Robot-Assisted Extravesical Ureteral Reimplantation (RALUR-EV) in Children: Initial Single-Center Experience at a Public Tertiary-Care Hospital in Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Surgical Technique
2.4. Variables and Definitions
2.5. Data Sources and Management
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Cohort Characteristics
3.2. Operative Metrics
3.3. Perioperative Outcomes
3.4. Follow-Up and Primary Outcome
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Läckgren, G.; Cooper, C.S.; Neveus, T.; Kirsch, A.J. Management of Vesicoureteral Reflux: What Have We Learned Over the Last 20 Years? Front. Pediatr. 2021, 9, 650326. [Google Scholar] [CrossRef]
- Finnell, S.M.E.; Carroll, A.E.; Downs, S.M.; the Subcommittee on Urinary Tract Infection. Diagnosis and Management of an Initial UTI in Febrile Infants and Young Children. Pediatrics 2011, 128, e749–e770. [Google Scholar] [CrossRef]
- Campbell Walsh Wein Urology-9780323546423. MEA Elsevier Health. Available online: https://www.eu.elsevierhealth.com/campbell-walsh-wein-urology-9780323546423.html (accessed on 7 October 2025).
- Gnech, M.; ’t Hoen, L.; Zachou, A.; Bogaert, G.; Castagnetti, M.; O’Kelly, F.; Quaedackers, J.; Rawashdeh, Y.F.; Silay, M.S.; Kennedy, U.; et al. Update and Summary of the European Association of Urology/European Society of Paediatric Urology Paediatric Guidelines on Vesicoureteral Reflux in Children. Eur. Urol. 2024, 85, 433–442. [Google Scholar] [CrossRef]
- Peters, C.A.; Skoog, S.J.; Arant, B.S.; Copp, H.L.; Elder, J.S.; Hudson, R.G.; Khoury, A.E.; Lorenzo, A.J.; Pohl, H.G.; Shapiro, E.; et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J. Urol. 2010, 184, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Masieri, L.; Steyaert, H.; Escolino, M.; Cerchione, R.; La Manna, A.; Cini, C.; Lendvay, T.S. Robot-assisted extravesical ureteral reimplantation (revur) for unilateral vesico-ureteral reflux in children: Results of a multicentric international survey. World J. Urol. 2018, 36, 481–488. [Google Scholar] [CrossRef]
- Chertin, L.; Kocherov, S.; Bakaleyshchik, P.; Baranov, Y.; Dubrov, V.; Kagantsov, I.; Karpachev, S.; Kuzovleva, G.; Pirogov, A.; Rudin, Y.; et al. Laparoscopic and Robot-assisted Laparoscopic Reimplantation for Lower Ureter Pathology. A Multi-institutional Comparative Study in 1343 Patients. Urology 2024, 186, 166–171. [Google Scholar] [CrossRef]
- Babajide, R.; Andolfi, C.; Kanabolo, D.; Wackerbarth, J.; Gundeti, M.S. Postoperative hydronephrosis following ureteral reimplantation: Clinical significance and importance of surgical technique and experience. J. Pediatr. Surg. 2023, 58, 574–579. [Google Scholar] [CrossRef]
- Boysen, W.R.; Ellison, J.S.; Kim, C.; Koh, C.J.; Noh, P.; Whittam, B.; Palmer, B.; Shukla, A.; Kirsch, A.; Gundeti, M.S. Multi-Institutional Review of Outcomes and Complications of Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation for Treatment of Primary Vesicoureteral Reflux in Children. J. Urol. 2017, 197, 1555–1561. [Google Scholar] [CrossRef]
- Aucatoma, F.C.; Pazmiño, M.C.B.; Ludeña, P.G. Características clínicas y resultados quirúrgicos de pacientes pediátricos intervenidos por cirugía robótica. Rev. Médica-Cient. CAMbios HECAM 2022, 21, e875. [Google Scholar]
- Rivero-Moreno, Y.; Cordova-Guilarte, J.; Echevarria, S.; Dorado-Avila, G.; Pianetti, L.; Acevedo-Rodríguez, J.; Chavez-Campos, C.; Paz-Castillo-Lopez, M.; Estrella-Gaibor, C.; Salcedo, Y.; et al. Innovation in Motion: Robotic Surgery’s status in Latin America. Ambul. Surg. 2023, 29, 47–50. [Google Scholar]
- Secin, F.P.; Coelho, R.; Monzó Gardiner, J.I.; Salcedo, J.G.C.; Puente, R.; Martínez, L.; Finkelstein, D.; Valero, R.; León, A.; Angeloni, D.; et al. Robotic surgery in public hospitals of Latin-America: A castle of sand? World J. Urol. 2018, 36, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Garibay González, F.; Navarrete Arellano, M.; Castillo Niño, J.C.; García González, F.M.; Sánchez Alejo, J.A. Robotic surgery in urology. First prospective pediatric case series in Latin America. Rev. Sanid. Mil. 2018, 72, 281–288. [Google Scholar]
- SciELO. Brasil-Robotics in Pediatric Urology Robotics in Pediatric Urology. Available online: https://www.scielo.br/j/ibju/a/ndRDtJL55DN4pfStbtQkYyw/?format=html&lang=en (accessed on 30 October 2025).
- Hospital de Especialidades Carlos Andrade Marín. Quienes Somos—Hospital Carlos Andrade Marín. Hospital de Especiali-dades Carlos Andrade Marín. 2024. Available online: https://hcam.iess.gob.ec/quienes-somos/ (accessed on 8 October 2025).
- Bustangi, N.; Kallas Chemaly, A.; Scalabre, A.; Khelif, K.; Luyckx, S.; Steyaert, H.; Varlet, F.; Lopez, M. Extravesical Ureteral Reimplantation Following Lich-Gregoir Technique for the Correction of Vesico-Ureteral Reflux Retrospective Comparative Study Open vs. Laparoscopy. Front. Pediatr. 2018, 6, 388. [Google Scholar] [CrossRef]
- Mei, H.; Tang, S. Robotic-assisted surgery in the pediatric surgeons’ world: Current situation and future prospectives. Front. Pediatr. 2023, 11, 1120831. [Google Scholar] [CrossRef]
- Lebowitz, R.L.; Olbing, H.; Parkkulainen, K.V.; Smellie, J.M.; Tamminen-Möbius, T.E. International system of radiographic grading of vesicoureteric reflux. Pediatr. Radiol. 1985, 15, 105–109. [Google Scholar] [CrossRef] [PubMed]
- ’t Hoen, L.A.; Bogaert, G.; Radmayr, C.; Dogan, H.S.; Nijman, R.J.M.; Quaedackers, J.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; Bhatt, N.R.; et al. Update of the EAU/ESPU guidelines on urinary tract infections in children. J. Pediatr. Urol. 2021, 17, 200–207. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Essamoud, S.; Ghidini, F.; Andolfi, C.; Gundeti, M.S. Robot-assisted laparoscopic extravesical ureteral reimplantation (RALUR-EV): A narrative review. Transl. Pediatr. 2024, 13, 1634–1640. [Google Scholar] [CrossRef]
- Hou, S.W.; Xing, M.H.; Gundeti, M.S. Pediatric robotic urologic procedures: Indications and outcomes. Indian J. Urol. 2023, 39, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Marco, B.B.; Haid, B.; Baydilli, N.; Donmez, M.I.; Spinoit, A.-F.; Paraboschi, I.; Masieri, L.; Steinkellner, L.; Comez, Y.I.; et al. A multi-institutional European comparative study of open versus robotic-assisted laparoscopic ureteral reimplantation in children with high grade (IV–V) vesicoureteral reflux. J. Pediatr. Urol. 2024, 20, 283–291. [Google Scholar] [CrossRef]
- Neheman, A.; Strine, A.C.; Concodora, C.W.; Schulte, M.E.; Noh, P.H. Outpatient Robotic Unilateral Extravesical Ureteral Reimplantation in the Pediatric Population: Short-Term Assessment of Safety. J. Urol. 2019, 201, 615–619. [Google Scholar] [CrossRef]
- Kim, J.K.; Batra, N.; Shavnore, R.; Szymanski, K.M.; Misseri, R.; Kaefer, M.; Cain, M.P.; Roth, J.; Dangle, P.; Meldrum, K.; et al. Attaining competency and proficiency in pediatric robot-assisted laparoscopic ureteric reimplantation: A learning curve configuration using cumulative sum analysis. World J. Urol. 2025, 43, 372. [Google Scholar] [CrossRef]
- Chen, C.J.; Peters, C.A. Robotic Assisted Surgery in Pediatric Urology: Current Status and Future Directions. Front. Pediatr. 2019, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Besner, A.-S.; Ferreira, J.L.; Ow, N.; Gaffar, R.; Guadagno, E.; Emil, S.; Poenaru, D. Patient-reported outcome measures in pediatric surgery—A systematic review. J. Pediatr. Surg. 2022, 57, 798–812. [Google Scholar] [CrossRef]
- Dixon, S.; Hill, H.; Flight, L.; Khetrapal, P.; Ambler, G.; Williams, N.R.; Brew-Graves, C.; Kelly, J.D.; Catto, J.W.F.; iROC Study Team. Cost-Effectiveness of Robot-Assisted Radical Cystectomy vs Open Radical Cystectomy for Patients with Bladder Cancer. JAMA Netw. Open 2023, 6, e2317255. [Google Scholar] [CrossRef]
- Hong, Y.E.; Shim, H.; Shin, M. Costs and cost-effectiveness of robotic-assisted surgery in South Korea: A systematic review and meta-analysis. Front. Public Health 2025, 13, 1683482. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Dou, B. Cost-effectiveness analysis of robotic surgery in healthcare for older individuals: A systematic review based on randomized controlled trials. Front. Public Health 2025, 13, 1614654. [Google Scholar] [CrossRef]
- Lai, T.-J.; Heggie, R.; Kamaruzaman, H.-F.; Bouttell, J.; Boyd, K. Economic Evaluations of Robotic-Assisted Surgery: Methods, Challenges and Opportunities. Appl. Health Econ. Health Policy 2025, 23, 35–49. [Google Scholar] [CrossRef]
- Lai, T.-J.; Roxburgh, C.; Boyd, K.A.; Bouttell, J. Clinical effectiveness of robotic versus laparoscopic and open surgery: An overview of systematic reviews. BMJ Open 2024, 14, e076750. [Google Scholar] [CrossRef] [PubMed]
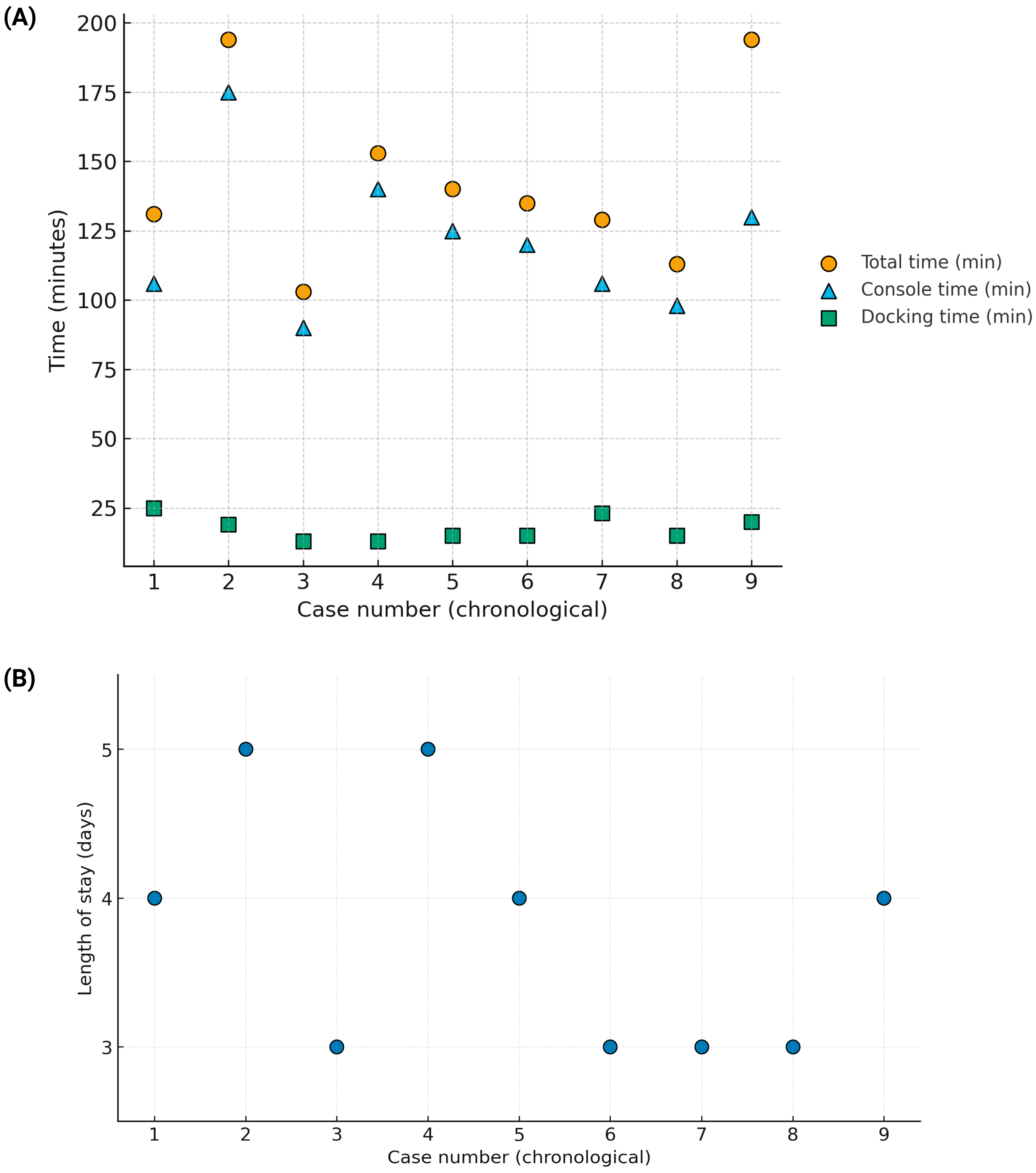
| Surgery Date | Diagnosis | Procedure | Total (min) | Docking (min) | Console (min) | Age | Sex | VUR Grade | Success | Postop UTI | Complications | Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 December 2021 | Left VUR | Left reimplant | 131 | 25 | 106 | 2 years, 9 months | M | III | Yes | No | No | 4 |
| 18 July 2022 | Right UVJ obstruction | Right reimplant | 194 | 19 | 175 | 13 years, 6 months | M | — | Yes | Yes | Clavien–Dindo II | 5 |
| 7 November 2022 | Left VUR | Left reimplant | 103 | 13 | 90 | 4 years, 5 months | M | III | Yes | No | No | 3 |
| 28 November 2022 | Left VUR | Left reimplant | 153 | 13 | 140 | 1 year, 4 months | F | III | Yes | Yes | Clavien–Dindo II | 5 |
| 6 March 2023 | Left VUR | Left reimplant | 140 | 15 | 125 | 9 years, 3 months | F | III | Yes | No | No | 4 |
| 16 December 2024 | Bilateral VUR | Left reimplant | 135 | 15 | 120 | 11 years, 2 months | F | R: I/L: III | Yes | No | No | 3 |
| 13 January 2025 | Bilateral VUR | Left reimplant | 129 | 23 | 106 | 2 years, 2 months | F | R: II/L: III | — | No | No | 3 |
| 25 April 2025 | Left VUR | Left reimplant | 113 | 15 | 98 | 6 years, 7 months | F | III | — | No | No | 3 |
| 19 May 2025 | Bilateral VUR | Right reimplant | 150 | 20 | 130 | 3 years, 0 months | M | R: IV/L: I | — | No | No | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Salazar, G.; Cruz-Álvarez, J.; Guamán-Ludeña, P.; Gaibor-Pazmiño, A.; Ortiz-Prado, E.; Izquierdo-Condoy, J.S. Robot-Assisted Extravesical Ureteral Reimplantation (RALUR-EV) in Children: Initial Single-Center Experience at a Public Tertiary-Care Hospital in Ecuador. J. Clin. Med. 2025, 14, 8120. https://doi.org/10.3390/jcm14228120
Sánchez-Salazar G, Cruz-Álvarez J, Guamán-Ludeña P, Gaibor-Pazmiño A, Ortiz-Prado E, Izquierdo-Condoy JS. Robot-Assisted Extravesical Ureteral Reimplantation (RALUR-EV) in Children: Initial Single-Center Experience at a Public Tertiary-Care Hospital in Ecuador. Journal of Clinical Medicine. 2025; 14(22):8120. https://doi.org/10.3390/jcm14228120
Chicago/Turabian StyleSánchez-Salazar, Giancarlo, Juan Cruz-Álvarez, Pablo Guamán-Ludeña, Alice Gaibor-Pazmiño, Esteban Ortiz-Prado, and Juan S. Izquierdo-Condoy. 2025. "Robot-Assisted Extravesical Ureteral Reimplantation (RALUR-EV) in Children: Initial Single-Center Experience at a Public Tertiary-Care Hospital in Ecuador" Journal of Clinical Medicine 14, no. 22: 8120. https://doi.org/10.3390/jcm14228120
APA StyleSánchez-Salazar, G., Cruz-Álvarez, J., Guamán-Ludeña, P., Gaibor-Pazmiño, A., Ortiz-Prado, E., & Izquierdo-Condoy, J. S. (2025). Robot-Assisted Extravesical Ureteral Reimplantation (RALUR-EV) in Children: Initial Single-Center Experience at a Public Tertiary-Care Hospital in Ecuador. Journal of Clinical Medicine, 14(22), 8120. https://doi.org/10.3390/jcm14228120







