Effectiveness of Controlled Ovarian Stimulation for Oocyte Preservation in Oncologic Patients: Insights from DuoStim Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Evaluation and Screening
2.3. Stimulation Phase Stratification
2.4. Controlled Ovarian Stimulation (COS) Protocols
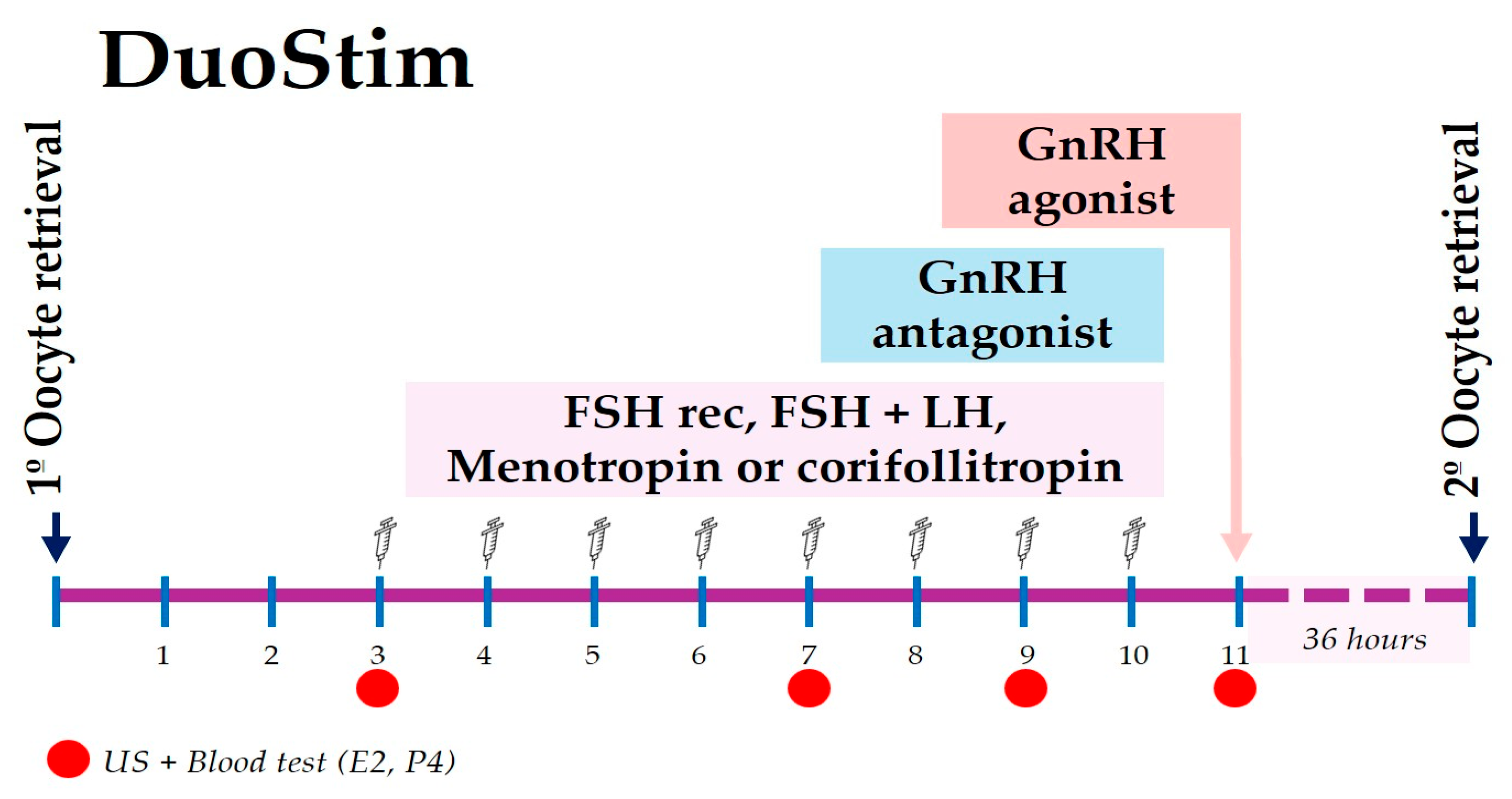
2.5. DuoStim Protocol
2.6. Outcome Variables
2.7. Ethical Approval
2.8. Statistical Analysis
2.8.1. Descriptive and Comparative Analyses
2.8.2. Multivariable Regression Analysis
3. Results
3.1. Baseline Characteristics and Oncological Distribution
3.2. Comparison of Stimulation Protocols Cohorts Across Oncological Patients
3.3. Outcomes in the DuoStim Subgroup
3.4. Outcomes in Breast Cancer Patients
3.5. Multivariable Regression Results
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COS | Controlled Ovarian Stimulation |
| DuoStim | Dual Stimulation |
| BC | Breast Cancer |
| EFP | Early Follicular Phase |
| LFP | Late Follicular Phase |
| LP | Luteal Phase |
| POF | Premature Ovarian Failure |
| AFC | Antral Follicle Count |
| AMH | Anti-Müllerian Hormone |
| MII | Metaphase II Oocytes |
| FORT | Follicular Output Rate |
| FOI | Follicle Oocyte Index |
| OSI | Ovarian Sensitivity Index |
References
- Bedaiwy, M.A.; Falcone, T. Fertility Preservation in Cancer Patients. Women’s Health 2006, 2, 479–489. [Google Scholar] [CrossRef]
- Bewtra, C.; Acharya, N. Preservation of Fertility in Cancer Patients: A Narrative Review. Cureus 2023, 15, e47910. [Google Scholar] [CrossRef]
- Baerwald, A.; Pierson, R. Ovarian Follicular Waves during the Menstrual Cycle: Physiologic Insights into Novel Approaches for Ovarian Stimulation. Fertil. Steril. 2020, 114, 443–457. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Cimadomo, D.; Petriglia, C.; Conforti, A.; Alviggi, C.; Ubaldi, N.; Ledda, S.; Ferrero, S.; Rienzi, L.; Ubaldi, F.M. DuoStim–A Reproducible Strategy to Obtain More Oocytes and Competent Embryos in a Short Time-Frame Aimed at Fertility Preservation and IVF Purposes. A Systematic Review. Upsala J. Med. Sci. 2020, 125, 121–130. [Google Scholar] [CrossRef]
- Marco, A.; Gargallo, M.; Ciriza, J.; Shikanov, A.; Baquedano, L.; García Pérez-Llantada, J.; Malo, C. Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 4360. [Google Scholar] [CrossRef]
- McClam, M.; Xiao, S. Preserving Oocytes in Oncofertility. Biol. Reprod. 2022, 106, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; De Sousa Lopes, S.M.C.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE Guideline: Female Fertility Preservation. Hum. Reprod. Open 2021, 2020, hoaa052. [Google Scholar] [CrossRef]
- Ozcan, M.C.H.; Snegovskikh, V.; Adamson, G.D. Oocyte and Embryo Cryopreservation before Gonadotoxic Treatments: Principles of Safe Ovarian Stimulation, a Systematic Review. Women’s Health 2022, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sönmezer, M.; Şükür, Y.E.; Ateş, C.; Saçıntı, K.G.; Sönmezer, M.; Aslan, B.; Atabekoğlu, C.S.; Özmen, B.; Oktay, K.H. Random Start Ovarian Stimulation before Gonadotoxic Therapies in Women with Cancer: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2023, 47, 103337. [Google Scholar] [CrossRef]
- Galati, G.; Somigliana, E.; Ciaffaglione, M.; Reschini, M.; Serra, N.; Sanzani, E.; Viganò, P.; Polledri, E.; Fustinoni, S.; Muzii, L.; et al. Follicular Steroidogenesis in Random Start Protocols for Oocyte Cryopreservation. J. Assist. Reprod. Genet. 2023, 40, 2149–2156. [Google Scholar] [CrossRef]
- La Marca, A.; Donno, V.; Longo, M.; Greco, P.; Cucinelli, F.; Varricchio, M.T.; Listorti, I.; Greco, E. Predicting the Total Number of Retrieved Oocytes Following Double Ovarian Stimulation (DuoStim). Hum. Reprod. 2023, 38, 1784–1788. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Cimadomo, D.; Argento, C.; Ubaldi, N.; Trabucco, E.; Drakopoulos, P.; Venturella, R.; Conforti, A.; Ggi, C.A.; Rienzi, L.; et al. Double Stimulation in the Same Ovarian Cycle (DuoStim) Is an Intriguing Strategy to Improve Oocyte Yield and the Number of Competent Embryos in a Short Timeframe. Minerva Ginecol. 2019, 71, 372–376. [Google Scholar] [CrossRef]
- Massin, N. New Stimulation Regimens: Endogenous and Exogenous Progesterone Use to Block the LH Surge during Ovarian Stimulation for IVF. Hum. Reprod. Update. 2017, 23, 211–220. [Google Scholar] [CrossRef]
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; Alshahrani, S.; Bashir, S.; Bukhari, N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep. Oncol. 2019, 12, 728. [Google Scholar] [CrossRef]
- Bonardi, B.; Massarotti, C.; Bruzzone, M.; Goldrat, O.; Mangili, G.; Anserini, P.; Spinaci, S.; Arecco, L.; Del Mastro, L.; Ceppi, M.; et al. Efficacy and Safety of Controlled Ovarian Stimulation With or Without Letrozole Co-Administration for Fertility Preservation: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 574669. [Google Scholar] [CrossRef] [PubMed]
- Vaiarelli, A.; Venturella, R.; Vizziello, D.; Bulletti, F.; Ubaldi, F.M. Dual Ovarian Stimulation and Random Start in Assisted Reproductive Technologies: From Ovarian Biology to Clinical Application. Curr. Opin. Obstet. Gynecol. 2017, 29, 153–159. [Google Scholar] [CrossRef]
- Fanton, M.; Cho, J.H.; Baker, V.L.; Loewke, K. A Higher Number of Oocytes Retrieved Is Associated with an Increase in Fertilized Oocytes, Blastocysts, and Cumulative Live Birth Rates. Fertil. Steril. 2023, 119, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.J.; Zhang, N.; Kolibianakis, E.M.; Costello, M.F.; Keller, E.; Chambers, G.M.; Venetis, C.A. Is There an Optimal Number of Oocytes Retrieved at Which Live Birth Rates or Cumulative Live Birth Rates per Aspiration Are Maximized after ART? A Systematic Review. Reprod. Biomed. Online 2021, 42, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Labrosse, J. Understanding Follicular Output Rate (FORT) and Its Implications for POSEIDON Criteria. Front. Endocrinol. 2019, 10, 246. [Google Scholar] [CrossRef]
- Alviggi, C.; Conforti, A.; Esteves, S.C.; Vallone, R.; Venturella, R.; Staiano, S.; Castaldo, E.; Andersen, C.Y.; De Placido, G. Understanding Ovarian Hypo-Response to Exogenous Gonadotropin in Ovarian Stimulation and Its New Proposed Marker-the Follicle-to-Oocyte (FOI) Index. Front. Endocrinol. 2018, 9, 589. [Google Scholar] [CrossRef]
- Carosso, A.R.; van Eekelen, R.; Revelli, A.; Canosa, S.; Mercaldo, N.; Benedetto, C.; Gennarelli, G. Women in Advanced Reproductive Age: Are the Follicular Output Rate, the Follicle-Oocyte Index and the Ovarian Sensitivity Index Predictors of Live Birth in an IVF Cycle? J. Clin. Med. 2022, 11, 859. [Google Scholar] [CrossRef]
- De Rosis, S.; Pennucci, F.; Lungu, D.A.; Manca, M.; Nuti, S. A Continuous PREMs and PROMs Observatory for Elective Hip and Knee Arthroplasty: Study Protocol. BMJ Open 2021, 11, e049826. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Bonciani, M.; Russo, E.; Mannella, P.; Simoncini, T.; Vainieri, M. Patient-reported Outcome Measures for Pregnancy-related Urinary and Fecal Incontinence: A Prospective Cohort Study in a Large Italian Population. Int. J. Gynecol. Obstet. 2022, 159, 435–443. [Google Scholar] [CrossRef]
- Mondschein, C.F.; Monda, C. The EU’s General Data Protection Regulation (GDPR) in a Research Context. In Chapter 5: Fundamentals of Clinical Data Science; Kubben, P., Dumontier, M., Dekker, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 55–71. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA 2025, 333, 71–74. [Google Scholar] [CrossRef]
- La Marca, A.; Grisendi, V.; Dondi, G.; Sighinolfi, G.; Cianci, A. The Menstrual Cycle Regularization Following D-Chiro-Inositol Treatment in PCOS Women: A Retrospective Study. Gynecol. Endocrinol. 2015, 31, 52–56. [Google Scholar] [CrossRef]
- De Mello Bianchi, P.H.; Serafini, P.; Monteiro Da Rocha, A.; Assad Hassun, P.; Alves Da Motta, E.L.; Sampaio Baruselli, P.; Chada Baracat, E. Review: Follicular Waves in the Human Ovary: A New Physiological Paradigm for Novel Ovarian Stimulation Protocols. Reprod. Sci. 2010, 17, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Moffat, R.; Pirtea, P.; Gayet, V.; Wolf, J.P.; Chapron, C.; De Ziegler, D. Dual Ovarian Stimulation Is a New Viable Option for Enhancing the Oocyte Yield When the Time for Assisted Reproductive Technnology Is Limited. Reprod. Biomed. Online 2014, 29, 659–661. [Google Scholar] [CrossRef]
- Petrone, P.; Vaiarelli, A.; Blockeel, C. Double Stimulation for the Management of Poor-Prognosis Patients: Where Are We Going? Curr. Opin. Obstet. Gynecol. 2023, 35, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, L.C.; Wright, J.D.; Brady, P.C. Triple Stimulation (TriStim) before Bilateral Oophorectomy in a Young Woman with Ovarian Cancer: A Case Report and Review of the Literature. Fertil. Res. Pract. 2020, 6, 17. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Cimadomo, D.; Cerrillo, M.; Ubaldi, F.M.; García Velasco, J.A. Surfing Follicular Waves in Ovarian Stimulation: Is There a Role for LH in DuoStim Protocols? A Narrative Review and SWOT Analysis. Reprod. Biol. Endocrinol. 2025, 23, 28. [Google Scholar] [CrossRef]
- Muteshi, C.; Child, T.; Ohuma, E.; Fatum, M. Ovarian Response and Follow-up Outcomes in Women Diagnosed with Cancer Having Fertility Preservation: Comparison of Random Start and Early Follicular Phase Stimulation—Cohort Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 10–14. [Google Scholar] [CrossRef]
- Tsampras, N.; Gould, D.; Fitzgerald, C.T. Double Ovarian Stimulation (DuoStim) Protocol for Fertility Preservation in Female Oncology Patients. Hum. Fertil. 2017, 20, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Santulli, P.; Maignien, C.; Pocate-Cheriet, K.; Marcellin, L.; Chen, Y.; Chapron, C. The Ovarian Response After Follicular Versus Luteal Phase Stimulation with a Double Stimulation Strategy. Reprod. Sci. 2020, 27, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Gica, C.; Maxim, B.-G.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M.; Iordachescu, D.; Gica, N. Double Ovarian Stimulation in the Same Ovarian Cycle. Maedica 2021, 16, 97–101. [Google Scholar] [CrossRef]
- Baig, A.S.; Camuñas, N.G.; Sánchez, P.P.; Nadal, J.S.; Fabuel, S.M.; Rubio Rubio, J.M. Controlled Ovarian Stimulation Initiated at Different Phases of the Menstrual Cycle for Fertility Preservation in Oncological Patients: A Retrospective Study. Reprod. Sci. 2023, 30, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Son, W.Y. In Vitro Maturation (IVM) of Human Immature Oocytes: Is It Still Relevant? Reprod. Biol. Endocrinol. 2023, 21, 110. [Google Scholar] [CrossRef]
- Engmann, L.; Benadiva, C.; Humaidan, P. GnRH Agonist Trigger for the Induction of Oocyte Maturation in GnRH Antagonist IVF Cycles: A SWOT Analysis. Reprod. Biomed. Online 2016, 32, 274–285. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Ruffa, A.; Cerrillo, M.; García-Velasco, J.A. GnRH Agonist Trigger in Poor Prognosis Patients Undergoing a Multicycle Approach through DuoStim or Consecutive Stimulations: A SWOT Analysis. Curr. Opin. Obstet. Gynecol. 2024, 36, 124–133. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Cimadomo, D.; Ruffa, A.; Rania, E.; Pittana, E.; Gallo, C.; Fiorenza, A.; Alviggi, E.; Alfano, S.; Carmelo, R.; et al. Oocyte Competence Is Comparable between Progestin Primed Ovarian Stimulation with Norethisterone Acetate (NETA-PPOS) and GnRH-Antagonist Protocols: A Matched Case-Control Study in PGT-A Cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 294, 4–10. [Google Scholar] [CrossRef]

| Diagnosis | Total Number of Cycles (%) | N patients (%) |
|---|---|---|
| Breast Cancer | 118 (48.4) | 96 (47.3) |
| Lymphoma | 70 (28.7) | 65 (32.0) |
| Gynecological cancer | 21 (8.6) | 14 (6.9) |
| Colon Cancer | 7 (2.9) | 7 (3.4) |
| Other Cancers | 15 (6.1) | 13 (6.4) |
| Chemotherapy for other Pathologies | 13 (5.3) | 8 (3.9) |
| Overall Cohort | EFP (a) | LPF (b) | LP (c) | DuoStim (d) | p-Value (FDR) | |
|---|---|---|---|---|---|---|
| Total Number of Cycles | 244 | 102 | 62 | 43 | 37 | |
| Age (years) | 31.0 (27.0–35.0) | 31.0 (26.0–35.0) | 32.0 (24.0–35.3) | 30.0 (26.0–34.0) | 32.0 (29.0–36.0) | ns |
| FSH (mUI/mL) | 6.80 (4.3–8.8) | 6.7 (4.2–8.7) | 6.3 (3.7–8.1) | 7.5 (5.9–11.7) | 7.6 (4.2–8.8) | ns |
| AMH (pg/mL) | 2.2 (1.0–3.6) | 2.0 (0.9–3.6) | 2.7 (1.8–3.8) | 2.3 (1.0–4.3) | 1.7 (0.9–3.1) | ns |
| AFC | 10 (7.0–15.0) | 10 (7.0–17.0) | 10.5 (8.0–15.0) | 11 (9.0–18.0) | 8 (5.0–10.0) | d vs. a **, b **, c ** |
| Gonadotropin (days) | 9 (8–10) | 8.0 (8.0–9.0) | 9.0 (9.0–9.0) | 10.0 (10.0–13.0) | 8.0 (8.0–9.0) | c vs. a ***, b ***, d *** a vs. b * |
| Total Gonadotropin (IU) | 2700 (2400–3000) | 2400 (2250–2700) | 2700 (2594–2700) | 3000 (2700–3300) | 2700 (2400–3000) | a vs. b **, d * c vs. a ***, b **, d ** |
| E2 trigger (pg/mL) | 572 (229–1176) | 619 (295–1188) | 693 (298–1295) | 577 (216–1099) | 232 (115–652) | d vs. a **, b **, c * |
| P4 trigger (pg/mL) | 1.34 (0.88–2.12) | 1.26 (0.58–1.97) | 1.53 (0.90–2.49) | 1.35 (0.98–2.39) | 1.40 (1.03–2.08) | ns |
| FOI | 0.60 (0.38–0.87) | 0.58 (0.39–0.90) | 0.63 (0.38–0.82) | 0.63 (0.45–0.89) | 0.43 (0.17–0.78) | ns |
| FORT | 0.40 (0.27–0.59) | 0.40 (0.29–0.60) | 0.40 (0.25–0.51) | 0.35 (0.21–0.56) | 0.42 (0.32–0.80) | ns |
| OSI | 2.45 (1.30–3.88) | 2.50 (1.40–4.70) | 2.50 (1.50–4.40) | 2.70 (1.50–3.90) | 1.40 (0.50–2.35) | d vs. a ***, b ***, c *** |
| Follicles ≥ 16 mm | 4.0 (2.0–6.0) | 4.0 (2.0–7.0) | 4.0 (2.0–5.25) | 4.0 (2.0–6.0) | 4.0 (2.0–5.0) | ns |
| Oocytes Retrieved | 6.0 (3.0–10.0) | 6.5 (3.8–10.0) | 7.0 (4.0–11.2) | 8.0 (4.0–12.0) | 3.0 (1.5–6.0) | d vs. a ***, b ***, c *** |
| MII Oocytes | 5.0 (3.0–8.0) | 5.0 (3.0–8.0) | 6.0 (3.0–9.0) | 6.0 (3.0–9.0) | 3.0 (2.0–5.0) | d vs. a *, b **, c ** |
| Maturity Rate % | 83.4 (60.3–100.0) | 87.5 (60.0–100.0) | 80.0 (58.3–100.0) | 75.0 (60.8–93.8) | 92.9 (65.6–100.0) | ns |
| Compliance | 10 (4.12%) | 4 (3.9%) | 4 (6.4%) | 1 (2.3%) | 1 (2.7%) | ns |
| EFP (a) | LPF (b) | LP (c) | DuoStim (d) | p-Value (FDR) | |
|---|---|---|---|---|---|
| Total Number of Cycles | 45 | 28 | 22 | 23 | |
| Age (years) | 34.5 (31.0–36.0) | 35.0 (32.0–37.8) | 34.0 (31.5–35.3) | 35 (31.0–37.0) | ns |
| FSH (mUI/mL) | 7.5 (4.1–9.65) | 7.4 (4.9–10.5) | 7.1 (4.13–10.6) | 8.2 (4.2–9.5) | ns |
| AMH (pg/mL) | 2.1 (0.9–4.5) | 2.4 (1.6–35) | 2.4 (0.9–4.2) | 1.9 (1.0–4.0) | ns |
| AFC | 10 (7.0–15.0) | 12.0 (8.0–15.0) | 11 (9.0–17.5) | 8 (5.0–10.0) | d vs. a *, b *, c ** |
| Gonadotropin (days) | 8.0 (8.0–9.0) | 9.0 (9.0–9.0) | 10.0 (9.0–11.0) | 8.0 (8.0–9.0) | c vs. a ***, b ***, c *** |
| Total Gonadotropin (IU) | 2400 (2400–2700) | 2700 (2700–2700) | 3000 (2981–3350) | 2700 (2400–3000) | c vs. a ***, b **, d *** a vs. b * |
| E2 trigger (pg/mL) | 326 (209–615) | 318 (195–530) | 272 (156–690) | 145 (77–367) | d vs. a * |
| P4 trigger (pg/mL) | 1.38 (0.92–2.22) | 1.79 (1.33–3.35) | 1.61 (1.06–2.38) | 1.5 (1.07–2.33) | ns |
| FOI | 0.62 (0.40–0.90) | 0.53 (0.32–0.80) | 0.63 (0.50–0.89) | 0.60 (0.40–1.00) | ns |
| FORT | 0.33 (0.20–0.50) | 0.36 (0.22–0.50) | 0.35 (0.26–0.46) | 0.50 (0.33–0.90) | ns |
| OSI | 2.80 (1.40–4.05) | 1.95 (1.30–3.05) | 2.70 (1.45–3.73) | 1.50 (0.80–2.50) | ns |
| Follicles ≥ 16 mm | 3.0 (2.0–5.0) | 4.5 (2.0–5.75) | 4.0 (2.0–6.0) | 3.0 (2.0–5.0) | ns |
| Oocytes Retrieved | 6.0 (3.0–10.0) | 5.0 (4.0–8.5) | 8.0 (4.0–11.2) | 4.0 (2.0–6.0) | d vs. c * |
| MII Oocytes | 5.0 (2.0–8.0) | 4.0 (2.0–7.2) | 6.0 (3.0–8.2) | 3.0 (1.0–6.0) | ns |
| Maturity Rate % | 81.5 (53.0–100.0) | 85.7 (55.3–100.0) | 73.9 (59.6–92.5) | 83.3 (50.0–100.0) | ns |
| Compliance | 2 (4.4%) | 2 (7.14%) | 0 (0.0%) | 1 (4.4%) | ns |
| Predictor MII Retrieved | Estimate β | 95% CI | p-Value |
|---|---|---|---|
| Year of cryopreservation | −0.023 | −0.061–0.015 | 0.351 |
| Age (years) | −0.003 | −0.019–0.013 | 0.673 |
| FSH (mIU/mL) | −0.014 | −0.036–0.007 | 0.233 |
| AMH (ng/mL) | 0.085 | 0.046–0.124 | <0.001 |
| AFC | 0.060 | 0.041–0.078 | <0.001 |
| E2 at trigger (pg/mL) | −2.6 × 10−5 | −9.3 × 10−9–3.8 × 10−5 | 0.424 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panattoni, A.; Montt Guevara, M.M.; Marzi, I.; Saçıntı, K.G.; Papini, F.; Maggiorano, C.; Macaluso, S.; Casarosa, E.; Simoncini, T.; Artini, P.G.; et al. Effectiveness of Controlled Ovarian Stimulation for Oocyte Preservation in Oncologic Patients: Insights from DuoStim Protocol. J. Clin. Med. 2025, 14, 8062. https://doi.org/10.3390/jcm14228062
Panattoni A, Montt Guevara MM, Marzi I, Saçıntı KG, Papini F, Maggiorano C, Macaluso S, Casarosa E, Simoncini T, Artini PG, et al. Effectiveness of Controlled Ovarian Stimulation for Oocyte Preservation in Oncologic Patients: Insights from DuoStim Protocol. Journal of Clinical Medicine. 2025; 14(22):8062. https://doi.org/10.3390/jcm14228062
Chicago/Turabian StylePanattoni, Andrea, Maria Magdalena Montt Guevara, Ilaria Marzi, Koray Görkem Saçıntı, Francesca Papini, Chiara Maggiorano, Sara Macaluso, Elena Casarosa, Tommaso Simoncini, Paolo Giovanni Artini, and et al. 2025. "Effectiveness of Controlled Ovarian Stimulation for Oocyte Preservation in Oncologic Patients: Insights from DuoStim Protocol" Journal of Clinical Medicine 14, no. 22: 8062. https://doi.org/10.3390/jcm14228062
APA StylePanattoni, A., Montt Guevara, M. M., Marzi, I., Saçıntı, K. G., Papini, F., Maggiorano, C., Macaluso, S., Casarosa, E., Simoncini, T., Artini, P. G., & Cela, V. (2025). Effectiveness of Controlled Ovarian Stimulation for Oocyte Preservation in Oncologic Patients: Insights from DuoStim Protocol. Journal of Clinical Medicine, 14(22), 8062. https://doi.org/10.3390/jcm14228062







