Systematic Review of Extracorporeal Membrane Oxygenation in Adult Sickle Cell Disease
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethics
2.2. Study Procedure
3. Results
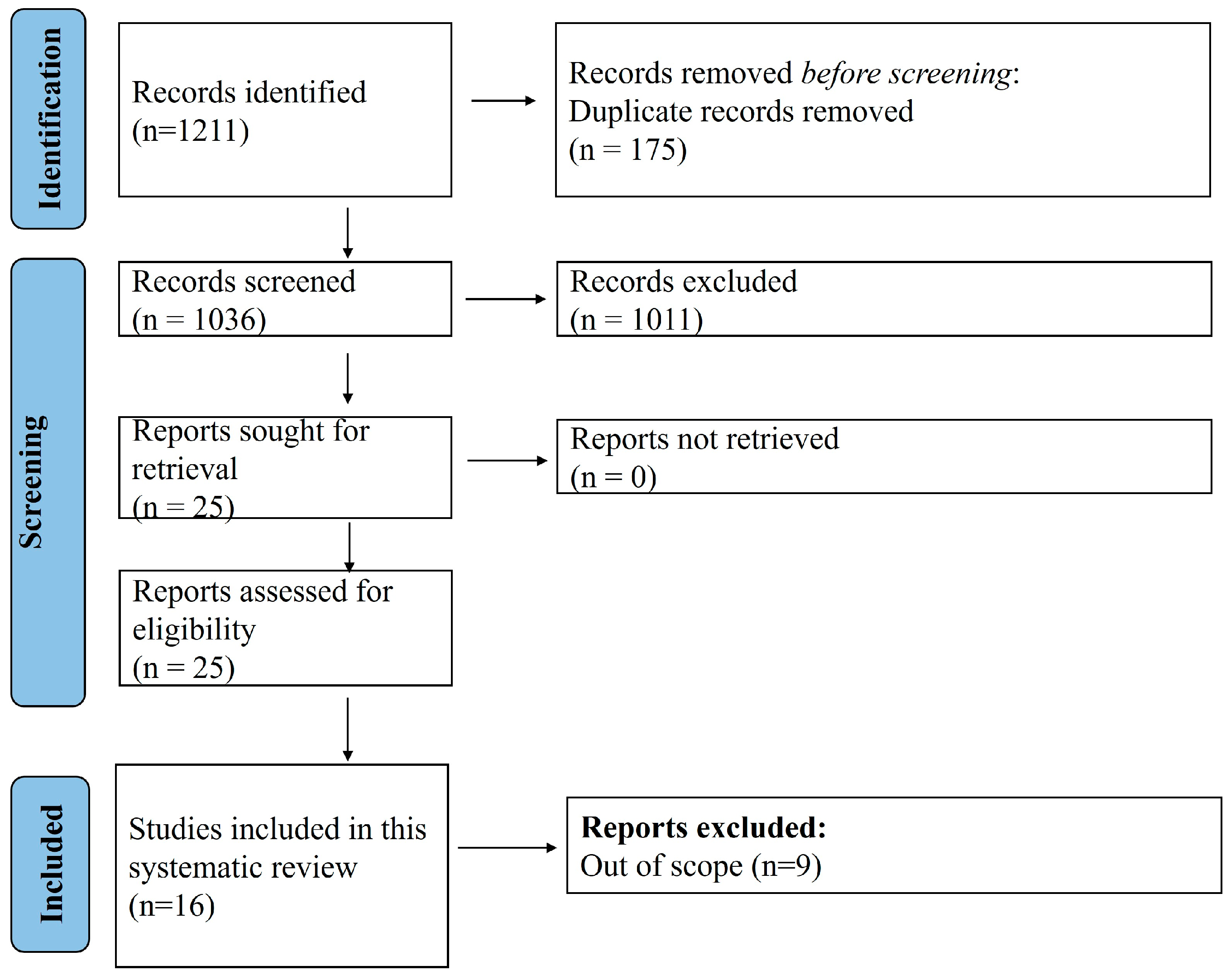
3.1. Search Results and Demographic Characteristics of Included Case Reports
3.2. Laboratory Investigation Findings in the Reported Cases
3.3. Management and Outcomes Reported with ECMO
4. Discussion
4.1. Key Findings
4.2. Comparison with Existing Literature
4.3. VA ECMO: A High-Risk Intervention with Limited Efficacy
4.4. VV ECMO: Improved Outcomes in Acute Chest Syndrome
4.5. Strengths, Limitations and Way Forward
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gladwin, M.T.; Sachdev, V. Cardiovascular Abnormalities in Sickle Cell Disease. J. Am. Coll. Cardiol. 2012, 59, 1123–1133. [Google Scholar] [CrossRef]
- Kim-Shapiro, D.B.; Gladwin, M.T. Nitric oxide pathology and therapeutics in sickle cell disease. Clin. Hemorheol. Microcirc. 2018, 68, 223–237. [Google Scholar] [CrossRef]
- Mehari, A.; Klings, E.S. Chronic Pulmonary Complications of Sickle Cell Disease. Chest 2016, 149, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Neumayr, L.D.; Earles, A.N.; Williams, R.; Lennette, E.T.; Dean, D.; Nickerson, B.; Orringer, E.; McKie, V.; Bellevue, R.; et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Engl. J. Med. 2000, 342, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef]
- Sachdev, V.; Rosing, D.R.; Thein, S.L. Cardiovascular complications of sickle cell disease. Trends Cardiovasc. Med. 2021, 31, 187–193. [Google Scholar] [CrossRef]
- Sheikh, A.B.; Nasrullah, A.; Lopez, E.D.; Tanveer Ud Din, M.; Sagheer, S.; Shah, I.; Javed, N.; Shekhar, R. Sickle Cell Disease-Induced Pulmonary Hypertension: A Review of Pathophysiology, Management, and Current Literature. Pulse 2021, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.G.; Hart, J.P. Current status of ECMO for massive pulmonary embolism. Front. Cardiovasc. Med. 2023, 10, 1298686. [Google Scholar] [CrossRef]
- Harnisch, L.-O.; Moerer, O. Contraindications to the Initiation of Veno-Venous ECMO for Severe Acute Respiratory Failure in Adults: A Systematic Review and Practical Approach Based on the Current Literature. Membranes 2021, 11, 584. [Google Scholar] [CrossRef]
- Filho, R.R.; Joelsons, D.; de Arruda Bravim, B. Extracorporeal membrane oxygenation in critically ill patients with active hematologic and non-hematologic malignancy: A literature review. Front. Med. 2024, 11, 1394051. [Google Scholar] [CrossRef]
- Jain, D.; Mohanty, D. Clinical manifestations of sickle cell disease in India: Misconceptions and reality. Curr. Opin. Hematol. 2018, 25, 171–176. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization (ELSO). ELSO Registry International Summary January 2022; ELSO: Ann Arbor, MI, USA, 2022. [Google Scholar]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar]
- Protocol for ECMO in SCD. Available online: https://osf.io/wrpx4/ (accessed on 3 January 2025).
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE Guidelines: Consensus Based Clinical Case Reporting Guideline Development. Headache J. Head Face Pain 2013, 53, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, A.; Rabin, J.; Rector, R.P.; Wu, Z.J.; Burke, A.P.; Sharifai, N.; Shah, A.; Taylor, B.S.; Gladwin, M.T. Venoarterial Extracorporeal Membrane Oxygenation Therapy in Patients with Sickle Cell Disease: Case Series and Review for Intensive Care Physicians. J. Intensive Care Med. 2025, 40, 929–936. [Google Scholar] [CrossRef]
- de Roux, Q.; Maghrebi, S.; Fiore, A.; Arminot-Fremaux, M.; Dessalle, T.; Lim, P.; Folliguet, T.; Bartolucci, P.; Langeron, O.; Mongardon, N. Venoarterial extracorporeal membrane oxygenation in sickle cell disease for urgent cardiac surgery. Ann. Thorac. Surg. 2020, 109, e161–e162. [Google Scholar] [CrossRef] [PubMed]
- Alashkar, F.; Herbstreit, F.; Carpinteiro, A.; Baum, J.; Tzalavras, A.; Aramayo-Singelmann, C.; Vance, C.; Lenz, V.; Gulbins, E.; Reinhardt, D.; et al. Veno-venous extracorporeal membrane oxygenation in adult patients with sickle cell disease and acute chest syndrome: A single-center experience. Hemoglobin 2020, 44, 71–77. [Google Scholar] [CrossRef]
- Belveyre, T.; Auchet, T.; Levy, B. Spontaneous breathing during extracorporeal membrane oxygenation treatment of sickle cell disease acute chest syndrome. Respir. Med. Case Rep. 2019, 28, 100924. [Google Scholar] [CrossRef]
- Parhar, K.; Parizkova, B.; Jones, N.; Valchanov, K.; Fowles, J.A.; Besser, M.; Telfer, P.; Kaya, B.; Vuylsteke, A.; Rubino, A. Extracorporeal membrane oxygenation for the treatment of adult sickle cell acute chest syndrome. Perfusion 2016, 31, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Grotberg, J.C.; Sullivan, M.; McDonald, R.K.; Despotovic, V.; Witt, C.A.; Reynolds, D.; Lee, J.S.; Kotkar, K.; Masood, M.F.; Kraft, B.D.; et al. Acute chest syndrome from sickle cell disease successfully supported with veno-venous extracorporeal membrane oxygenation. Artif. Organs 2024, 48, 789. [Google Scholar] [CrossRef]
- Sewaralthahab, S.S.; Menaker, J.; Law, J.Y. Successful use of veno-venous extracorporeal membrane oxygenation in an adult patient with sickle cell anemia and severe acute chest syndrome. Hemoglobin 2018, 42, 65–67. [Google Scholar] [CrossRef]
- Chambers, J.; Smith, N.; Sehring, M.; Chittivelu, S. Acute chest syndrome progressing to ARDS in a patient of 25-week gestation. Case Rep. Crit. Care 2018, 2018, 4243569. [Google Scholar] [CrossRef]
- Tanenbaum, M.T.; Shvygina, A.; Sridhar, V.; Vaughn, J.E.; Joseph, M. The use of extracorporeal membrane oxygenation during acute chest crisis in an adult sickle cell disease patient: Identification of pearls and pitfalls. Am. Surg. 2021, 87, 1833–1835. [Google Scholar] [PubMed]
- Al-Sawaf, O.; Köhler, P.; Eichenauer, D.A.; Böll, B.; Kochanek, M.; Shimabukuro-Vornhagen, A. Management of an adult patient with sickle cell disease and acute chest syndrome by veno-venous extracorporeal membrane oxygenation. Ann. Hematol. 2019, 98, 789–791. [Google Scholar] [CrossRef]
- Gillett, D.S.; Gunning, K.E.; Sawicka, E.H.; Bellingham, A.J.; Ware, R.J. Life threatening sickle chest syndrome treated with extracorporeal membrane oxygenation. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 81–82. [Google Scholar] [PubMed]
- Ciociolo, G.; Reid, S.; Halligan, K. Extracorporeal membrane oxygenation (ecmo) therapy in an adult patient with sickle cell disease presenting with severe acute chest syndrome. Chest 2024, 166, A2236–A2237. [Google Scholar] [CrossRef]
- Offer, K.; Sara, D.; Avraham, M.; Gorfil, D.M.; Nisim, I. Severe Acute Respiratory Distress Syndrome in a Patient with Sickle-Cell Anemia Requiring Veno-Venous Extracorporeal Membrane Oxygenation Therapy: Case Report and Review of the Literature. Case Rep. Clin. Med. 2022, 11, 499–506. [Google Scholar] [CrossRef]
- Yalamarti, T.; Ayyoub, J.; Darnell, M.; Kinniry, P. Case Series of Adult Sickle Cell Patient Outcomes on Veno-Venous Extracorporeal Membrane Oxygenation. In D59. Sickle Cell Disease; American Thoracic Society: New York, NY, USA, 2020; p. A7210. [Google Scholar]
- Avgeridou, S.; Djordjevic, I.; Sabashnikov, A.; Eghbalzadeh, K.; Suhr, L.; Ivanov, B.; Merkle, J.; Rustenbach, C.; Mader, N.; Kröner, A.; et al. ECMO therapy in acute chest syndrome for patients with sickle cell disease: A case report and literature review. SN Compr. Clin. Med. 2021, 3, 2356–2361. [Google Scholar] [CrossRef]
- Hoffmann, M.; Geldner, G.; Leschke, M. Lebensbedrohliches akutes Thoraxsyndrom mit hämolytischer Krise bei Sichelzellerkrankung [Life-threatening acute chest syndrome with hemolytic crisis in sickle cell disease. Treatment using a venovenous extracorporeal membrane oxygenation (ECMO)]. Dtsch. Med. Wochenschr. 2011, 136, 2192–2195. [Google Scholar] [CrossRef]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; Mcmullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Remy, T.; Jegard, J.; Chenouard, A.; Maminirina, P.; Liet, J.M.; Couec, M.L.; Joram, N.; Bourgoin, P. ECMO in sickle cell disease: Updated ELSO registry analysis. Artif. Organs 2025, 49, 508–515. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Hebbel, R.P.; Vercellotti, G.M.; Nath, K.A. Endothelial biology in sickle cell disease. Clin. Hemorheol. Microcirc. 2004, 30, 195–208. [Google Scholar] [CrossRef]
- Ataga, K.I.; Key, N.S. Hypercoagulability in sickle cell disease: The role of inflammation and endothelial dysfunction. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 47–53. [Google Scholar]
- Vichinsky, E.P. Pulmonary hypertension in sickle cell disease. N. Engl. J. Med. 2004, 350, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Sachdev, V. Pulmonary hypertension in sickle cell disease: Pathophysiology and treatment. Blood 2008, 112, 918–925. [Google Scholar]
- Novelli, E.M.; Gladwin, M.T. Pulmonary complications of sickle cell disease. Clin. Chest Med. 2016, 37, 479–496. [Google Scholar]
- Abrams, D.; Combes, A.; Brodie, D. ECMO for adult respiratory failure: Current use and evolving applications. Lancet Respir. Med. 2015, 3, 505–516. [Google Scholar]
- van Zuuren, E.J.; Fedorowicz, Z. Low-molecular-weight heparins for managing vaso-occlusive crises in people with sickle cell disease. Cochrane Database Syst. Rev. 2015, 2015, CD010155. [Google Scholar]
- Thelin, S.; Bagge, L.; Hultman, J.; Borowiec, J.; Nilsson, L.; Thorelius, J. Heparin-coated cardiopulmonary bypass circuits reduce blood cell trauma. Experiments in the pig. Eur. J. Cardiothorac. Surg. 1991, 5, 486–491. [Google Scholar] [CrossRef]
- Connes, P.; Renoux, C.; Joly, P.; Nader, E. Vascular pathophysiology of sickle cell disease. Presse Médicale 2023, 52, 104202. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Bishop, M.A. Extracorporeal Membrane Oxygenation in Adults. In StatPearls [Internet]; Updated 21 June 2023; Stat Pearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576426/ (accessed on 21 September 2025).
- Karami, M.; Mandigers, L.; Miranda, D.D.; Rietdijk, W.J.; Binnekade, J.M.; Knijn, D.C.; Lagrand, W.K.; den Uil, C.A.; Henriques, J.P.; Vlaar, A.P.; et al. Survival of patients with acute pulmonary embolism treated with venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J. Crit. Care 2021, 64, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.H.; Gordon, M.; Vender, R.; Pettigrew, S.; Desai, P.; Marchetti, N.; Mamary, A.J.; Panaro, J.; Cohen, G.; Bashir, R.; et al. Venoarterial extracorporeal membrane oxygenation in massive pulmonary embolism-related cardiac arrest: A systematic review. Crit. Care Med. 2021, 49, 760–769. [Google Scholar] [CrossRef]
- Baldetti, L.; Beneduce, A.; Cianfanelli, L.; Falasconi, G.; Pannone, L.; Moroni, F.; Venuti, A.; Sacchi, S.; Gramegna, M.; Pazzanese, V.; et al. Use of extracorporeal membrane oxygenation in high-risk acute pulmonary embolism: A pooled analysis. Artif. Organs 2021, 45, 569–576. [Google Scholar] [CrossRef]
- Mendes, P.D.; Chequer, K.M.; Thomaz, C.M.A.E.; Assunção, G.M.S.; Augusto, F.D.; Fonseca Filho, G.A. Management of refractory chronic pain in sickle cell disease with intrathecal drug delivery system. Hematol. Transfus. Cell Ther. 2023, 45, 399–402. [Google Scholar] [CrossRef]
- Ballas, S.K. Indications for RBC Exchange Transfusion in Patients with Sickle Cell Disease: Revisited. Ann. Clin. Lab. Sci. 2019, 49, 836–837. [Google Scholar]
- Braaten, J.A.; Dillon, B.S.; Wothe, J.K.; Olson, C.P.; Lusczek, E.R.; Sather, K.J.; Beilman, G.J.; Brunsvold, M.E. Extracorporeal Membrane Oxygenation Patient Outcomes Following Restrictive Blood Transfusion Protocol. Crit. Care Explor. 2023, 5, e1020. [Google Scholar] [CrossRef] [PubMed]
- Klings, E.S.; Machado, R.F.; Barst, R.J.; Morris, C.R.; Mubarak, K.K.; Gordeuk, V.R.; Kato, G.J.; Ataga, K.I.; Gibbs, J.S.; Castro, O.; et al. An official American Thoracic Society clinical practice guideline: Diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am. J. Respir. Crit. Care Med. 2014, 189, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
| Author | Country | Case | Age (Years) | Gender | Concomitant Diseases | Initial Diagnosis |
|---|---|---|---|---|---|---|
| Grazioli et al. [16] | USA | 1 | 38 | F | None | VOC, Pulmonary Embolism |
| USA | 2 | 48 | M | Beta thalassemia | VOC | |
| USA | 3 | 60 | F | Beta Thalassemia and ESRD. | Fulminant colitis requiring colectomy | |
| de Roux et al. [17] | France | 4 | 35 | F | CKD; RHD; Aortic and Mitral Valve replacement | Cardiogenic shock, complicated with anuria and acute respiratory failure. |
| Alashkara et al. [18] | Germany | 5 | 18 | F | None | VOC, ACS |
| Germany | 6 | 20 | F | Beta thalassemia, cholecystectomy, splenectomy, opioid-induced paralytic ileus, left lower DVT | VOC | |
| Germany | 7 | 38 | F | left artificial hip joint replacement, previous pneumonia, and Escherichia coli-associated urosepsis | VOC | |
| Belveyre et al. [19] | France | 8 | 19 | F | chronic pulmonary hypertension (with no right heart dysfunction) and mitral annuloplasty for the treatment of rheumatic valvopathy. Transfusion iron overload. | VOC and UTI |
| France | 9 | 20 | F | VOC | ||
| Parhar et al. [20] | UK | 10 | 18 | F | Exchange for stroke | AGE Salmonella septicemia |
| Grotberg et al. [21] | USA | 11 | 25 | M | None | CAP |
| Sewaralthahab et al. [22] | USA | 12 | 25 | F | Hip AVN, PE, iron overload | ARF |
| Chambers et al. [23] | USA | 13 | 20 | F | Pregnant G1P0 25 WKs | CAP/ACS |
| Tanenbaum et al. [24] | USA | 14 | 32 | F | None | ACS |
| Al-Sawaf et al. [25] | Germany | 15 | 21 | M | H/O Salmonella sepsis with multiple organ failure, intracranial bleedings requiring surgical evacuation and bone infarction of hips and vertebral bodies | VOC |
| Gillett et al. [26] | UK | 16 | 20 | F | None | VOC |
| Ciociolo et al. [27] | USA | 17 | 21 | F | None | VOC |
| Offer et al. [28] | Israel | 18 | 31 | M | beta-thalassemia, G6PD deficiency, chronic leukocytosis and thrombocytosis | ACS |
| Yalamarti et al. [29] | USA | 19 | 24 | M | None | MRSA Bacteremia |
| USA | 20 | 22 | F | Lupus nephritis, recurrent ACS, cardiomegaly | ARF | |
| USA | 21 | 32 | M | Nephrogenic DI | ACS | |
| Avgeridou et al. [30] | Germany | 22 | 19 | M | None | PE |
| Hoffmann et al. [31] | France | 23 | 28 | F | None | ACS |
| Author | Case | Initial Echo Findings | CT Chest Findings | HbS% | Other Labs |
|---|---|---|---|---|---|
| Grazioli et al. [16] | 1 | Severe RV dilation | Diffuse GGO and bilateral subsegmental pulmonary embolisms | 70% | Hb 6.5 g/dL; Lactate > 17 mmol/L; HCO3− 10 mmol; Creatinine 1.9 mg/dL |
| 2 | Severe RV dilation with akinesia of the mid-free wall and normal apical motion. The LV was underfilled with normal but hyperdynamic function. | Bilateral infiltrate on CXR | Not mentioned | Hb 5.7 mg/dL; platelet count 31/L | |
| 3 | Severely dilated RV and severely reduced RV systolic function decreased LV size and filling with normal wall thickness and EF 65–70% | Bilateral upper lobe subsegmental pulmonary emboli and bilateral lower lobe consolidation consistent with pneumonia | Not mentioned | Lactate 5–7 mmol/L; Total bilirubin 8.3 mg/dL | |
| de Roux et al. [17] | 4 | Left ventricular ejection fraction of 0.40, pulmonary systolic arterial pressure of 75 mm Hg, aortic bioprosthetic valve degeneration (mean gradient 68 mm Hg, valve area 0.4 cm2), mitral bioprosthetic valve stenosis (mean gradient 9 mm Hg, valve area 1.3 cm2), and moderate tricuspid regurgitation. | none | Not mentioned | none |
| Alashkara et al. [18] | 5 | Normal PCWP | Bilateral pulmonary consolidations indicating ACS | <30.0% after Ex | WBC 34.3 /L; Hb 7.8 g/dL; Platelets 157/L; LDH 1067 U/L; Total serum bilirubin 1.6 mg/dL; Serum creatinine 0.46 mg/dL; Creatine kinase 59 U/L; Troponin I 224 ng/L; CRP 12 mg/dL. |
| 6 | Normal (EF) of 60.0% mild tricuspid valve insufficiency | Bilateral pulmonary consolidations with a positive air-bronchogram in part with ground-glass components | Not mentioned | Hb 4.1 g/dL; WBC 27.3 /L; Platelets 139.0 /L; LDH 944.0 U/L; Total serum bilirubin 2.8 mg/dL; Serum creatinine 1.39 mg/dL; CK 117 U/L; Troponin I 5321.0 ng/L; CRP 1.5 mg/dL; Procalcitonin 7.17 ng/mL | |
| 7 | Right ventricular strain with dilation of the right ventricle and severe tricuspid regurgitation | Severe bilateral pulmonary infiltrates in addition to a right segmental pulmonary embolism | <5.0% after Exchange | WBC 9.1 /L; Hb 6.4 g/dL; Platelet count 98.0 109/L; LDH 2467 U/L; Total serum bilirubin 2.9 mg/dL; Serum creatinine 0.52 mg/dL; CK 170 U/L; Troponin I 998 ng/L; CRP 22.4 mg/dL; Procalcitonin 1.42 ng/mL | |
| Belveyre et al. [19] | 8 | Acute cor pulmonale and right ventricular failure | Not mentioned | 17% and changed to >5% after exchange therapy | Not mentioned |
| 9 | Acute cor pulmonale with increased SPAP > 60 mmHg, paradoxical septum, mild right ventricular dysfunction (TAPSE at 10 mm), while cardiac output was increased along with moderate mitral regurgitation | Major and bilateral alveolar condensation, cardiomegaly and moderate pleural effusion | 31% | Urea 0.97 g/L—Creatinine 18.3 mg/L | |
| Parhar et al. [20] | 10 | Not mentioned | bilateral lung consolidation ACS | 32% | Hb 6.3 g/dL; WBC 37 /L |
| Grotberg et al. [21] | 11 | RV dilation (5 cm) with preserved systolic function. | right lower lobe consolidation | 77% | Hb 6.3 g/dL; WBC 15.8 /L; Platelet count 93 /L |
| Sewaralthahab et al. [22] | 12 | Normal biventricular function, ejection fraction of 60.0% and mild pulmonary hypertension. | Widespread consolidations in all five lobes | Not mentioned | Hb 8.4 g/dL; WBC 16.8 /L; Platelet count 235 /L; Total bilirubin 1 mg/dL; LDH 284 U/L; Lactate 0.6 mmol/L |
| Chambers et al. [23] | 13 | Not mentioned | Bilateral airspace opacifications, worse on the left | Not mentioned | WBC 53.3 /L-Hb of 8.1 g/dL; Reticulocyte index of 21.8% |
| Tanenbaum et al. [24] | 14 | Right heart failure with a severely enlarged right ventricle, right ventricular volume and pressure overload, mild–moderate tricuspid regurgitation, and a moderately dilated right atrium. Left heart function was normal (ejection fraction approximately 60–65%) | Bilateral lung field opacification. | Difficult exchange | WBC 23.4 /L; Aspartate amino transferase 768 IU/L; Alanine aminotransferase 385 IU/L; Prothrombin time 32.1; INR 3; Troponin O 0.26 ng/mL |
| Al-Sawaf et al. [25] | 15 | Severely reduced left ventricular function with an ejection fraction of less than 20% | Bilateral consolidations | 96% | Chlamydia pneumoniae |
| Gillett et al. [26] | 16 | High pulmonary artery pressure | Bilateral patchy consolidation | 35% | Hb 105 g/L-WBC 7.3 /L; Platelet count 46 /L |
| Ciociolo et al. [27] | 17 | preserved left ventricular ejection fraction, with reduced right ventricular systolic function and right ventricular overload | bilateral pulmonary opacities | 45% | Not mentioned |
| Offer et al. [28] | 18 | increased pulmonary pressure and right ventricular failure | bilateral infiltrate, no PE | 90% | Not mentioned |
| Yalamarti et al. [29] | 19 | elevated PASP with LVEF 65–70 | Not mentioned | Not mentioned | Not mentioned |
| 20 | elevated PASP 41% with LVEF 45% | Not mentioned | Not mentioned | Not mentioned | |
| 21 | elevated PASP 35% with LVEF normal | Not mentioned | Not mentioned | Not mentioned | |
| Avgeridou et al. [30] | 22 | a massive impairment of right heart function | PE repeated 2 days later, bilateral infiltrate | 49% | Platelet count 50 /L |
| Hoffmann et al. [31] | 23 | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Author | Initial Treatment | Indication for ECMO | Type of ECMO | Anticoagulation | Echo Post ECMO | Complications | Duration | Outcome | LOS | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Grazioli et al. [16] | Antibiotics Blood Transfusion Alteplase | Pulmonary embolism with shock | VA | None | LV EF 20% with good contractility, RV size was normal with severely reduced systolic function | Retroperitoneal and peritoneal hemorrhage, coagulopathy, CRRT, Brain edema and herniation | 3 days | death | 3 days | Autopsy: -microscopic pulmonary emboli and fat emboli in alveolar capillaries consistent with ACS. -Fat emboli in glomeruli in the kidneys -Widespread hypoxic–ischemic encephalopathy with occasional petechial hemorrhages and distended capillaries in the cerebrum, suggestive of potential cerebral fat embolism |
| Pain management Intubation Anticoagulation | suspected PE with shock + 3 CPRs | VA | Yes | Not repeated | AKI, ALF, Brain herniation | 3 days | death | 3 days | Autopsy: -Diffuse thrombi and fat emboli in lung capillaries consistent with ACS. -Diffuse fat emboli in the kidneys with acute tubular necrosis. -Petechial hemorrhages and diffuse fat emboli in the brain consistent with cerebral fat embolism. | |
| Intubation-colectomy | Pulmonary Embolism with shock | VA | None | RV decompression and improved function | abdominal compartment syndrome requiring a decompressive laparotomy, bleeding from the inferior border of the pancreas and diffuse raw surface bleeding consistent with coagulopathy | 4 days | death | 4 days | ||
| de Roux et al. [17] | Initial treatment with diuretics and dobutamine, Vasopressor administration and cautious fluid infusion, Exchange transfusion | Cardiac Surgery—post CPB | VA | Yes | none | post-CPB hemorrhage | 17 days | survival | 25 days in ICU and 42 in hospital | |
| Alashkara et al. [18] | Antibiotics Hydration Opioids Nebulized heparin | ACS | VV | unclear | none | None | 49 h | survival | 5 days | allogeneic PBSCT from her HLA-identical brother. |
| Fluids Opioids, Antibiotics, Exchange Tx, Nebulized heparin | ACS | VV | unclear | none | None | 251 h, 30 min | survival | Extubated on day 15 | ||
| Hydration Opioids Antibiotics, Exchange Tx | ACS | VV | Yes | none | transfusion-dependent progressive thrombocytopenia with evidence of a bilateral SDH prior to decannulation | 98 h | survival | unclear | subsequent cerebral CT scan, SDH regressed and the patient was transferred to the normal unit. | |
| Belveyre et al. [19] | Antibiotics, Hydration, Opioids, Intubation, Inhalational nitric oxide | ACS | VV | unclear | Regression of the acute cor pulmonale at day 1 | None | 7 days | survival | Extubated day 16 | Had two ECMO |
| ACS | VV-spontaneous breathing | Yes | increase RV strain treated with diuretics | Hemorrhagic shock occurred within a few hours due to a large femoral hematoma | 19 days | survival | 30 days | |||
| Parhar et al. [20] | Antibiotics, Hydration, Opioids, Intubation, Exchange Tx, IVIG | ACS | VV | Yes | none | None | 10 days | survival | unclear | |
| Grotberg et al. [21] | Antibiotics, Hydration, Opioids, Intubation, Exchange Tx, Paralysis, Proning, Inhaled epoprostenol. | ARDS | VV | None | RV function improved | worsened hemolysis (plasma-free Hb of 110 mg/dL) improved with reducing the flow | 20 | survived | 43 days | |
| Sewaralthahab et al. [22] | Antibiotics, Hydration, Opioids, Intubation, Exchange, Paralysis, Proning | ARDS | VV | Yes | None | spontaneous right pneumothorax, bleeding from tracheostomy site, a urinary tract infection, and DVT | 20 days | survival | 31 days | |
| Chambers et al. [23] | Antibiotics, hydration, opioids, intubation, Exchange, delivery | ARDS | VV | unclear | None | none | <10 days | survival | 12 days | |
| Tanenbaum et al. [24] | Antibiotics, hydration, opioids, intubation, Exchange, | ACS | VA thenplus VV | Unclear | None | irreversible severe brain damage. | 12 days | death | 40 days of withdrawal due to poor neurological recovery | CPR following intubation + transfer + another CPR |
| Al-Sawaf et al. [25] | Antibiotics, hydration, opioids, intubation, Exchange, SLED | ACS | VV | Unclear | None | None | 7 days | survival | Extubated 14 days from decannulation | |
| Gillett et al. [26] | Antibiotics, Hydration, Opioids, Intubation, Exchange Tx Epoprostenol | ACS | VV | Unclear | Improved PAP | Candidemia | 10 days | survival | Not mentioned | |
| Ciociolo et al. [27] | Antibiotics, Hydration, Opioids, Intubation, Exchange Tx | ACS + barotrauma | VV then VA due to high intorpic support | Unclear | None | None | 16 days | survival | Extubated 16 days later and discharged home 44 days later | bilateral subcutaneous emphysema and minor pneumomediastinum after second intubation |
| Offer et al. [28] | Antibiotics, Hydration, Opioids, Intubation, Exchange, Proning | ARDS + Aspiration pneumonitis | VV | Unclear | None | DVT, watershed brain infarct | 7 days | survival | 1 month | |
| Yalamarti et al. [29] | none | ARDS | VV | unclear | not reported | CPR | 21 days | death | ||
| none | ARF | VV than VA | unclear | not reported | cardiogenic shock | >48 days | death | |||
| none | ARF | VV | unclear | not reported | Brain death with severe hypernatremia and nephrogenic DI—refractory hypoxia | 6 | death | |||
| Avgeridou et al. [30] | Alteplase, Antibiotics | PE with Cardiogenic shock | Awake VA ECMO + Thrombectomy | Heparin then Argatroban | not reported | HIT | 6 | survival | 22 days | |
| Hoffmann et al. [31] | ACS | VV | Unclear | 12 | survival | 19 days back to primary hospital | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Taitoon, S.K.E.; Sridharan, K. Systematic Review of Extracorporeal Membrane Oxygenation in Adult Sickle Cell Disease. J. Clin. Med. 2025, 14, 6725. https://doi.org/10.3390/jcm14196725
Al Taitoon SKE, Sridharan K. Systematic Review of Extracorporeal Membrane Oxygenation in Adult Sickle Cell Disease. Journal of Clinical Medicine. 2025; 14(19):6725. https://doi.org/10.3390/jcm14196725
Chicago/Turabian StyleAl Taitoon, Safa Khalil Ebrahim, and Kannan Sridharan. 2025. "Systematic Review of Extracorporeal Membrane Oxygenation in Adult Sickle Cell Disease" Journal of Clinical Medicine 14, no. 19: 6725. https://doi.org/10.3390/jcm14196725
APA StyleAl Taitoon, S. K. E., & Sridharan, K. (2025). Systematic Review of Extracorporeal Membrane Oxygenation in Adult Sickle Cell Disease. Journal of Clinical Medicine, 14(19), 6725. https://doi.org/10.3390/jcm14196725








