The Impact of Cangrelor in the UK for the Treatment of STEMI Patients with Gastric Absorption Issues Undergoing Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Inputs and Data Sources
2.3. Target Population
2.4. Efficacy and Safety Data
2.5. Length of Stay Data
2.6. GPI Bailout Use
2.7. Cost Data
3. Results
3.1. Sensitivity Analysis
3.1.1. One Way Sensitivity Analysis
3.1.2. Scenario Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| BCIS | British Cardiovascular Intervention Society |
| BI | Budget impact |
| BIM | Budget impact model |
| BNF | British National Formulary |
| CVD | Cardiovascular diseases |
| EMA | European Medicines Agency |
| ESC | European Society of Cardiology |
| GPI | Glycoprotein IIb/IIIa receptor inhibitors |
| HES | Hospital episode statistics |
| IDR | Ischemia-driven revascularisation |
| ISPOR | The Profession Society for Health Economics and Outcomes Research |
| IV | Intravenous |
| LOS | Length of stay |
| MI | Myocardial infarction |
| NHS | National Health Service |
| NICE | National Institute of Clinical Excellence |
| OHCA | Out-of-hospital cardiac arrest |
| OWSA | One-way sensitivity analysis |
| PCI | Percutaneous coronary intervention |
| PPCI | Primary percutaneous coronary intervention |
| PSS | Personal Social Services |
| ST | Stent thrombosis |
| STEMI | ST-elevation myocardial infarction |
| TIMI | Thrombolysis in myocardial infarction |
References
- British Heart Foundation. UK Factsheet 2023. Available online: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-uk-factsheet.pdf (accessed on 29 August 2023).
- Silber, S.; Albertsson, P.; Avilés, F.F.; Camici, P.G.; Colombo, A.; Hamm, C.; Jørgensen, E.; Marco, J.; Nordrehaug, J.E.; Ruzyllo, W.; et al. Guidelines for Percutaneous Coronary Interventions: The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur. Heart J. 2005, 26, 804–847. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of Platelet Inhibition with Cangrelor during PCI on Ischemic Events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Ludman, P. BCIS National Audit Adult Interventional Procedures—2022–2023|British Cardiovascular Intervention Society. Available online: https://www.bcis.org.uk/audit-results/ (accessed on 24 April 2024).
- Parodi, G.; Bellandi, B.; Xanthopoulou, I.; Capranzano, P.; Capodanno, D.; Valenti, R.; Stavrou, K.; Migliorini, A.; Antoniucci, D.; Tamburino, C.; et al. Morphine Is Associated with a Delayed Activity of Oral Antiplatelet Agents in Patients with ST-Elevation Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2015, 8, e001593. [Google Scholar] [CrossRef]
- A Byrne, R.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Farag, M. Optimal intravenous antiplatelet therapy in patients with ST-elevation myocardial infarction: Is the picture becoming clearer? J. Thromb. Thrombolysis 2024, 57, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Galli, M.; Collet, J.P.; Kastrati, A.; O’Donoghue, M.L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022, 17, e1371–e1396. [Google Scholar] [CrossRef]
- Tavenier, A.H.; Hermanides, R.S.; Ottervanger, J.P.; Tolsma, R.; van Beurden, A.; Slingerland, R.J.; Ter Horst, P.G.J.; Gosselink, A.T.M.; Dambrink, J.E.; van Leeuwen, M.A.H.; et al. Impact of opioids on P2Y12 receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 4–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angiolillo, D.J.; Schneider, D.J.; Bhatt, D.L.; French, W.J.; Price, M.J.; Saucedo, J.F.; Shaburishvili, T.; Huber, K.; Prats, J.; Liu, T.; et al. Pharmacodynamic effects of cangrelor and clopidogrel: The platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J. Thromb. Thrombolysis 2012, 34, 44–55. [Google Scholar] [CrossRef]
- European Medicines Agency. Kengrexal|European Medicines Agency (EMA). 12 June 2015. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kengrexal (accessed on 25 March 2025).
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V.J.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet Inhibition with Cangrelor in Patients Undergoing PCI. N. Engl. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous Platelet Blockade with Cangrelor during PCI. N. Engl. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef]
- NHS Digital. Hospital Episode Statistics (HES) to Map Percutaneous Coronary Intervention. 2021. Available online: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed on 11 July 2024).
- Farag, M.F.; Spinthakis, N.; Srinivasan, M.; Sullivan, K.; Wellsted, D.; Gorog, D.A. P6084Morphine use in STEMI associated with enhanced platelet reactivity and larger infarct size, and this is negated by GPI use peri-PPCI. Eur. Heart J. 2017, 38 (Suppl. S1), ehx493.P6084. [Google Scholar] [CrossRef]
- Thompson, P.; Campbell, M.; McNeice, A. 44 A review of glycoprotein IIB/IIIA inhibitors in primary PCI within the belfast HSC trust. Heart 2023, 109 (Suppl. S6), A50–A51. [Google Scholar] [CrossRef]
- Gallen, R.; Shand, J.; Mulvey, P. 45 Patterns of use and the impact on intra-procedural decision-making of intra-coronary imaging during percutaneous coronary intervention in an Irish tertiary referral hospital. Heart 2023, 109 (Suppl. S6), A51–A52. [Google Scholar] [CrossRef]
- Ludman, P. BCIS National Audit Adult Interventional Procedures—2021–2022|British Cardiovascular Intervention Society. Available online: http://www.bcis.org.uk/wp-content/uploads/2023/02/BCIS-Audit-2021-22-data-for-web-05-02-2023.pdf (accessed on 14 August 2023).
- The National Institute for Cardiovascular Outcomes Research (NICOR). Percutaneous Coronary Intervention (PCI)—2020 Summary Report. HQIP. Available online: https://www.hqip.org.uk/resource/percutaneous-coronary-intervention-pci-2020-summary-report/ (accessed on 25 March 2025).
- The National Institute for Cardiovascular Outcomes Research (NICOR). National Audit of Percutaneous Coronary Intervention: 2021 Summary Report. Available online: https://www.hqip.org.uk/resource/national-audit-of-percutaneous-coronary-intervention-2021-summary-report/ (accessed on 25 March 2025).
- The National Institute for Cardiovascular Outcomes Research (NICOR). Percutaneous Coronary Intervention (PCI)—2022 Summary Report. Available online: https://www.nicor.org.uk/national-cardiac-audit-programme/previous-reports/pci-1/2022-5/napci-domain-report-2022-final?layout=file (accessed on 25 March 2025).
- Westman, P.C.; Lipinski, M.J.; Torguson, R.; Waksman, R. A comparison of cangrelor, prasugrel, ticagrelor, and clopidogrel in patients undergoing percutaneous coronary intervention: A network meta-analysis. Cardiovasc. Revasc Med. 2017, 18, 79–85. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Harrington, R.A.; Stone, G.W.; Deliargyris, E.N.; Steg, P.G.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Menozzi, A.; Prats, J.; et al. Evaluation of Ischemic and Bleeding Risks Associated with 2 Parenteral Antiplatelet Strategies Comparing Cangrelor with Glycoprotein IIb/IIIa Inhibitors: An Exploratory Analysis from the CHAMPION Trials. JAMA Cardiol. 2017, 2, 127–135. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Hamm, C.W.; Stone, G.W.; Gibson, C.M.; Mahaffey, K.W.; Leonardi, S.; Liu, T.; Skerjanec, S.; Day, J.R.; et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 2013, 382, 1981–1992. [Google Scholar] [CrossRef]
- Yerasi, C.; Case, B.C.; Chezar-Azerrad, C.; Forrestal, B.J.; Medranda, G.A.; Shea, C.; Zhang, C.; Ben-Dor, I.; Satler, L.F.; Bernardo, N.L.; et al. Cangrelor vs. glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention. Am. Heart J. 2021, 238, 59–65. [Google Scholar] [CrossRef] [PubMed]
- NICE Recommendations|Acute Coronary Syndromes|Guidance NICE. 18 November 2020. Available online: https://www.nice.org.uk/guidance/ng185/chapter/Recommendations (accessed on 25 March 2025).
- Thim, T.; Jakobsen, L.; Jensen, R.V.; Støttrup, N.; Eftekhari, A.; Grove, E.L.; Larsen, S.B.; Sørensen, J.T.; Carstensen, S.; Amiri, S.; et al. Real-World Experience with Cangrelor as Adjuvant to Percutaneous Coronary Intervention: A Single-Centre Observational Study. Cardiol. Res. Pract. 2023, 2023, 3197512. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.C.; Weatherly, H.; Birch, S.; Castelli, A.; Chalkley, M.; Dargan, A.; Forder, J.E.; Gao, M.; Hinde, S.; Markham, S.; et al. Unit Costs of Health and Social Care 2023 Manual; Personal Social Services Research Unit (University of Kent) & Centre for Health Economics (University of York): Kent, UK, 2024. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Mauskopf, J.A.; Augustovski, F.; Caro, J.J.; Lee, K.M.; Minchin, M.; Orlewska, E.; Penna, P.; Barrios, J.-M.R.; Shau, W.-Y. Budget Impact Analysis—Principles of Good Practice: Report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014, 17, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Shore, J.; Russell, J.; Jenks, M. Evidence Standards Framework for Digital Health Technologies: Cost Consequences and Budget Impact Analyses and Data Sources. Natl. Inst. Health Care Excell. 2019. Available online: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/evidence-standards-framework/budget-impact-guide.pdf (accessed on 25 March 2025).
- Danese, M.D.; Gleeson, M.; Kutikova, L.; I Griffiths, R.; Azough, A.; Khunti, K.; Seshasai, S.R.K.; Ray, K.K. Estimating the economic burden of cardiovascular events in patients receiving lipid-modifying therapy in the UK. BMJ Open 2016, 6, e011805. [Google Scholar] [CrossRef]
- Mamas, M.A.; Tosh, J.; Hulme, W.; Hoskins, N.; Bungey, G.; Ludman, P.; de Belder, M.; Kwok, C.S.; Verin, N.; Kinnaird, T.; et al. Health Economic Analysis of Access Site Practice in England During Changes in Practice: Insights from the British Cardiovascular Interventional Society. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004482. [Google Scholar] [CrossRef]
- Lizano-Díez, I.; Paz Ruiz, S. Analysis of the Financial Impact of Using Cangrelor on the Safety and Efficacy Outcomes in Patients Undergoing Percutaneous Coronary Intervention in Whom Oral Therapy with P2Y12 Inhibitors is Not Feasible or Desirable, in Spain. Clin. Outcomes Res. 2021, 13, 77–87. [Google Scholar] [CrossRef]
- Cangrelor|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/cangrelor/ (accessed on 11 July 2024).
- Clopidogrel|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/clopidogrel/ (accessed on 24 June 2024).
- Clopidogrel 75 mg Film-Coated Tablets—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/5207/smpc#gref (accessed on 24 June 2024).
- Prasugrel|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/prasugrel/ (accessed on 24 June 2024).
- Prasugrel 10 mg Film-Coated Tablets—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/15829/smpc#gref (accessed on 24 June 2024).
- Ticagrelor|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/ticagrelor/ (accessed on 24 June 2024).
- Brilique 90 mg Film Coated Tablets—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/5767/smpc#gref (accessed on 24 June 2024).
- Eptifibatide|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/eptifibatide/ (accessed on 11 July 2024).
- Integrilin—Direct Healthcare Professional Communication (DHPC)|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/dhpc/integrilin (accessed on 26 June 2024).
- Tirofiban|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/tirofiban/ (accessed on 11 July 2024).
- Aggrastat 50 mcg/mL Solution for Infusion—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/566/smpc#gref (accessed on 11 July 2024).
- NHS Improvement. 2017/18 Reference Costs|Archived Reference Costs. 2020. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed on 11 July 2024).
- Lizano-Diez, I.; Paz, S. Budget impact analysis of antiplatelet therapy with cangrelor in patients with acute coronary artery disease undergoing percutaneous coronary intervention in Portugal. Eur. Heart J. Acute Cardiovasc. Care 2021, 10 (Suppl. S1), zuab020-105. [Google Scholar] [CrossRef]
- Lizano-Díez, I.; Paz, S.; Cortes, J.G. PCV26 Budget IMPACT Analysis of Antiplatelet Therapy with Cangrelor in Patients with ACUTE Coronary Artery Disease Undergoing Percutaneous Coronary Intervention in Germany. Value Health 2020, 23, S491. [Google Scholar] [CrossRef]
- Lizano-Diez, I.; Paz, S. Budget impact analysis of antiplatelet therapy with cangrelor in patients with acute coronary artery disease undergoing percutaneous coronary intervention in Belgium. Eur. Heart J. Acute Cardiovasc. Care 2021, 10 (Suppl. S1), zuab020-106. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Dobrzycki, S.; Gajda, R.; Gąsior, M.; Gierlotka, M.; Jaguszewski, M.; Legutko, J.; Lesiak, M.; Navarese, E.P.; et al. Cangrelor—Expanding therapeutic options in patients with acute coronary syndrome. Cardiol. J. 2024, 31, 133–146. [Google Scholar] [CrossRef]
- Ferlini, M.; Raone, L.; Bendotti, S.; Currao, A.; Primi, R.; Bongiorno, A.; Fava, C.; Dall’oglio, L.; Adamo, M.; Ghiraldin, D.; et al. Cangrelor in Patients Undergoing Percutaneous Coronary Intervention After Out-of-Hospital Cardiac Arrest. J. Clin. Med. 2024, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Price, S.; Sibbing, D.; Baumbach, A.; Capodanno, D.; Gigante, B.; Halvorsen, S.; Huber, K.; Lettino, M.; Leonardi, S.; et al. Antithrombotic therapy in patients with acute coronary syndrome complicated by cardiogenic shock or out-of-hospital cardiac arrest: A joint position paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 125–140. [Google Scholar] [CrossRef]
- Kordis, P.; Bozic Mijovski, M.; Berden, J.; Steblovnik, K.; Blinc, A.; Noc, M. Cangrelor for comatose survivors of out-of-hospital cardiac arrest undergoing percutaneous coronary intervention: The CANGRELOR-OHCA study. EuroIntervention 2023, 18, 1269–1271. [Google Scholar] [CrossRef]
- Kordis, P.; Berden, J.; Mikuz, U.; Noc, M. Immediate Platelet Inhibition Strategy for Comatose Out-of-Hospital Cardiac Arrest Survivors Undergoing Percutaneous Coronary Intervention and Mild Therapeutic Hypothermia. J. Clin. Med. 2024, 13, 2121. [Google Scholar] [CrossRef]
- White, H.D.; Chew, D.P.; Dauerman, H.L.; Mahaffey, K.W.; Gibson, C.M.; Stone, G.W.; Gruberg, L.; Harrington, R.A.; Bhatt, D.L. Reduced immediate ischemic events with cangrelor in PCI: A pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am. Heart J. 2012, 163, 182–190.e4. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Steg, P.G.; Bhatt, D.L.; Capodanno, D.; Angiolillo, D.J. Cangrelor: Clinical Data, Contemporary Use, and Future Perspectives. J. Am. Heart Assoc. 2021, 10, e022125. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, L.; Betti, M.; D’AScenzo, F.; De Ferrari, G.; De Filippo, O.; Gaudio, C.; Collet, C.; Sabouret, P.; Agostoni, P.; Zivelonghi, C.; et al. Impact of In-Hospital Bleeding on Postdischarge Therapies and Prognosis in Acute Coronary Syndromes. J. Cardiovasc. Pharmacol. 2025, 85, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Suppliers for Eptifibatide 75 mg/100 mL Solution for Infusion Vials. Available online: https://dmd-browser.nhsbsa.nhs.uk/vmp/view/5849/suppliers?ref=JUVGJUJGJUJEJUVGJUJGJUJEZSZwYWdlPTE1MTAmc2l6ZT0yMCZzb3J0T3JkZXI9YWxwaGE%3D (accessed on 26 June 2024).
- Medicinal Forms|Eptifibatide|Drugs|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/drugs/eptifibatide/medicinal-forms/ (accessed on 26 June 2024).
| Cangrelor | Clopidogrel | Prasugrel | Ticagrelor | Eptifibatide | Tirofiban | Aspirin and Heparin Alone | ||
|---|---|---|---|---|---|---|---|---|
| Current scenario a | 0% | 10% | 50% | 30% | 2.5% | 2.5% | 5% | |
| Future scenario b | Year 1 | 10% | 9% | 45% | 27% | 2.25% | 2.25% | 4.5% |
| Year 2 | 15% | 8.5% | 42.5% | 24% | 2% | 2% | 4% | |
| Year 3 | 20% | 8% | 40% | 21% | 1.75% | 1.75% | 3.5% | |
| Year 4 | 25% | 7.5% | 37.5% | 18% | 1.5% | 1.5% | 3% | |
| Year 5 | 30% | 7% | 35% | 15% | 1.25% | 1.25% | 2.5% | |
| Efficacy at 48 h of PCI Shown by % Rates of Each Event | Safety Outcomes, TIMI, at 48 h of PCI | Reference | |||||
|---|---|---|---|---|---|---|---|
| ST | MI | IDR | Death | Major | Minor | ||
| Cangrelor | 0.10% | 2.06% | 0.29% | 0.20% | 0.20% | 0.49% | Westman et al., 2017 [22] |
| Clopidogrel | 0.85% | 3.65% | 0.74% | 0.36% | 0.22% | 0.41% | Steg et al., 1992 [24] |
| Prasugrel | 0.47% | 2.74% | 0.55% | 0.19% | 0.20% | 0.44% | Westman et al., 2017 [22] |
| Ticagrelor | 0.53% | 2.66% | 0.64% | 0.36% | 0.21% | 0.65% | Westman et al., 2017 [22] |
| Eptifibatide | 0.59% | 2.45% | 0.78% | 0.39% | 0.78% | 1.57% | Vaduganathan et al., 2017 [23] |
| Tirofiban | 0.59% | 2.45% | 0.78% | 0.39% | 0.78% | 1.57% | 2.5% |
| Aspirin & heparin a | 0.85% | 3.65% | 0.74% | 0.36% | 0.22% | 0.41% | Steg et al., 1992 [24] |
| Event | Cost Per Event | Reference |
|---|---|---|
| Ischemic events a | ||
| ST | GBP 309.06 | Danese et al., 2016 [31] |
| MI | GBP 309.06 | Danese et al., 2016 [31] |
| IDR | GBP 309.06 | Danese et al., 2016 [31] |
| Bleeding events b | ||
| Major | GBP 381.74 | Mamas et al., 2018 [32] |
| Minor | GBP 381.74 | Mamas et al., 2018 [32] |
| Cardiac death | GBP 0.00 | Lizano-Diez et al. [33] |
| Antiplatelet therapy c | ||
| Cangrelor | GBP 250.00 | BNF [34] |
| Clopidogrel | GBP 0.16 | BNF [35], SmPC [36] |
| Prasugrel | GBP 0.88 | BNF [37], SmPC [38] |
| Ticagrelor | GBP 1.95 | BNF [39], SmPC [40] |
| Eptifibatide d | GBP 897.00 | BNF [41], SmPC [42] |
| Tirofiban d | GBP 292.22 | BNF [43], SmPC [44] |
| Aspirin and heparin alone e | GBP 0.00 | |
| Cangrelor | GBP 250.00 | BNF [34] |
| Hospital stay cost | ||
| Post-operative day in hospital | GBP 498.50 | NHS reference costs [45] |
| Parameter | Total over 5 Years | Average over 5 Years |
|---|---|---|
| Patients treated with cangrelor, N | 10,903 | 2181 |
| Total costs in scenario without cangrelor, GBP | 74,948,153 | 14,989,631 |
| Total costs in scenario with cangrelor, GBP | 74,730,151 | 14,946,030 |
| Net budget impact, GBP | −218,002 | −43,600 |
| Budget impact, % | −0.29 | −0.29 |
| Parameter | Base Case Value | −20% | +20% | Lower Bound BI | Upper Bound BI | Difference Between Upper and Lower Bound BI Result |
|---|---|---|---|---|---|---|
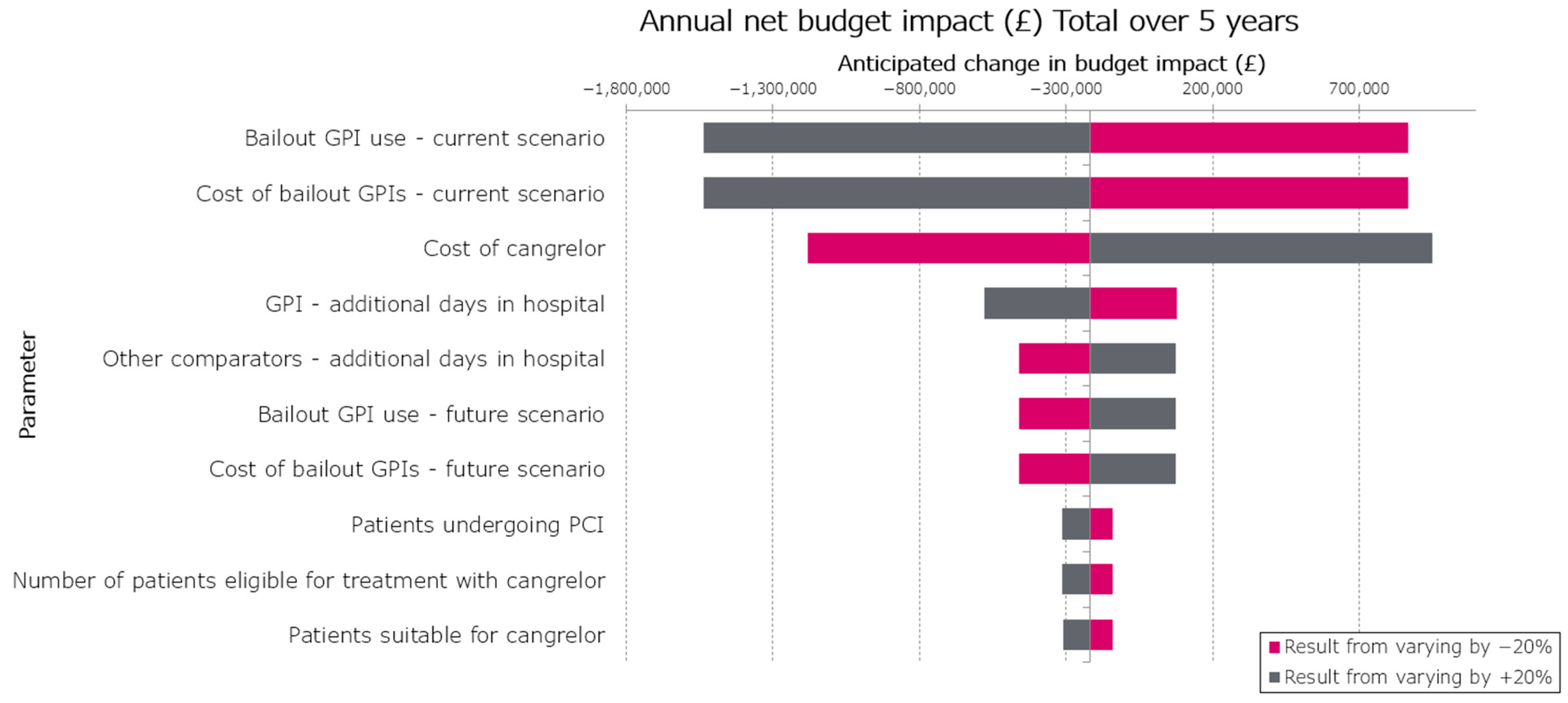
| Bailout GPI use—current scenario | 0.10 | 0.06 | 0.14 | GBP 868,552 | −GBP 1,537,205 | GBP 2,405,756 |
| Cost of bailout GPIs—current scenario | 59.461 | 38.48 | 84.93 | GBP 868,552 | −GBP 1,537,205 | GBP 2,405,756 |
| Cost of cangrelor | 250 | 161.79 | 357.10 | −GBP 1,179,760 | GBP 949,682 | GBP 2,129,442 |
| GPI—additional days in hospital | 3.10 | 2.01 | 4.43 | GBP 79,248 | −GBP 578,898 | GBP 658,146 |
| Other comparators—additional days in hospital | 2.52 | 1.63 | 3.60 | −GBP 459,950 | GBP 75,750 | GBP 535,700 |
| Bailout GPI use—future scenario | 0.02 | 0.01 | 0.03 | −GBP 459,561 | GBP 75,277 | GBP 534,838 |
| Cost of bailout GPIs—future scenario | 13.081 | 8.466 | 18.69 | −GBP 459,561 | GBP 75,277 | GBP 534,838 |
| Patients undergoing PCI | GBP 94,103.00 | GBP 60,898.50 | GBP 134,417.09 | −GBP 141,080 | −GBP 311,396 | GBP 170,316 |
| Number of patients eligible for treatment with cangrelor | GBP 10,902.65 | GBP 7,055.62 | GBP 15,573.39 | −GBP 141,080 | −GBP 311,396 | GBP 170,316 |
| Patients suitable for cangrelor | 0.12 | 0.07 | 0.16 | −GBP 140,067 | −GBP 310,339 | GBP 170,271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, B.; Cain, R.; Stork, R.; Tarpey, G.; Colucciello, A.; Olivier, D.; Barwood, C.; Wright, W.; McAtamney, R. The Impact of Cangrelor in the UK for the Treatment of STEMI Patients with Gastric Absorption Issues Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2025, 14, 7564. https://doi.org/10.3390/jcm14217564
Modi B, Cain R, Stork R, Tarpey G, Colucciello A, Olivier D, Barwood C, Wright W, McAtamney R. The Impact of Cangrelor in the UK for the Treatment of STEMI Patients with Gastric Absorption Issues Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2025; 14(21):7564. https://doi.org/10.3390/jcm14217564
Chicago/Turabian StyleModi, Bhavik, Rob Cain, Richard Stork, Gina Tarpey, Alessia Colucciello, Danielle Olivier, Caroline Barwood, Will Wright, and Rory McAtamney. 2025. "The Impact of Cangrelor in the UK for the Treatment of STEMI Patients with Gastric Absorption Issues Undergoing Percutaneous Coronary Intervention" Journal of Clinical Medicine 14, no. 21: 7564. https://doi.org/10.3390/jcm14217564
APA StyleModi, B., Cain, R., Stork, R., Tarpey, G., Colucciello, A., Olivier, D., Barwood, C., Wright, W., & McAtamney, R. (2025). The Impact of Cangrelor in the UK for the Treatment of STEMI Patients with Gastric Absorption Issues Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine, 14(21), 7564. https://doi.org/10.3390/jcm14217564





