ Biofilms Microbiomes 2025, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Stewart, P.S.; Williamson, K.S.; Boegli, L.; Hamerly, T.; White, B.; Scott, L.; Hu, X.; Mumey, B.M.; Franklin, M.J.; Bothner, B.; et al. Search for a Shared Genetic or Biochemical Basis for Biofilm Tolerance to Antibiotics Across Bacterial Species. Antimicrob. Agents Chemother. 2022, 66, e00021–e00022. [Google Scholar] [CrossRef]
- Matheus, G.G.; Chamoun, M.N.; Khosrotehrani, K.; Sivakumaran, Y.; Wells, T.J. Understanding the pathophysiology of Pseudomonas aeruginosa colonization as a guide for future treatment for chronic leg ulcers. Burn. Trauma 2025, 13, tkae083. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, S.; Wang, H.; Wang, Y. Biofilm therapy for chronic wounds. Int. Wound J. 2024, 21, e14667. [Google Scholar] [CrossRef]
- Kim, J.H.; Ruegger, P.R.; Lebig, E.G.; VanSchalkwyk, S.; Jeske, D.R.; Hsiao, A.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress Create a Microenvironment That Significantly Decreases the Diversity of the Microbiota in Diabetic Chronic Wounds and Promotes Biofilm Formation. Front. Cell. Infect. Microbiol. 2020, 10, 259. [Google Scholar] [CrossRef]
- Bach, M.S.; de Vries, C.R.; Khosravi, A.; Sweere, J.M.; Popescu, M.C.; Chen, Q.; Demirdjian, S.; Hargil, A.; Van Belleghem, J.D.; Kaber, G.; et al. Filamentous bacteriophage delays healing of Pseudomonas-infected wounds. Cell Rep. Med. 2022, 3, 100656. [Google Scholar] [CrossRef]
- Chen, X.; Lorenzen, J.; Xu, Y.; Jonikaite, M.; Thaarup, I.C.; Bjarnsholt, T.; Kirketerp-Møller, K.; Thomsen, T.R. A novel chronic wound biofilm model sustaining coexistence of Pseudomonas aeruginosa and Staphylococcus aureus suitable for testing of antibiofilm effect of antimicrobial solutions and wound dressings. Wound Repair Regen. 2021, 29, 820–829. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.; Wu, J.; Ahamed, A.I.; Wang, Y.; Martins-Green, M. N-Acetyl-cysteine and Mechanisms Involved in Resolution of Chronic Wound Biofilm. J. Diabetes Res. 2020, 2020, 9589507. [Google Scholar] [CrossRef]
- Johani, K.; Malone, M.; Jensen, S.; Gosbell, I.; Dickson, H.; Hu, H.; Vickery, K. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int. Wound J. 2017, 14, 1160–1169. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Bernardi, T.; Provot, C.; Tasse, J.; Sotto, A.; Lavigne, J.-P. A Relevant Wound-Like In Vitro Media to Study Bacterial Cooperation and Biofilm in Chronic Wounds. Front. Microbiol. 2022, 13, 705479. [Google Scholar] [CrossRef]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2020, 271, 1174–1185. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair. Regen. 2012, 20, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Y.; Zhao, M.; Jiang, J. Collective cell migration: Implications for wound healing and cancer invasion. Burn. Trauma 2013, 1, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Jeffery Marano, R.; Jane Wallace, H.; Wijeratne, D.; William Fear, M.; San Wong, H.; O’Handley, R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci. Rep. 2015, 5, 13296. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.B.; Shruptha, P.; Prabhu, V.; Srujan, C.; Nayak, U.Y.; Anuradha, C.K.R.; Ramachandra, L.; Keerthana, P.; Joshi, M.B.; Murali, T.S. Pseudomonas aeruginosa virulence proteins pseudolysin and protease IV impede cutaneous wound healing. Lab. Investig. 2020, 100, 1532–1550. [Google Scholar] [CrossRef]
- Roy, S.; Elgharably, H.; Sinha, M.; Ganesh, K.; Chaney, S.; Mann, E.; Miller, C.; Khanna, S.; Bergdall, V.K.; Powell, H.M. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J. Pathol. 2014, 233, 331–343. [Google Scholar] [CrossRef]
- Trøstrup, H.; Lerche, C.J.; Christophersen, L.J.; Thomsen, K.; Jensen, P.Ø.; Hougen, H.P.; Høiby, N.; Moser, C. Chronic Pseudomonas aeruginosa biofilm infection impairs murine S100A8/A9 and neutrophil effector cytokines—Implications for delayed wound closure? Pathog. Dis. 2017, 75, ftx068. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Seth, A.K.; Hong, S.J.; Geringer, M.R.; Xie, P.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. Deficient cytokine expression and neutrophil oxidative burst contribute to impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Wound Repair Regen. 2013, 21, 833–841. [Google Scholar] [CrossRef]
- Roy, R.; Mahmud, F.; Zayas, J.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. Reduced Bioactive Microbial Products (Pathogen-Associated Molecular Patterns) Contribute to Dysregulated Immune Responses and Impaired Healing in Infected Wounds in Mice with Diabetes. J. Investig. Dermatol. 2024, 144, 387–397.e11. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Gurjala, A.N.; Hong, S.J.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A. Treatment of Pseudomonas aeruginosa biofilm–infected wounds with clinical wound care strategies: A quantitative study using an in vivo rabbit ear model. Plast. Reconstr. Surg. 2012, 129, 262e–274e. [Google Scholar] [CrossRef]
- Pastar, I.; Nusbaum, A.G.; Gil, J.; Patel, S.B.; Chen, J.; Valdes, J.; Stojadinovic, O.; Plano, L.R.; Tomic-Canic, M.; Davis, S.C. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE 2013, 8, e56846. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.K.S.; Lichtenberg, M.; Fritz, B.G.; Díaz-Pinés Cort, I.; Al-Zoubaidi, D.F.; Gottlieb, H.; Kirketerp-Møller, K.; Bjarnsholt, T.; Jakobsen, T.H. The chronic wound characterisation study and biobank: A study protocol for a prospective observational cohort investigation of bacterial community composition, inflammatory responses and wound-healing trajectories in non-healing wounds. BMJ Open 2024, 14, e084081. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.C.; Cheong, J.Z.A.; Radzietza, M.; Fritz, B.; Malone, M.; Bjarnsholt, T.; Ousey, K.; Swanson, T.; Schultz, G.; Gibson, A.L.F.; et al. What is slough? Defining the proteomic and microbial composition of slough and its implications for wound healing. Wound Repair Regen. 2024, 32, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.; Blanz, E.; Gaddy, J. Clinical investigation of biofilm in non-healing wounds by high resolution microscopy techniques. J. Wound Care 2016, 25, S11–S22. [Google Scholar] [CrossRef]
- Barbosa, A.; Miranda, S.; Azevedo, N.F.; Cerqueira, L.; Azevedo, A.S. Imaging biofilms using fluorescence in situ hybridization: Seeing is believing. Front. Cell. Infect. Microbiol. 2023, 13, 1195803. [Google Scholar] [CrossRef]
- Mikziński, P.; Kraus, K.; Widelski, J.; Paluch, E. Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms 2024, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Mayer, P.; Smith, A.C.; Hurlow, J.; Morrow, B.R.; Bohn, G.A.; Bowler, P.G. Assessing biofilm at the bedside: Exploring reliable accessible biofilm detection methods. Diagnostics 2024, 14, 2116. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Edmonds, M.E.; Serena, T.E. Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers. Int. Wound J. 2023, 20, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial biofilms affect chronic wound healing: A narrative review. Int. J. Surg. Glob. Health 2020, 3, e16. [Google Scholar] [CrossRef]
- Torkington-Stokes, R.; Moran, K.; Martinez, D.S.; Granara, D.C.; Metcalf, D.G. Improving outcomes for patients with hard-to-heal wounds following adoption of the Wound Hygiene Protocol: Real-world evidence. J. Wound Care 2024, 33, 304–310. [Google Scholar] [CrossRef]
- Patel, M.P.T.; Yang, K.C.; Goss, S.; Alcantara, S.; Lantis, J.C., II. The Effects of Debridement and Secondary Dressing on Planktonic and Biofilm Protected Bacteria: An Analysis of 3 Studies. World J. Vasc. Surg. 2018, 1, 1015. [Google Scholar]
- Walker, M.; Metcalf, D.; Parsons, D.; Bowler, P. A real-life clinical evaluation of a next-generation antimicrobial dressing on acute and chronic wounds. J. Wound Care 2015, 24, 11–22. [Google Scholar] [CrossRef]
- Mori, Y.; Nakagami, G.; Kitamura, A.; Minematsu, T.; Kinoshita, M.; Suga, H.; Kurita, M.; Hayashi, C.; Kawasaki, A.; Sanada, H. Effectiveness of biofilm-based wound care system on wound healing in chronic wounds. Wound Repair Regen. 2019, 27, 540–547. [Google Scholar] [CrossRef]
- Yang, C.; Goss, S.G.; Alcantara, S.; Schultz, G.; Lantis Ii, J. Effect of negative pressure wound therapy with instillation on bioburden in chronically infected wounds. Wounds A Compend. Clin. Res. Pract. 2017, 29, 240–246. [Google Scholar]
- Serena, T.E.; Jalodi, O.; Serena, L.; Patel, K.; Mynti, M. Evaluation of the combination of a biofilm-disrupting agent and negative pressure wound therapy: A case series. J. Wound Care 2021, 30, 9–14. [Google Scholar] [CrossRef]
- Namgoong, S.; Jung, S.-Y.; Han, S.-K.; Kim, A.-R.; Dhong, E.-S. Clinical experience with surgical debridement and simultaneous meshed skin grafts in treating biofilm-associated infection: An exploratory retrospective pilot study. J. Plast. Surg. Hand Surg. 2020, 54, 47–54. [Google Scholar] [CrossRef]
- Jacob, A.; Jones, L.M.; Abdo, R.J.; Cruz-Schiavone, S.F.; Skerker, R.; Caputo, W.J.; Krehbiel, N.; Moyer-Harris, A.K.; McAtee, A.; Baker, I. Lights, fluorescence, action—Influencing wound treatment plans including debridement of bacteria and biofilms. Int. Wound J. 2023, 20, 3279–3288. [Google Scholar] [CrossRef]
- Watson, F.; Chen, R.; Percival, S.L. In vitro prevention and inactivation of biofilms using controlled-release iodine foam dressings for wound healing. Int. Wound J. 2024, 21, e14365. [Google Scholar] [CrossRef]
- Parsons, D.; Meredith, K.; Rowlands, V.J.; Short, D.; Metcalf, D.G.; Bowler, P.G. Enhanced Performance and Mode of Action of a Novel Antibiofilm Hydrofiber® Wound Dressing. BioMed Res. Int. 2016, 2016, 7616471. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Barki, K.G.; Das, A.; Dixith, S.; Ghatak, P.D.; Mathew-Steiner, S.; Schwab, E.; Khanna, S.; Wozniak, D.J.; Roy, S.; Sen, C.K. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann. Surg. 2019, 269, 756–766. [Google Scholar] [CrossRef]
- Barrigah-Benissan, K.; Ory, J.; Sotto, A.; Salipante, F.; Lavigne, J.P.; Loubet, P. Antiseptic Agents for Chronic Wounds: A Systematic Review. Antibiotics 2022, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Lenselink, E.; Andriessen, A. A cohort study on the efficacy of a polyhexanide-containing biocellulose dressing in the treatment of biofilms in wounds. J. Wound Care 2011, 20, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of wound dressings containing silver on skin and immune cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Namen Ii, W.; Moore, J.; Buchanan, M.; Hayes, V.; Myntti, M.F.; Hakaim, A. Clinical Assessment of a Biofilm-Disrupting Agent for the Management of Chronic Wounds Compared with Standard of Care: A Therapeutic Approach. Wounds 2018, 30, 120–130. [Google Scholar] [PubMed]
- Wei, M.; Jiang, Q.; Niu, N.; Dong, L.; Wang, L.; Chen, W.; Fu, Q.; Chen, Y.; Wang, J. Reduction of biofilm in chronic wounds by antibacteriaprotease combined with silver dressing. Int. J. Clin. Exp. Med. 2019, 12, 12293–12302. [Google Scholar]
- Mañas, C.R.; Rodríguez, R.A.; Sánchez, J.P.; González, C.M.P.; Gonzalez, J.G.; Mullor, M. Treating diabetic foot ulcers with antimicrobial wound dressing impregnated with dialkylcarbamoyl chloride. J. Wound Care 2025, 34, 278–284. [Google Scholar] [CrossRef]
- Mariana Peroni, M.D.A.; Minervini, S.; Pancheri, O.; Baratto, F.; Bonvini, S.; Pertile, R.; Bertasi, G.; Fantin, F. The use of p-toluenesulfonic acid in the removal of biofilm in chronic ulcers. Int. J. Sci. Res. Updates 2024, 8, 77–89. [Google Scholar] [CrossRef]
- Astrada, A.; Pamungkas, R.A.; Abidin, K.R. Advancements in Managing Wound Biofilm: A Systematic Review and Meta-analysis of Randomized Controlled Trials on Topical Modalities. Foot Ankle Spec. 2024, 19386400231225708. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, S.; Al-Kurdi, D.; Ologun, Y.; Ovington, L.G.; Martyn-St James, M.; Richardson, R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst. Rev. 2014, 2014, CD003557. [Google Scholar] [CrossRef]
- Kim, J.S.; Lim, M.C.; Kim, S.M.; Lee, J.Y. Extracellular matrix-degrading enzymes as a biofilm control strategy for food-related microorganisms. Food Sci. Biotechnol. 2023, 32, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Dawan, J.; Zhang, S.; Ahn, J. Recent Advances in Biofilm Control Technologies for the Food Industry. Antibiotics 2025, 14, 254. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Nair, A.V.; Parmar, K.; Rajmani, R.S.; Chakravortty, D.; Das, D. Combating biofilm-associated Klebsiella pneumoniae infections using a bovine microbial enzyme. NPJ Biofilms Microbiomes 2024, 10, 119. [Google Scholar] [CrossRef]
- Miller, K.G.; Tran, P.L.; Haley, C.L.; Kruzek, C.; Colmer-Hamood, J.A.; Myntti, M.; Hamood, A.N. Next science wound gel technology, a novel agent that inhibits biofilm development by gram-positive and gram-negative wound pathogens. Antimicrob. Agents Chemother. 2014, 58, 3060–3072. [Google Scholar] [CrossRef]
- Swanson, T.; Ousey, K.; Haesler, E.; Bjarnsholt, T.; Carville, K.; Idensohn, P.; Kalan, L.; Keast, D.H.; Larsen, D.; Percival, S.; et al. IWII Wound Infection in Clinical Practice consensus document: 2022 update. J. Wound Care 2022, 31, S10–S21. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef] [PubMed]
| Anti-Biofilm Mechanism | Reported Observation | Cautions | Integration | Ref. | |
|---|---|---|---|---|---|
| Debridement | Physically disrupts biofilms and affect EPS | Structured repeated debridement linked to improved closure, enables antibiotics to penetrate | Requires expertise, pain and bleeding risks | Immediate topical antiseptic and dressing, post procedure | [56,58] |
| Topical antiseptics (iodine, polyhexanide, silver) | Bactericidal (broad spectrum), prevents biofilm reformation | Improved management of infection risk | Local irritation, products variability | After debridement | [10,63] |
| Advanced dressings (e.g., AQUACEL Ag+) | Prolonged antimicrobial activity, sequestration of exudate | Higher wound closure rates | Cost | As primary dressing post debridement | |
| [57,64] | |||||
| NPWT | Macro/micro-deformation, fluid exchange disrupts biofilm | Reduction in bioburden and exudate | Device availability, Reduce biofilm thickness and mass. Needs antibiotics to eliminate viable bacteria. | Between dressing change | [59,60,61] |
| Enzyme-based(e.g., glycoside hydrolases, DNase, dispersin B). | Targeted biofilm degradation | Improved bacterial killing and susceptibility | Stability formulation | After debridement, before topical antiseptic and systemic antimicrobial. | [65,66] |
| Electroceutical/E-stim | modify bacterial gene expression | Accelerate wound closure | Device access, protocol differences | [67] | |
| Adjunct to standard care |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuhanna, Y. Microbial Biofilms as Barriers to Chronic Wound Healing: Diagnostic Challenges and Therapeutic Advances. J. Clin. Med. 2025, 14, 8121. https://doi.org/10.3390/jcm14228121
Almuhanna Y. Microbial Biofilms as Barriers to Chronic Wound Healing: Diagnostic Challenges and Therapeutic Advances. Journal of Clinical Medicine. 2025; 14(22):8121. https://doi.org/10.3390/jcm14228121
Chicago/Turabian StyleAlmuhanna, Yasir. 2025. "Microbial Biofilms as Barriers to Chronic Wound Healing: Diagnostic Challenges and Therapeutic Advances" Journal of Clinical Medicine 14, no. 22: 8121. https://doi.org/10.3390/jcm14228121
APA StyleAlmuhanna, Y. (2025). Microbial Biofilms as Barriers to Chronic Wound Healing: Diagnostic Challenges and Therapeutic Advances. Journal of Clinical Medicine, 14(22), 8121. https://doi.org/10.3390/jcm14228121






