No-Reflow During Coronary Interventions: A Narrative Review
Abstract
1. Clinical Implications
- No-reflow phenomenon represents a complex, multifactorial challenge that profoundly compromises myocardial reperfusion and independently predicts adverse clinical outcomes post-coronary intervention in ACS patients.
- Although biomarker identification and imaging modalities have improved early diagnosis and risk stratification, effective, standardized preventive therapies are still elusive.
- Current pharmacological and mechanical management strategies yield inconsistent results, emphasizing the critical need for personalized, mechanism-driven treatment paradigms.
- Emerging technologies, including artificial intelligence-driven risk prediction and novel microvascular-targeted therapies, offer promising avenues for precision medicine to mitigate no-reflow and improve patient prognosis.
2. Introduction
3. Methodology
3.1. Prevalence and Epidemiology
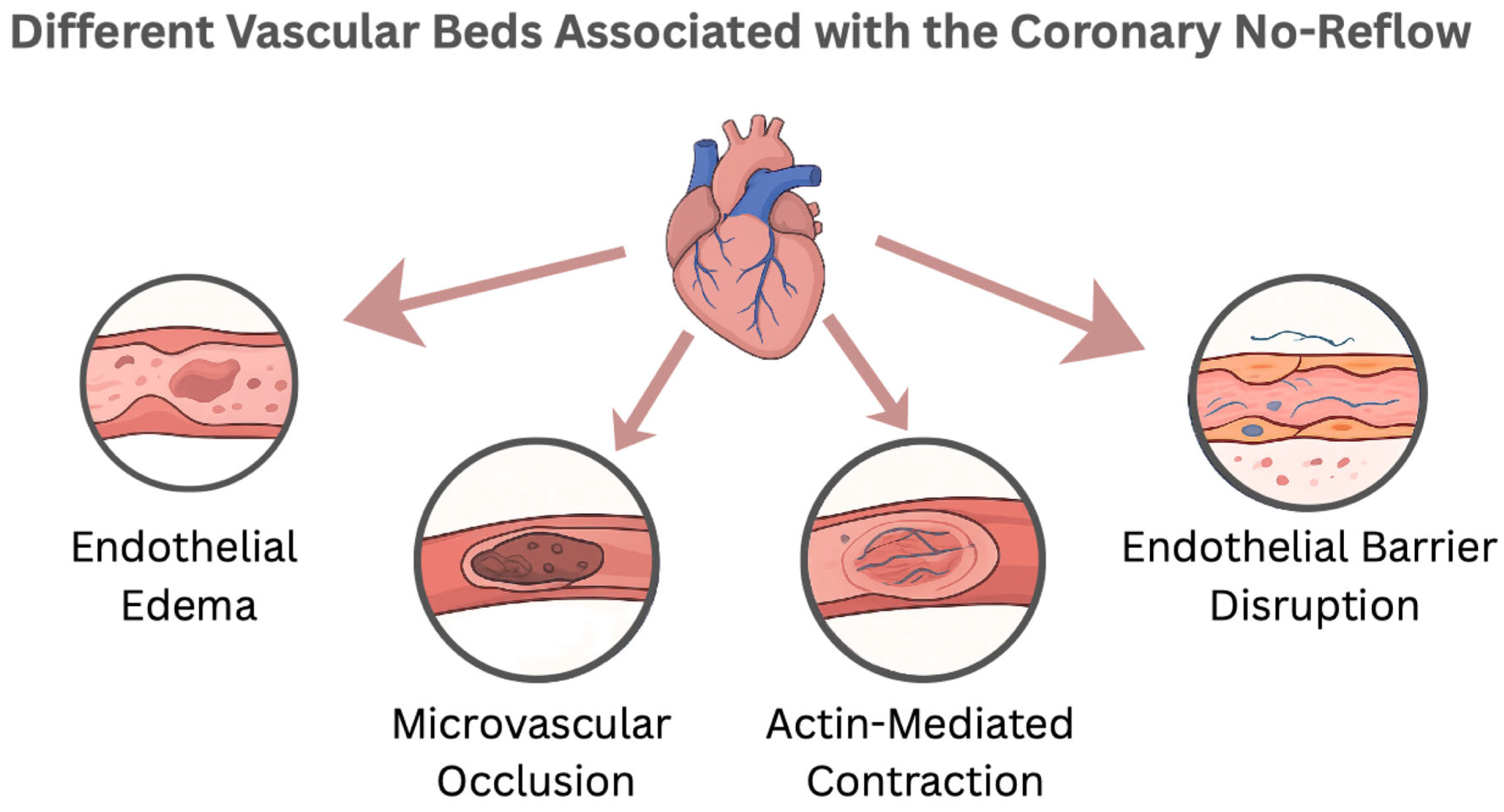
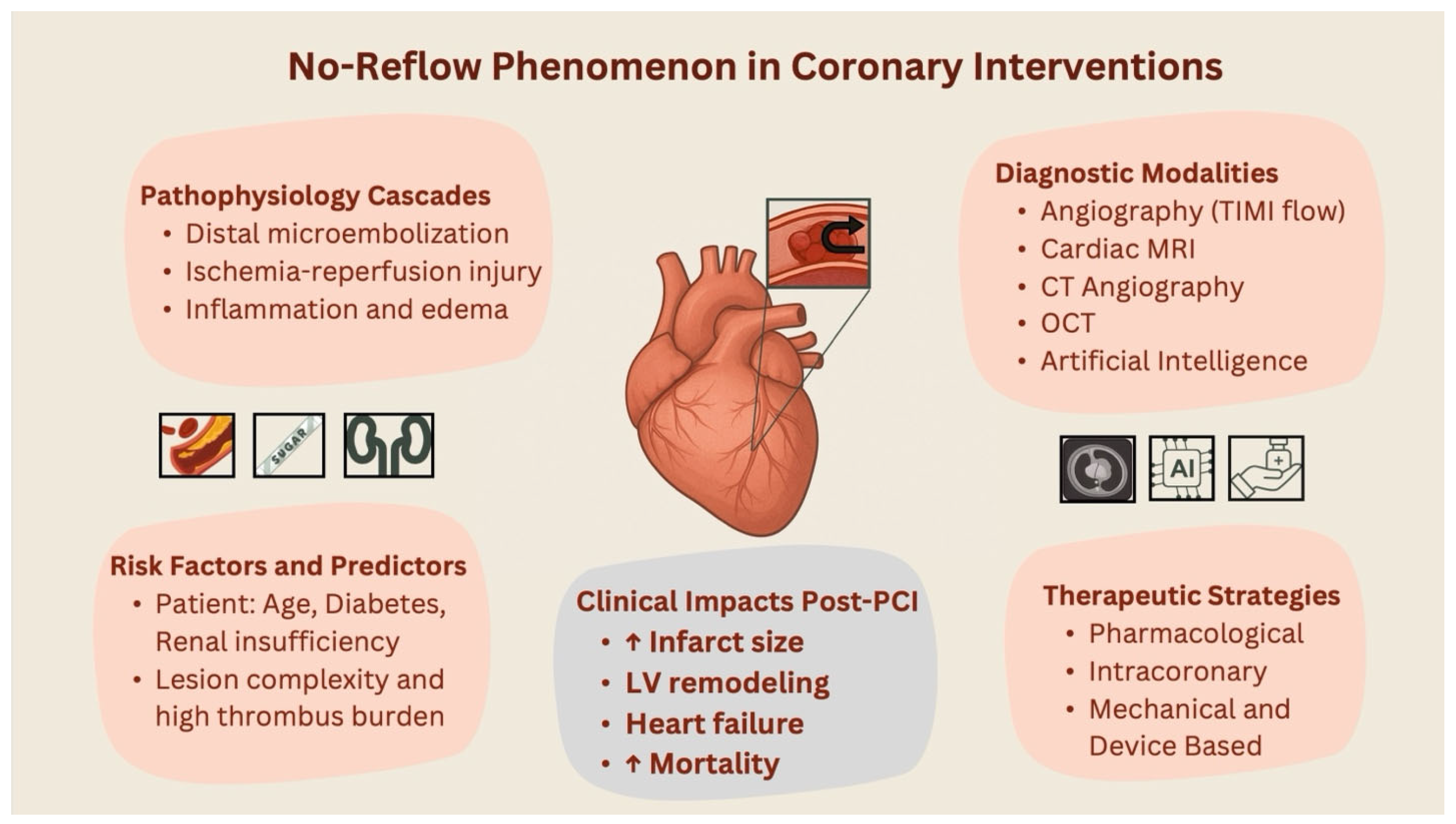
3.2. Pathophysiology
3.3. Risk Factors
Procedural-Related Risk Factors
3.4. Patient-Related Risk Factors
3.5. Imaging Predictors and Advances
4. Management
4.1. Pharmacologic Therapies
Antiplatelet Therapy
4.2. Anticoagulation and the No-Reflow Phenomenon
4.3. Inflammation Modulation
4.4. Mechanical and Device-Based Interventions
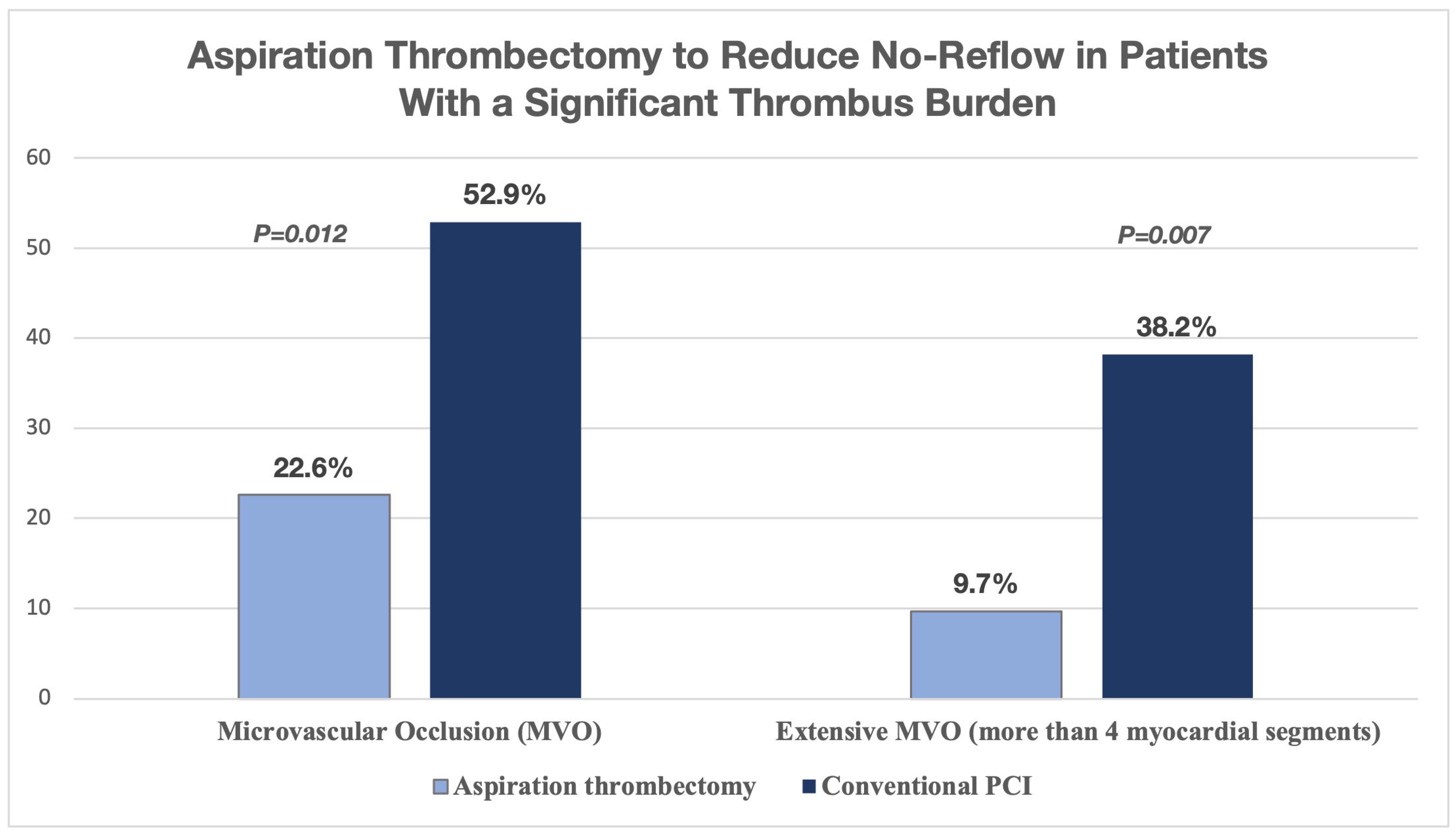
Aspiration Thrombectomy
4.5. Distal Protection Devices (DPD)
Emerging Device Technologies for Thrombus Management
4.6. Stent and Balloon Technologies Impacting No-Reflow Incidence
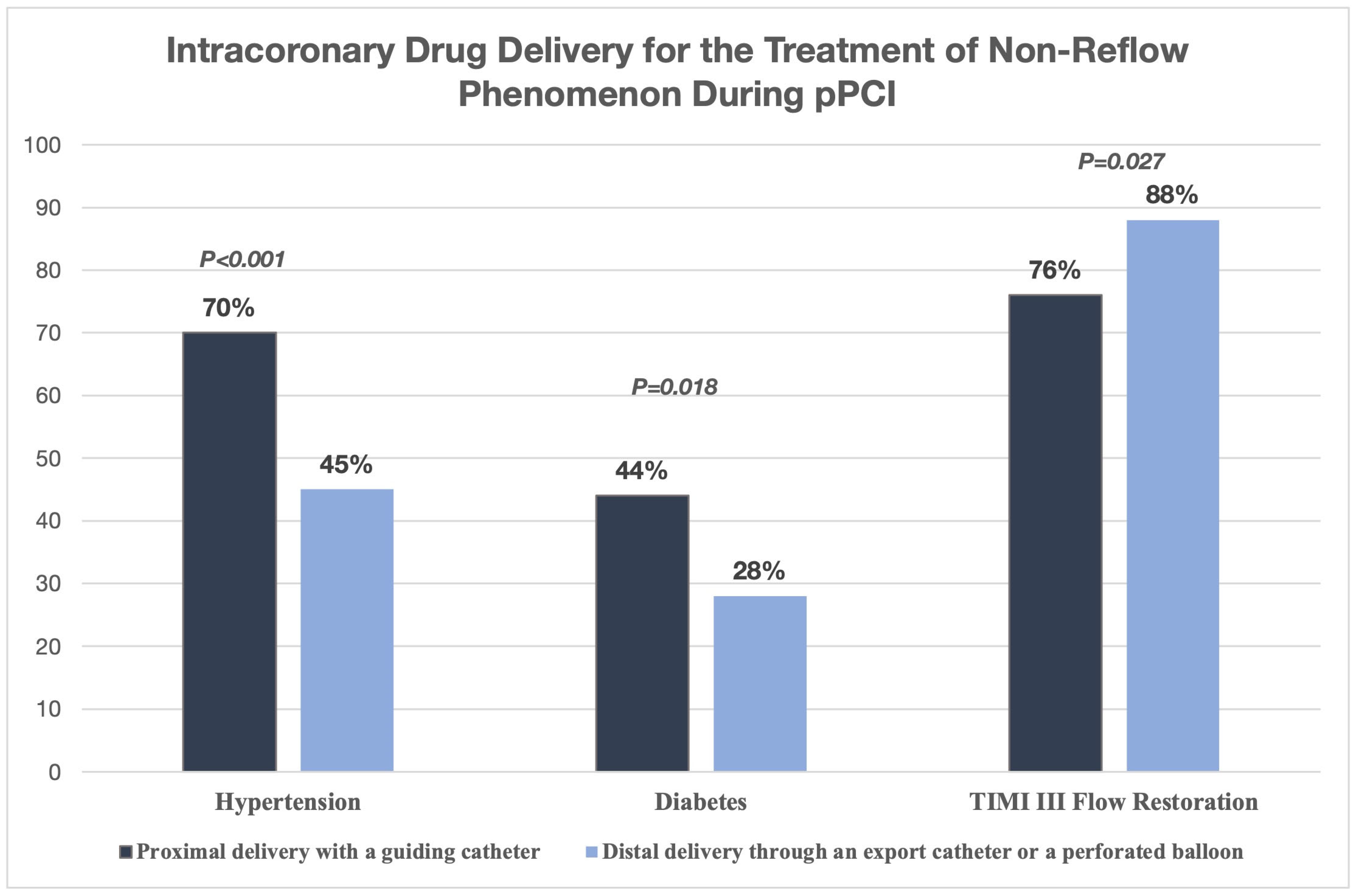
4.7. Intracoronary Epinephrine for Refractory No-Reflow During Primary PCI
4.8. Artificial Intelligence in Predicting and Managing No-Reflow After PCI
4.9. Special Populations
4.10. Prognostic Implications and Short- and Long-Term Outcomes
4.11. Controversies and Unmet Needs
4.12. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| AI | Artificial Intelligence |
| CAD | Coronary Artery Disease |
| CHF | Congestive Heart Failure |
| CMR | Cardiac Magnetic Resonance |
| CNR | Coronary No-Reflow |
| cTFC | Corrected TIMI Frame Count |
| CTA | Computed Tomography Angiography |
| Hs-CRP | High-Sensitivity C-Reactive Protein |
| IC | Intracoronary |
| IMH | Intramyocardial Hemorrhage |
| MACE | Major Adverse Cardiac Events |
| MI | Myocardial Infarction |
| MRI | Magnetic Resonance Imaging |
| MVO | Microvascular Occlusion |
| OCT | Optical Coherence Tomography |
| PBT | Perforated Balloon Technique |
| PCI | Percutaneous Coronary Intervention |
| PLR | Platelet-to-Lymphocyte Ratio |
| RDW | Red Cell Distribution Width |
| STEMI | ST-Elevation Myocardial Infarction |
| TCFA | Thin-Cap Fibroatheroma |
| UFH | Unfractionated Heparin |
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” Phenomenon: A Contemporary Review. J. Clin. Med. 2022, 11, 2233. [Google Scholar] [CrossRef]
- Bouleti, C.; Mewton, N.; Germain, S. The No-Reflow Phenomenon: State of the Art. Arch. Cardiovasc. Dis. 2015, 108, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Cassese, S.; Xhepa, E.; Joner, M.; Sager, H.B.; Kufner, S.; Laugwitz, K.-L.; Schunkert, H.; Kastrati, A. Coronary No-Reflow and Adverse Events in Patients with Acute Myocardial Infarction after Percutaneous Coronary Intervention with Current Drug-Eluting Stents and Third-Generation P2Y12 Inhibitors. Clin. Res. Cardiol. 2024, 113, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, Y.; Wu, X.; Wang, J.; Wang, C. Risk Factors for No-Reflow in Patients with ST-Elevation Myocardial Infarction Who Underwent Percutaneous Coronary Intervention: A Case-Control Study. Cardiol. Res. Pract. 2022, 2022, 3482518. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Dharmashankar, K.C.; Abdalrahman, I.B.; Kloner, R.A. No-Reflow Phenomenon Following Percutaneous Coronary Intervention for Acute Myocardial Infarction: Incidence, Outcome, and Effect of Pharmacologic Therapy. J. Intervent. Cardiol. 2010, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Avalos, J.A.; Bazán-Rodríguez, L.; Espinoza-Escobar, G.; Martínez-Villa, F.A.; Ornelas-Aguirre, J.M. Predictors in No-Reflow Phenomenon in Acute Myocardial Infarction with ST-Segment Elevation. Arch. Cardiol. Mex. 2022, 92, 461–468. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Chen, Y.; Wang, C.; Zhu, X. A Risk Score for No Reflow in Patients With ST-Segment Elevation Myocardial Infarction After Primary Percutaneous Coronary Intervention. Clin. Cardiol. 2015, 38, 208–215. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, T.; Chen, K.; Wu, G.; Xia, Y.; Wang, X.; Zong, G. A Nomogram Risk Prediction Model for No-Reflow after Primary Percutaneous Coronary Intervention Based on Rapidly Accessible Patient Data among Patients with ST-Segment Elevation Myocardial Infarction and Its Relationship with Prognosis. Front. Cardiovasc. Med. 2022, 9, 966299. [Google Scholar] [CrossRef]
- Xie, E.; Li, Q.; Ye, Z.; Guo, Z.; Li, Y.; Shen, N.; Yu, C.; Gao, Y.; Zheng, J. Canada Acute Coronary Syndrome Risk Score Predicts No-/Slow-Reflow in ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Heliyon 2023, 9, e21276. [Google Scholar] [CrossRef]
- Tasar, O.; Karabay, A.K.; Oduncu, V.; Kirma, C. Predictors and Outcomes of No-Reflow Phenomenon in Patients with Acute ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Coron. Artery Dis. 2019, 30, 270–276. [Google Scholar] [CrossRef]
- Setiha, E.A.E.H.E.; Guindy, E.A.E.; Sheikh, R.G.E.; Elsaid, A.M.; Ashmawy, M.M. Incidence and Predictors of No Reflow Phenomenon in Patient Undergoing Primary Percutaneous Coronary Intervention. J. Adv. Med. Med. Res. 2022, 34, 437–449. [Google Scholar] [CrossRef]
- Galli, M.; Niccoli, G.; De Maria, G.; Brugaletta, S.; Montone, R.A.; Vergallo, R.; Benenati, S.; Magnani, G.; D’Amario, D.; Porto, I.; et al. Coronary Microvascular Obstruction and Dysfunction in Patients with Acute Myocardial Infarction. Nat. Rev. Cardiol. 2024, 21, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Pepine, C.J. Physiology and Functional Significance of the Coronary Microcirculation: An Overview of Its Implications in Health and Disease. Am. Heart J. Plus Cardiol. Res. Pract. 2024, 40, 100381. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Kastrati, A. Coronary No-Reflow after Primary Percutaneous Coronary Intervention-Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. J. Clin. Med. 2023, 12, 5592. [Google Scholar] [CrossRef]
- Steenbergen, C.; Hill, M.L.; Jennings, R.B. Volume Regulation and Plasma Membrane Injury in Aerobic, Anaerobic, and Ischemic Myocardium in Vitro. Effects of Osmotic Cell Swelling on Plasma Membrane Integrity. Circ. Res. 1985, 57, 864–875. [Google Scholar] [CrossRef]
- Kasseckert, S.A.; Schäfer, C.; Kluger, A.; Gligorievski, D.; Tillmann, J.; Schlüter, K.-D.; Noll, T.; Sauer, H.; Piper, H.M.; Abdallah, Y. Stimulation of cGMP Signalling Protects Coronary Endothelium against Reperfusion-Induced Intercellular Gap Formation. Cardiovasc. Res. 2009, 83, 381–387. [Google Scholar] [CrossRef][Green Version]
- Maxwell, L.; Gavin, J.B. The Role of Post-Ischaemic Reperfusion in the Development of Microvascular Incompetence and Ultrastructural Damage in the Myocardium. Basic Res. Cardiol. 1991, 86, 544–553. [Google Scholar] [CrossRef]
- Schnoor, M.; García Ponce, A.; Vadillo, E.; Pelayo, R.; Rossaint, J.; Zarbock, A. Actin Dynamics in the Regulation of Endothelial Barrier Functions and Neutrophil Recruitment during Endotoxemia and Sepsis. Cell. Mol. Life Sci. CMLS 2017, 74, 1985–1997. [Google Scholar] [CrossRef]
- Vink, H.; Duling, B.R. Identification of Distinct Luminal Domains for Macromolecules, Erythrocytes, and Leukocytes within Mammalian Capillaries. Circ. Res. 1996, 79, 581–589. [Google Scholar] [CrossRef]
- Ostergaard, L.; Kristiansen, S.B.; Angleys, H.; Frøkiær, J.; Michael Hasenkam, J.; Jespersen, S.N.; Bøtker, H.E. The Role of Capillary Transit Time Heterogeneity in Myocardial Oxygenation and Ischemic Heart Disease. Basic Res. Cardiol. 2014, 109, 409. [Google Scholar] [CrossRef]
- van den Berg, B.M.; Vink, H.; Spaan, J.A.E. The Endothelial Glycocalyx Protects against Myocardial Edema. Circ. Res. 2003, 92, 592–594. [Google Scholar] [CrossRef]
- Nees, S.; Weiss, D.R.; Senftl, A.; Knott, M.; Förch, S.; Schnurr, M.; Weyrich, P.; Juchem, G. Isolation, Bulk Cultivation, and Characterization of Coronary Microvascular Pericytes: The Second Most Frequent Myocardial Cell Type in Vitro. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H69–H84. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, F.M.; Mastitskaya, S.; Hammond-Haley, M.; Freitas, F.; Wah, W.R.; Attwell, D. Capillary Pericytes Mediate Coronary No-Reflow after Myocardial Ischaemia. eLife 2017, 6, e29280. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The Coronary Circulation in Acute Myocardial Ischaemia/Reperfusion Injury: A Target for Cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Canty, J.M.; Klocke, F.J. Reduced Regional Myocardial Perfusion in the Presence of Pharmacologic Vasodilator Reserve. Circulation 1985, 71, 370–377. [Google Scholar] [CrossRef]
- Lønborg, J.; Kelbæk, H.; Helqvist, S.; Holmvang, L.; Jørgensen, E.; Saunamäki, K.; Kløvgaard, L.; Kaltoft, A.; Bøtker, H.E.; Lassen, J.F.; et al. The Impact of Distal Embolization and Distal Protection on Long-Term Outcome in Patients with ST Elevation Myocardial Infarction Randomized to Primary Percutaneous Coronary Intervention--Results from a Randomized Study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 180–188. [Google Scholar] [CrossRef]
- Stone, G.W.; Webb, J.; Cox, D.A.; Brodie, B.R.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Dulas, D.; Rutherford, B.D.; et al. Distal Microcirculatory Protection during Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial Infarction: A Randomized Controlled Trial. JAMA 2005, 293, 1063–1072. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Heusch, G. A Fresh Look at Coronary Microembolization. Nat. Rev. Cardiol. 2022, 19, 265–280. [Google Scholar] [CrossRef]
- Falk, E.; Thuesen, L. Pathology of Coronary Microembolisation and No Reflow. Heart 2003, 89, 983–985. [Google Scholar] [CrossRef]
- Carlsson, M.; Martin, A.J.; Ursell, P.C.; Saloner, D.; Saeed, M. Magnetic Resonance Imaging Quantification of Left Ventricular Dysfunction Following Coronary Microembolization. Magn. Reson. Med. 2009, 61, 595–602. [Google Scholar] [CrossRef]
- Fröhlich, G.M.; Meier, P.; White, S.K.; Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury: Looking beyond Primary PCI. Eur. Heart J. 2013, 34, 1714–1722. [Google Scholar] [CrossRef]
- Kidambi, A.; Mather, A.N.; Motwani, M.; Swoboda, P.; Uddin, A.; Greenwood, J.P.; Plein, S. The Effect of Microvascular Obstruction and Intramyocardial Hemorrhage on Contractile Recovery in Reperfused Myocardial Infarction: Insights from Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2013, 15, 58. [Google Scholar] [CrossRef]
- Adamson, R.H.; Zeng, M.; Adamson, G.N.; Lenz, J.F.; Curry, F.E. PAF- and Bradykinin-Induced Hyperpermeability of Rat Venules Is Independent of Actin-Myosin Contraction. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H406–H417. [Google Scholar] [CrossRef] [PubMed]
- Tarikuz Zaman, A.K.M.; Spees, J.L.; Sobel, B.E. Attenuation of Cardiac Vascular Rhexis: A Promising Therapeutic Target. Coron. Artery Dis. 2013, 24, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.P.; Rashid, M.; Dinh, D.T.; Brennan, A.; Bloom, J.E.; Biswas, S.; Lefkovits, J.; Shaw, J.A.; Chan, W.; Clark, D.J.; et al. No-Reflow Prediction in Acute Coronary Syndrome During Percutaneous Coronary Intervention: The NORPACS Risk Score. Circ. Cardiovasc. Interv. 2024, 17, e013738. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ammar, A.; Saghir, T.; Sial, J.A.; Khan, K.A.; Shah, J.A.; Shaikh, A.H.; Rizvi, S.N.H.; Qamar, N.; Karim, M. Development and Validation of a Novel Risk Stratification Model for Slow-Flow/No-Reflow During Primary Percutaneous Coronary Intervention (the RK-SF/NR Score). Am. J. Cardiol. 2022, 171, 32–39. [Google Scholar] [CrossRef]
- Vatan, M.B.; Çakmak, A.C.; Ağaç, S.; Eynel, E.; Erkan, H. The Systemic Immune-Inflammation Index Predicts Impaired Myocardial Perfusion and Short-Term Mortality in ST-Segment Elevation Myocardial Infarction Patients. Angiology 2023, 74, 365–373. [Google Scholar] [CrossRef]
- Gong, X.; Lei, X.; Huang, Z.; Song, Y.; Wang, Q.; Qian, J.; Ge, J. D-Dimer Level Predicts Angiographic No-Reflow Phenomenon After Percutaneous Coronary Intervention Within 2–7 Days of Symptom Onset in Patients with ST-Segment Elevation Myocardial Infarction. J. Cardiovasc. Transl. Res. 2021, 14, 728–734. [Google Scholar] [CrossRef]
- Wang, L.; Huang, S.; Zhou, Q.; Dou, L.; Lin, D. The Predictive Value of Laboratory Parameters for No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Following Primary Percutaneous Coronary Intervention: A Meta-Analysis. Clin. Cardiol. 2024, 47, e24238. [Google Scholar] [CrossRef]
- Harigaya, H.; Motoyama, S.; Sarai, M.; Inoue, K.; Hara, T.; Okumura, M.; Naruse, H.; Ishii, J.; Hishida, H.; Ozaki, Y. Prediction of the No-Reflow Phenomenon during Percutaneous Coronary Intervention Using Coronary Computed Tomography Angiography. Heart Vessel. 2011, 26, 363–369. [Google Scholar] [CrossRef]
- Ozaki, Y.; Tanaka, A.; Tanimoto, T.; Kitabata, H.; Kashiwagi, M.; Kubo, T.; Takarada, S.; Ishibashi, K.; Komukai, K.; Ino, Y.; et al. Thin-Cap Fibroatheroma as High-Risk Plaque for Microvascular Obstruction in Patients with Acute Coronary Syndrome. Circ. Cardiovasc. Imaging 2011, 4, 620–627. [Google Scholar] [CrossRef]
- Okutsu, M.; Horio, T.; Tanaka, H.; Akiyama, M.; Okimoto, N.; Tsubouchi, T.; Kawajiri, K.; Ohashi, Y.; Sumitsuji, S.; Ikari, Y. Predictive Performance of Dual Modality of Computed Tomography Angiography and Intravascular Ultrasound for No-Reflow Phenomenon after Percutaneous Coronary Stenting in Stable Coronary Artery Disease. Heart Vessel. 2018, 33, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Carrick, D.; Haig, C.; Rauhalammi, S.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; et al. Prognostic Significance of Infarct Core Pathology Revealed by Quantitative Non-Contrast in Comparison with Contrast Cardiac Magnetic Resonance Imaging in Reperfused ST-Elevation Myocardial Infarction Survivors. Eur. Heart J. 2016, 37, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.-G.; Pavon, A.G.; Muller, O.; Iglesias, J.-F.; Vincenti, G.; Monney, P.; Harbaoui, B.; Eeckhout, E.; Schwitter, J. Relationship between CMR-Derived Parameters of Ischemia/Reperfusion Injury and the Timing of CMR after Reperfused ST-Segment Elevation Myocardial Infarction. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Dil, S.; Ryabov, V.; Maslov, L.; Mochula, O.; Mochula, A.; Kercheva, M.; Zavadovsky, K.; Vyshlov, E. Assessing Coronary Microvascular Dysfunction in Refractory No-Reflow: Insights from Dynamic Myocardial Perfusion Scintigraphy and Cardiac MRI. Microvasc. Res. 2025, 162, 104862. [Google Scholar] [CrossRef]
- Dai, W.; Ye, Z.; Li, L.; Su, Q. Effect of Preoperative Loading Dose Ticagrelor and Clopidogrel on No-Reflow Phenomenon during Intervention in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Drug Des. Devel. Ther. 2018, 12, 2039–2049. [Google Scholar] [CrossRef]
- Wöhrle, J.; Merkle, N.; Kunze, M.; Cristea, E.; Mehran, R.; Rottbauer, W.; Stone, G.W. Effect of Bivalirudin Compared with Unfractionated Heparin plus Abciximab on Infarct Size and Myocardial Recovery after Primary Percutaneous Coronary Intervention: The Horizons-AMI CMRI Substudy. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2012, 79, 1083–1089. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, Z.; Xu, B.; Chen, B.; Ge, H.; Ding, S.; Pu, J. Impact of Bivalirudin on Ischemia/Reperfusion Injury in Patients with Reperfused STEMI Assessed by Cardiac Magnetic Resonance. Pharmaceuticals 2024, 17, 196. [Google Scholar] [CrossRef]
- Yu, L.; Chen, J.; Zhang, J. Meta-Analysis of the Correlation between Inflammatory Response Indices and No-Reflow after PCI in Patients with Acute STEMI. Am. J. Transl. Res. 2024, 16, 5168–5181. [Google Scholar] [CrossRef]
- Ismaiel, M.; Shehata, M.; Elwasify, T.; Hosny, M.; Anwar, A.; Ahmed, T.A.N.; Elagha, A. Impact of Aspiration Thrombectomy on Microvascular Obstruction in Patients With ST-Segment Elevation Myocardial Infarction. J. Saudi Heart Assoc. 2025, 37, 9. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, R.; Sakata, K.; Miwa, K.; Matsubara, T.; Yasuda, T.; Inoue, M.; Okada, H.; Kanaya, H.; Kawashiri, M.; Yamagishi, M.; et al. Impact of Distal Protection with Filter-Type Device on Long-Term Outcome after Percutaneous Coronary Intervention for Acute Myocardial Infarction: Clinical Results with Filtrap®. J. Atheroscler. Thromb. 2016, 23, 1313–1323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, L.; Wright, A.R.; Hynynen, K.; Goertz, D.E. Self-Sensing with Hollow Cylindrical Transducers for Histotripsy-Enhanced Aspiration Mechanical Thrombectomy Applications. Sensors 2025, 25, 5417. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.; Veliky, M.; Abah, C.P.; Dietrich, M.S.; Chitale, R.; Simaan, N. Endovascular Detection of Catheter-Thrombus Contact by Vacuum Excitation. IEEE Trans. Biomed. Eng. 2024, 71, 1926–1936. [Google Scholar] [CrossRef]
- Freixa, X.; Belle, L.; Joseph, L.; Tanguay, J.-F.; Souteyrand, G.; LAllier, P.L.; Jolicœur, E.M. Immediate vs. Delayed Stenting in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. EuroIntervention 2013, 8, 1207–1216. [Google Scholar] [CrossRef]
- Sinha, S.K.; Kumar, P.; Sharma, A.K.; Razi, M.; Pandey, U.; Sachan, M.; Shukla, P.; Aggarwal, P.; Jha, M.J.; Thakur, R.; et al. Perforated Balloon Technique Mediated Intracoronary Delivery of Nicorandil to Treat Coronary No-Reflow Phenomenon: A Novel Pharmacological Solution to Precarious Situation. Am. J. Cardiovasc. Dis. 2021, 11, 544–554. [Google Scholar]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Muçaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and Safety of Intracoronary Epinephrine versus Conventional Treatments Alone in STEMI Patients with Refractory Coronary No-Reflow during Primary PCI: The Restore Observational Study. Catheter. Cardiovasc. Interv. 2021, 97, 602–611. [Google Scholar] [CrossRef]
- Wang, L.; Bao, P.; Wang, X.; Xu, B.; Liu, Z.; Hu, G. Machine Learning Prediction of No Reflow in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Cardiovasc. Diagn. Ther. 2024, 14, 547–562. [Google Scholar] [CrossRef]
- Del Turco, S.; Basta, G.; De Caterina, A.R.; Sbrana, S.; Paradossi, U.; Taddei, A.; Trianni, G.; Ravani, M.; Palmieri, C.; Berti, S.; et al. Different Inflammatory Profile in Young and Elderly STEMI Patients Undergoing Primary Percutaneous Coronary Intervention (PPCI): Its Influence on No-Reflow and Mortality. Int. J. Cardiol. 2019, 290, 34–39. [Google Scholar] [CrossRef]
- Malmberg, K.; Rydén, L.; Efendic, S.; Herlitz, J.; Nicol, P.; Waldenström, A.; Wedel, H.; Welin, L. Randomized Trial of Insulin-Glucose Infusion Followed by Subcutaneous Insulin Treatment in Diabetic Patients with Acute Myocardial Infarction (DIGAMI Study): Effects on Mortality at 1 Year. J. Am. Coll. Cardiol. 1995, 26, 57–65. [Google Scholar] [CrossRef]
- Kaya, A.; Keskin, M.; Tatlisu, M.A.; Uzman, O.; Borklu, E.; Cinier, G.; Yildirim, E.; Kayapinar, O. Atrial Fibrillation: A Novel Risk Factor for No-Reflow Following Primary Percutaneous Coronary Intervention. Angiology 2020, 71, 175–182. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Kang, M.G.; Jung, M.K.; Ahn, J.-H.; Koh, J.-S.; Guevarra, P.I.; Kim, S.-W.; Tantry, U.S.; Gurbel, P.A.; Hwang, J.-Y.; et al. Association and Prognostic Implications of “No-Reflow Phenomenon” and Hypercoagulability in Patients With ST-Segment Elevation Myocardial Infarction. JACC Asia 2025, 5, 1478–1501. [Google Scholar] [CrossRef] [PubMed]
- Godínez, L.; González-Pacheco, H.; Gopar-Nieto, R.; Eid-Lidt, G.; Cruz, J.; Araiza-Garaygordobil, D.; Mendoza-Garcia, S.; Altamirano-Castillo, A.; Ontiveros-Mercado, H.; Mendoza, A. Prevalence and Prognostic Implications of the No-Reflow Phenomenon in Patients Undergoing Primary Percutaneous Coronary Intervention in a University Center in a Middle-Income Country. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.2066. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Abbott, J.D.; Al-Azizi, K.; Barman, N.; Bortnick, A.E.; Cohen, M.G.; Dehghani, P.; Henry, T.D.; Latif, F.; Madjid, M.; et al. SCAI Expert Consensus Statement on the Management of Patients With STEMI Referred for Primary PCI. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102294. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2025, 85, 2135–2237. [Google Scholar] [CrossRef]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between Microvascular Obstruction and Adverse Events Following Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction: An Individual Patient Data Pooled Analysis from Seven Randomized Trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients With Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, e011408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malakouti, S.; Hashim, A.; Frazzetto, M.; Cortese, B. No-Reflow During Coronary Interventions: A Narrative Review. J. Clin. Med. 2025, 14, 7976. https://doi.org/10.3390/jcm14227976
Malakouti S, Hashim A, Frazzetto M, Cortese B. No-Reflow During Coronary Interventions: A Narrative Review. Journal of Clinical Medicine. 2025; 14(22):7976. https://doi.org/10.3390/jcm14227976
Chicago/Turabian StyleMalakouti, Sara, Ahmed Hashim, Marco Frazzetto, and Bernardo Cortese. 2025. "No-Reflow During Coronary Interventions: A Narrative Review" Journal of Clinical Medicine 14, no. 22: 7976. https://doi.org/10.3390/jcm14227976
APA StyleMalakouti, S., Hashim, A., Frazzetto, M., & Cortese, B. (2025). No-Reflow During Coronary Interventions: A Narrative Review. Journal of Clinical Medicine, 14(22), 7976. https://doi.org/10.3390/jcm14227976




