Predicting Outcome and Duration of Mechanical Ventilation in Acute Hypoxemic Respiratory Failure: The PREMIER Study
Abstract
1. Introduction
2. Methods
2.1. Patient Population and Study Design
2.2. Variables and Outcomes
2.3. Predefined Rules and Statistical Analysis
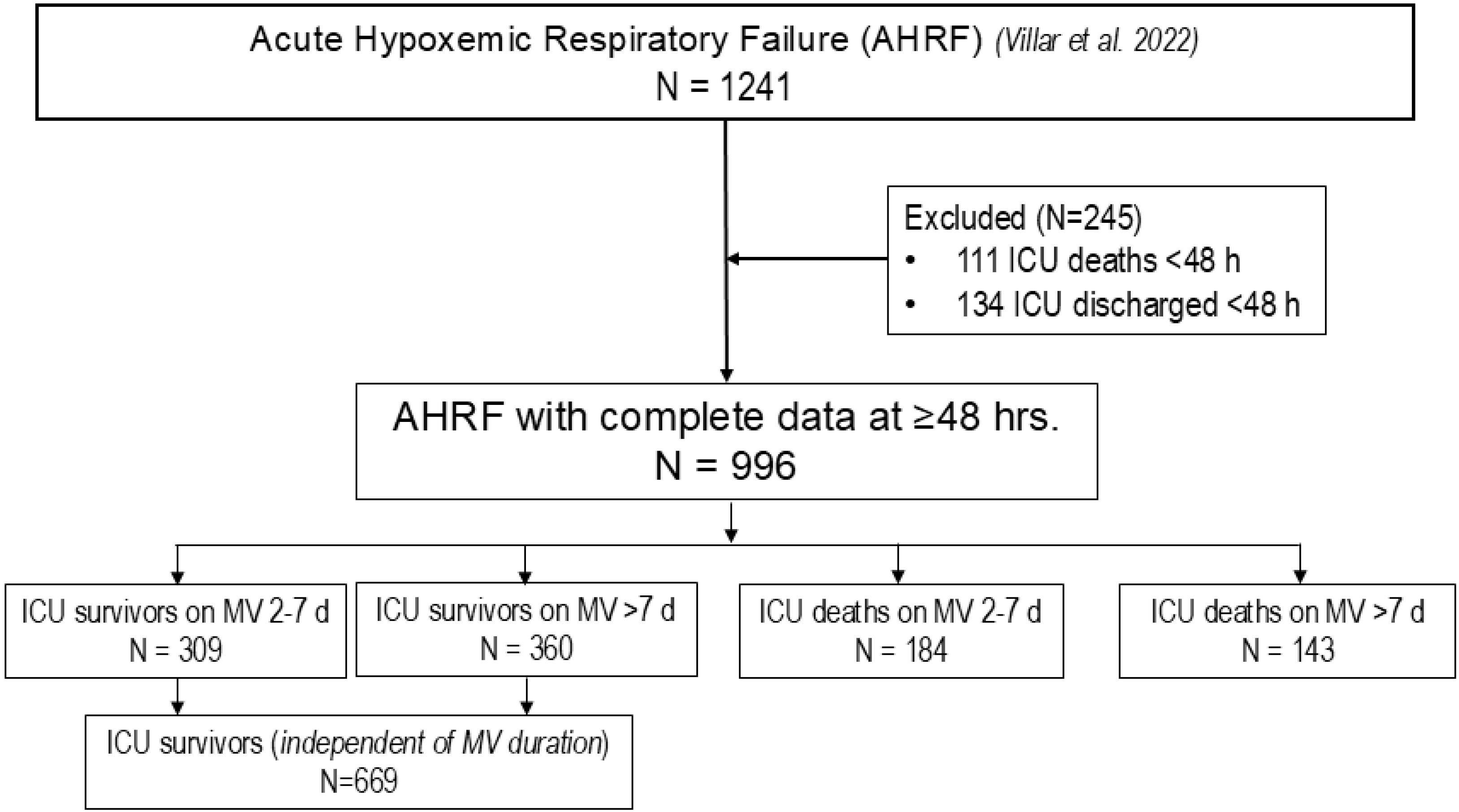
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, V.D.; Memish, Z.A.; Bearman, G. Preventing ventilator-associated pneumonia: A position paper of the International Society for Infectious Diseases, 2024 update. Int. J. Infect. Dis. 2025, 151, 107305. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Dasta, J.F.; McLaughlin, T.P.; Mody, S.H.; Piech, C.T. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit. Care Med. 2005, 33, 1266–1271. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Villar, J. Early paralytic agents for ARDS? Yes, no, and sometimes. N. Engl. J. Med. 2019, 380, 2061–2063. [Google Scholar] [CrossRef]
- Figueroa-Casas, J.B.; Connery, S.M.; Montoya, R.; Dwivedi, A.K.; Lee, S. Accuracy of early prediction of duration of mechanical ventilation by intensivists. Ann. Am. Thorac. Soc. 2014, 11, 182–185. [Google Scholar] [CrossRef]
- Troché, G.; Moine, P. Is the duration of mechanical ventilation predictable? Chest 1997, 112, 745–751. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, J.; Deng, Y.; Tu, L.; Ding, Y.; Zhang, Y.; Yuan, X.; Xu, K.; Guo, L.; Gao, N. Advances in machine learning for mechanically ventilated patients. Int. J. Gen. Med. 2025, 18, 3301–3311. [Google Scholar] [CrossRef]
- Villar, J.; González-Martín, J.M.; Fernández, C.; Soler, J.A.; Ambrós, A.; Pita-García, L.; Fernández, L.; Ferrando, C.; Arocas, B.; González-Vaquero, M.; et al. Predicting the length of mechanical ventilation in acute respiratory disease syndrome using machine learning: The PIONEER study. J. Clin. Med. 2024, 13, 1811. [Google Scholar] [CrossRef]
- Mendiratta, S.; Mukkelli, V.G.; Kayal, E.B.; Khanna, P.; Mehndiratta, A. Predicting mechanical ventilation duration I ICU patients: A data-driven machine learning approach for clinical decision-making. Digit. Health 2025, 11, 20552076251352988. [Google Scholar] [CrossRef]
- Dam, T.A.; Roggeveen, L.F.; van Diggelen, F.; Fleuren, L.M.; Jagesar, A.R.; Otten, M.; de Vries, H.J.; Gommers, D.; Cremer, O.L.; Bosman, R.J.; et al. Predicting responders to prone positioning in mechanically ventilated patients with COVID-19 using machine learning. Ann. Intensive Care 2022, 12, 99. [Google Scholar] [CrossRef]
- Parreco, J.; Hidalgo, A.; Parks, J.J.; Kozol, R.; Rattan, R. Using artificial intelligence to predict prolonged mechanical ventilation and tracheostomy placement. J. Surg. Res. 2018, 228, 179–187. [Google Scholar] [CrossRef]
- Ding, X.F.; Li, J.B.; Liang, H.Y.; Wang, Z.Y.; Jiao, T.T.; Liu, Z.; Yi, L.; Bian, W.S.; Wang, S.P.; Zhu, X.; et al. Predictive model for acute respiratory distress syndrome events in ICU patients in China using machine learning algorithms: A secondary analysis of a cohort study. J. Transl. Med. 2019, 17, 326. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Huang, T.; Yang, R.; Cheng, H.; Wang, H.; Yin, H.; Lyu, J. Developing an explainable machine learning model to predict the mechanical ventilation duration of patients with ARDS in intensive care units. Heart Lung 2023, 58, 74–81. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- Villar, J.; Mora-Ordoñez, J.M.; Soler, J.A.; Mosteiro, F.; Vidal, A.; Ambrós, A.; Fernández, L.; Murcia, I.; Civantos, B.; Romera, M.A.; et al. The PANDORA study: Prevalence and outcome of acute hypoxemic respiratory failure in the pre-Covid-19 era. Crit. Care Explor. 2022, 4, e0684. [Google Scholar] [CrossRef]
- Calster, B.V.; Vergouwe, Y.; Looman, C.W.N.; Belle, V.V.; Timmerman, D.; Steyerberg, E.W. Assessing the discriminative ability of risk models for more than two outcome categories. Eur. J. Epidemiol. 2012, 27, 761–770. [Google Scholar] [CrossRef]
- Villar, J.; Martínez, D.; Mosteiro, F.; Ambrós, A.; Añón, J.M.; Ferrando, C.; Soler, J.A.; Montiel, R.; Vidal, A.; Conesa-Cayuela, L.A.; et al. Is overall mortality the right composite endpoint in clinical trials of acute respiratory distress syndrome? Crit. Care Med. 2018, 46, 892–899. [Google Scholar] [CrossRef]
- Villar, J.; Ambrós, A.; Mosteiro, F.; Martínez, D.; Fernández, L.; Ferrando, C.; Carriedo, D.; Soler, J.A.; Parrilla, D.; Hernández, M.; et al. A prognostic enrichment strategy for selection of patients with acute respiratory distress syndrome in clinical trials. Crit. Care Med. 2019, 47, 377–385. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, S.; Liu, R.; Wang, H.; Zheng, J.; Yu, K. Risk factors for outcomes of acute respiratory distress syndrome patients: A retrospective study. J. Thorac. Dis. 2019, 11, 673–685. [Google Scholar] [CrossRef]
- Le, S.; Pellegrini, E.; Green-Saxena, A.; Summers, C.; Hoffman, J.; Calvert, J.; Das, R. Supervised machine learning for the early prediction of acute respiratory distress syndrome (ARDS). J. Crit. Care 2020, 60, 96–102. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in Intensive Care Units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, S.I. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 2012, 17, 228–243. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The proposal to lower p value thresholds to 0.005. JAMA 2018, 319, 1429–1430. [Google Scholar] [CrossRef]
- Rashid, M.; Ramakrishnan, M.; Chandran, V.P.; Nandish, S.; Nair, S.; Shanbhag, V.; Thunga, G. Artificial intelligence in acute respiratory distress syndrome: A systematic review. Artif. Intell. Med. 2022, 131, 102361. [Google Scholar] [CrossRef]
- Maslove, D.M.; Tang, B.; Shankar-Hari, M.; Lawler, P.R.; Angus, D.C.; Baillie, J.K.; Baron, R.M.; Bauer, M.; Buchmann, T.G.; Calfee, C.S.; et al. Redefining critical illness. Nat. Med. 2022, 28, 1141–1148. [Google Scholar] [CrossRef]
- Smit, M.R.; Reddy, K.; Munshi, L.; Bos, L.D.J. Towards precision medicine in respiratory failure. Crit. Care Med. 2025, 53, e656–e664. [Google Scholar] [CrossRef]
- Gee, M.H.; Gotlieb, J.E.; Albertine, K.H.; Kubis, J.M.; Peters, S.P.; Fish, J.E. Physiology of aging related to outcome in the adult respiratory distress syndrome. J. Appl. Physiol. 1990, 69, 822–829. [Google Scholar] [CrossRef]
- Heybati, K.; Deng, J.; Bhandarkar, A.; Zhou, F.; Zamanian, C.; Arya, N.; Bydon, M.; Bauer, P.R.; Gajic, O.; Walkey, A.J.; et al. Outcomes of acute respiratory failure in patients with cancer in the United States. Mayo Clin. Proc. 2024, 99, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Fernández, C.; González-Martín, J.M.; Ferrando, C.; Añón, J.M.; Del Saz-Ortíz, A.M.; Díaz-Lamas, A.; Bueno-González, A.; Fernández, L.; Domínguez-Berrot, A.M.; et al. Respiratory subsets in patients with moderate to severe acute respiratory distress syndrome for early prediction of death. J. Clin. Med. 2022, 11, 5724. [Google Scholar] [CrossRef] [PubMed]
- Shiu, K.K.; Rosen, M.J. Is there a safe plateau pressure threshold for patients with acute lung injury and acute respiratory distress syndrome? Am. J. Respir. Crit. Care Med. 2006, 173, 686, author reply 687. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Battaglini, D.; Pelosi, P. Postoperative respiratory disorders. Curr. Opin. Crit. Care 2016, 22, 379–385. [Google Scholar] [CrossRef]
- Yoshida, R.; Komukai, K.; Kubota, T.; Kinoshita, K.; Fukushima, K.; Yamamoto, H.; Niijima, A.; Matsumoto, T.; Nakayama, R.; Watanabe, M.; et al. The relationship between the initial pH and neurological outcome in patients with out-of-hospital cardiac arrest is affected by the status of recovery of spontaneous circulation on hospital arrival. Heart Vessels 2024, 39, 446–453. [Google Scholar] [CrossRef]
- Martin, D.S.; Gould, D.W.; Shahid, T.; Doidge, J.C.; Cowden, A.; Sadique, Z.; Camsooksai, J.; Charles, W.N.; Davey, M.; Francis-Johnson, A.; et al. Conservative oxygen therapy in mechanically ventilated critically ill patients: The UK-ROX randomized clinical trial. JAMA 2025, 334, 398–408. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, D.; Chao, Y.; Zhang, R.; Guo, L.; Xie, J.; Liu, S.; Zhao, Z.; Huang, Y.; Yang, Y.; et al. Effect of individualized positive end-expiratory pressure titrated by electrical impedance tomography in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2025, 211, 418–421. [Google Scholar] [CrossRef]
- Churpek, M.M.; Gupta, S.; Spicer, A.B.; Parker, W.F.; Fahrenbach, J.; Brenner, S.K.; Leaf, D.E.; for the STOP-COVID investigators. Hospital-level variation in death for critically ill patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 403–411. [Google Scholar] [CrossRef]
- Szakmany, T.; Russell, P.; Wilkes, A.R.; Hall, J.E. Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: Meta-Analysis of randomized controlled trials. Brit. J. Anaesth. 2015, 114, 396–405. [Google Scholar] [CrossRef]
- Young, D.; Harison, D.A.; Cuthbertson, B.H.; Rowan, K.; TracMan Collaborators. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: The TracMan randomized trial. JAMA 2013, 309, 2121–2129. [Google Scholar] [CrossRef]
- Retel Helmrich, I.R.A.; Mikolić, A.; Kent, D.M.; Lingsm, H.F.; Wynants, L.; Steyerberg, E.W.; van Klaveren, D. Does poor methodological quality of prediction modeling studies translate to poor model performance? An illustration in traumatic brain injury. Diagn. Progn. Res. 2022, 6, 8. [Google Scholar] [CrossRef]
- Rose, L.; McGinlay, M.; Amin, R.; Burns, K.E.; Connolly, B.; Hart, N.; Jouvet, P.; Katz, S.; Leasa, D.; Mawdsley, C.; et al. Variation in definition of prolonged mechanical ventilation. Respir. Care 2017, 62, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, N.K.; Hill, A.D.; Shiah, M.S.; Hua, M.; Gershengorn, H.B.; Ferrando-Vivas, P.; Harrison, D.; Rowan, K.; Lindenauer, P.K.; Wunsch, H. Use of mechanical ventilation across 3 countries. JAMA Intern. Med. 2023, 183, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Romano, T.G.; Correia, M.D.T.; Mendes, P.V.; Zampieri, F.G.; Maciel, A.T.; Park, M. Metabolic acid-base adaptation triggered by acute persistent hypercapnia in mechanically ventilated patients with acute respiratory distress syndrome. Rev. Bras. Ter. Intensiva 2016, 28, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef]
- Perkins, G.D.; Mistay, D.; Gates, S.; Gao, F.; Suelson, C.; Hart, N.; Camporota, L.; Karley, J.; Carle, C.; Paramasivana, E.; et al. Effect of protocolized weaning with early extubation to noninvasive ventilation vs. invasive weaning on time to liberation from mechanical ventilation among patients with respiratory failure. The Breathe randomized clinical trial. JAMA 2018, 320, 1881–1888. [Google Scholar] [CrossRef]
- Burns, K.E.A.; Rizvi, L.; Cook, D.J.; Lebovic, G.; Dodek, P.; Villar, J.; Slutsky, A.S.; Jones, A.; Kapadia, F.N.; Gattas, D.J.; et al. Ventilator weaning and discontinuation practice for critically ill patients. JAMA 2021, 325, 1173–1184. [Google Scholar] [CrossRef]

| Variables | N = 1241 T0 | N = 996 T0 | p-Value |
|---|---|---|---|
| Age, years, median (IQR) Age, years, mean ± SD | 65 (54–74) 62.8 ± 14.3 | 65 (54–73) 62.1 ± 14.6 | 0.254 0.254 |
| Sex | n (%: 95%CI) | N (%: 95%CI) | |
| Male | 834 (67.2: 64.6 to 69.8) | 680 (68.3: 65.4 to 71.2) | 0.580 |
| Female | 407 (32.8: 30.2 to 35.4) | 316 (31.7; 28,8 to 34.6) | 0.580 |
| Etiology (reasons for invasive MV), n (%: 95%CI) | |||
| Post-surgery | 208 (16.8: 14.7 to 18.8) | 144 (14.5: 12.3 to 16.6) | 0.138 |
| Stroke or coma | 191 (15.4: 13.4 to 17.4) | 162 (16.3: 14.0 to 18.6) | 0.562 |
| Pneumonia | 169 (13.6: 11.7 to 15.5) | 149 (15.0: 12.7 to 17.2) | 0.346 |
| Sepsis/acute pancreatitis | 152 (12.3;10.4 to 14.1) | 118 (11.8: 9.8 to 13.9) | 0.718 |
| Trauma | 151 (12.2: 10.4 to 14.0) | 135 (13.6: 11.4 to 15.7) | 0.325 |
| Cardiac arrest | 117 (9.4: 7.8 to 11.1) | 88 (8.8: 7.1 to 10.6) | 0.625 |
| Cardiac failure/fluid overload | 62 (5.0: 3.8 to 6.2) | 49 (4.9: 3.6 to 6.3) | 0.914 |
| Aspiration/inhalation | 49 (4.0: 2.9 to 5.0) | 45 (4.5: 3.2 to 5.8) | 0.559 |
| Others | 137 (11.0: 9.3 to 12.8) | 101 (10.1: 8.3 to 12.0) | 0.492 |
| Unknown etiology | 5 (0.4: 0 to 0.7) | 5 (0.5: 0.1 to 0.9) | 0.724 |
| APACHE II score, mean ± SD | 21.0 ± 8.0 § | 20.7 ± 7.5 | 0.365 |
| SOFA score, mean ± SD | 8.95 ± 3.47 | 8.93 ± 3.27 | 0.890 |
| FiO2, mean ± SD | 0.63 ± 0.22 | 0.64 ± 0.22 | 0.285 |
| PaO2, mmHg, mean ± SD | 98.9 ± 34.6 | 98.8 ± 34.7 | 0.946 |
| PaO2/FiO2, mmHg, mean ± SD | 170.5 ± 64.1 | 169.9 ± 64.7 | 0.827 |
| PaCO2, mmHg, mean ± SD | 46.1 ±12.4 | 46.1 ± 12.1 | 1.0 |
| pH, mean ± SD | 7.32 ± 0.11 | 7.32 ± 0.11 | 1.0 |
| VT, mL/kg PBW, mean ± SD | 6.88 ± 1.07 | 6.90 ± 1.06 | 0.659 |
| Respiratory rate, ventilator cycles/min, mean ± SD | 19.7 ± 4.4 | 19.9 ± 4.4 | 0.285 |
| Minute ventilation, L/min, mean ± SD | 8.6 ± 2.1 | 8,7 ± 2.1 | 0.263 |
| PEEP, cmH2O, mean ± SD | 7.8 ± 2.8 | 8.0 ± 2.9 | 0.100 |
| Plateau pressure, cmH2O, mean ± SD | 22.3 ± 5.5 | 22.3 ± 5.4 | 1.0 |
| Driving pressure, cmH2O, mean ± SD | 14.5 ± 4.9 | 14.3 ± 4.7 | 0.329 |
| No. extrapulmonary OF, mean ± SD | 1.72 ± 1.05 | 1.70 ± 1.01 | 0.649 |
| Days from last day of MV to ICU discharge, median (IQR) | 2 (0–5) | 2 (0–6) | 0.247 |
| All-cause ICU mortality, n (%: 95%CI) | 438 (35.3: 32.6 to 38.0) | 327 (32.8: 29.9 to 35.8) | 0.216 |
| All-cause hospital mortality, n (%: 95%CI) | 514 (41.4: 38.7 to 44.2) | 393 (39.5: 36.4 to 42.5) | 0.363 |
| Variables at T48 | Selected Variables |
|---|---|
| Age | X |
| Sex | |
| Arterial hypertension (comorbidity) | X |
| Diabetes (comorbidity) | X |
| Obesity (comorbidity) | |
| COPD (comorbidity) | |
| Cardiac failure (comorbidity) | |
| Malignancy (comorbidity) | X |
| Immunocompromised (comorbidity) | |
| Chronic renal failure (comorbidity) | X |
| SOFA score | X |
| VT (kg/min PBW) | X |
| FiO2 | |
| Respiratory rate | |
| PEEP | X |
| Plateau pressure | X |
| PaO2 | X |
| PaO2/FiO2 ratio | |
| PaCO2 | |
| pH | X |
| Number of organ failures | X |
| Minute ventilation (liters/min) |
| Variables | ICU Survivors vs. ICU Deaths on MV ≤ 7 Days | ICU Survivors vs. ICU Deaths on MV > 7 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | OR (95% CI) | p-Value | β | SE | OR (95% CI) | p-Value | |
| Intercept | 35.6 | 0.02 | 2.78 × 1015 (2.68–2.89) | <0.001 | 1.47 | 0.02 | 4.33 (4.16–4.50) | <0.001 |
| Age | 0.03 | 0.01 | 1.03 (1.02–1.05) | <0.001 | 0.04 | 0.01 | 1.04 (1.02–1.06) | <0.001 |
| Arterial hypertension: No | 0 (ref) | - | 1 (ref) | - | 0 (ref) | - | 1 (ref) | - |
| Arterial hypertension: Yes | 0.5 | 0.23 | 1.65 (1.04–2.62) | 0.032 | 0.05 | 0.25 | 1.05 (0.64–1.71) | 0.856 |
| Diabetes: No | 0 (ref) | - | 1 (ref) | - | 0 (ref) | - | 1 (ref) | - |
| Diabetes: Yes | 0.04 | 0.23 | 1.04 (0.66–1.65) | 0.853 | 0.63 | 0.24 | 1.88 (1.17–3.03) | 0.009 |
| Malignancy: No | 0 (ref) | - | 1 (ref) | - | 0 (ref) | - | 1 (ref) | - |
| Malignancy: Yes | 0.01 | 0.30 | 1.01 (0.56–1.81) | 0.969 | 0.56 | 0.29 | 1.74 (0.99–3.08) | 0.056 |
| Chronic renal failure: No | 0 (ref) | - | 1 (ref) | - | 0 (ref) | - | 1 (ref) | - |
| Chronic renal failure: Yes | −0.7 | 0.38 | 0.49 (0.24–1.04) | 0.062 | 0.10 | 0.35 | 1.10 (0.55–2.21) | 0.783 |
| SOFA T48 | 0.1 | 0.06 | 1.14 (1.02–1.28) | 0.026 | 0.01 | 0.06 | 1.01 (0.89–1.15) | 0.846 |
| VT (kg/min/PBW) T48 | −0.2 | 0.10 | 0.78 (0.64–0.96) | 0.019 | −0.39 | 0.11 | 0.68 (0.55–0.83) | <0.001 |
| PEEP T48 | −0.3 | 0.04 | 0.73 (0.67–0.80) | <0.001 | −0.24 | 0.04 | 0.79 (0.72–0.86) | <0.001 |
| Pplat T48 | 0.2 | 0.02 | 1.24 (1.19–1.30) | <0.001 | 0.26 | 0.02 | 1.30 (1.23–1.36) | <0.001 |
| PaO2 T48 | 0.01 | 0 | 1.01 (1–1.01) | 0.019 | 0 | 0 | 1 (1–1.01) | 0.293 |
| pH T48 | −5.7 | 0.14 | 0 (0–0) | <0.001 | −1.10 | 0.15 | 0.33 (0.25–0.45) | <0.001 |
| Number of organ failures T48 | 0.6 | 0.19 | 1.73 (1.18–2.52) | 0.005 | 0.73 | 0.21 | 2.08 (1.38–3.13) | <0.001 |
| Techniques | Global AUC (95%CI) | Accuracy AUC (95% CI) | ICU Survivors vs. ICU Deaths on MV ≤ 7 Days AUC (95% CI) | ICU Survivors vs. ICU Deaths on MV > 7 Days AUC (95% CI) |
|---|---|---|---|---|
| Multilayer Perceptron | 0.78 (0.74–0.81) | 0.74 (0.68–0.78) | 0.86 (0.80–0.92) | 0.86 (0.80–0.93) |
| Random Forest | 0.73 (0.70–0.76) | 0.70 (0.65–0.75) | 0.79 (0.72–0.87) | 0.78 (0.69–0.86) |
| Support Vector Machine | 0.66 (0.60–0.69) | 0.67 (0.63–0.71) | 0.66 (0.0.58–0.75) | 0.73 (0.64–0.82) |
| Multinomial Regression | 0.75 (0.73–0.78) | 0.72 (0.67–0.76) | 0.83 (0.76–0.90) | 0.84 (0.77–0.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar, J.; González-Martín, J.M.; Fernández, C.; Soler, J.A.; Rey-Abalo, M.; Mora-Ordóñez, J.M.; Ortiz-Díaz-Miguel, R.; Fernández, L.; Murcia, I.; Robaglia, D.; et al. Predicting Outcome and Duration of Mechanical Ventilation in Acute Hypoxemic Respiratory Failure: The PREMIER Study. J. Clin. Med. 2025, 14, 7903. https://doi.org/10.3390/jcm14227903
Villar J, González-Martín JM, Fernández C, Soler JA, Rey-Abalo M, Mora-Ordóñez JM, Ortiz-Díaz-Miguel R, Fernández L, Murcia I, Robaglia D, et al. Predicting Outcome and Duration of Mechanical Ventilation in Acute Hypoxemic Respiratory Failure: The PREMIER Study. Journal of Clinical Medicine. 2025; 14(22):7903. https://doi.org/10.3390/jcm14227903
Chicago/Turabian StyleVillar, Jesús, Jesús M. González-Martín, Cristina Fernández, Juan A. Soler, Marta Rey-Abalo, Juan M. Mora-Ordóñez, Ramón Ortiz-Díaz-Miguel, Lorena Fernández, Isabel Murcia, Denis Robaglia, and et al. 2025. "Predicting Outcome and Duration of Mechanical Ventilation in Acute Hypoxemic Respiratory Failure: The PREMIER Study" Journal of Clinical Medicine 14, no. 22: 7903. https://doi.org/10.3390/jcm14227903
APA StyleVillar, J., González-Martín, J. M., Fernández, C., Soler, J. A., Rey-Abalo, M., Mora-Ordóñez, J. M., Ortiz-Díaz-Miguel, R., Fernández, L., Murcia, I., Robaglia, D., Añón, J. M., Ferrando, C., Parrilla, D., Dominguez-Berrot, A. M., Cobeta, P., Martínez, D., Amaro-Harpigny, A., Andaluz-Ojeda, D., Fernández, M. M., ... Szakmany, T. (2025). Predicting Outcome and Duration of Mechanical Ventilation in Acute Hypoxemic Respiratory Failure: The PREMIER Study. Journal of Clinical Medicine, 14(22), 7903. https://doi.org/10.3390/jcm14227903








