Comparison of the 36-Item WHODAS 2.0 Functional Assessment in Older Adults by Face-to-Face or Telephone Interviews: A Randomised Crossover Study
Abstract
1. Introduction
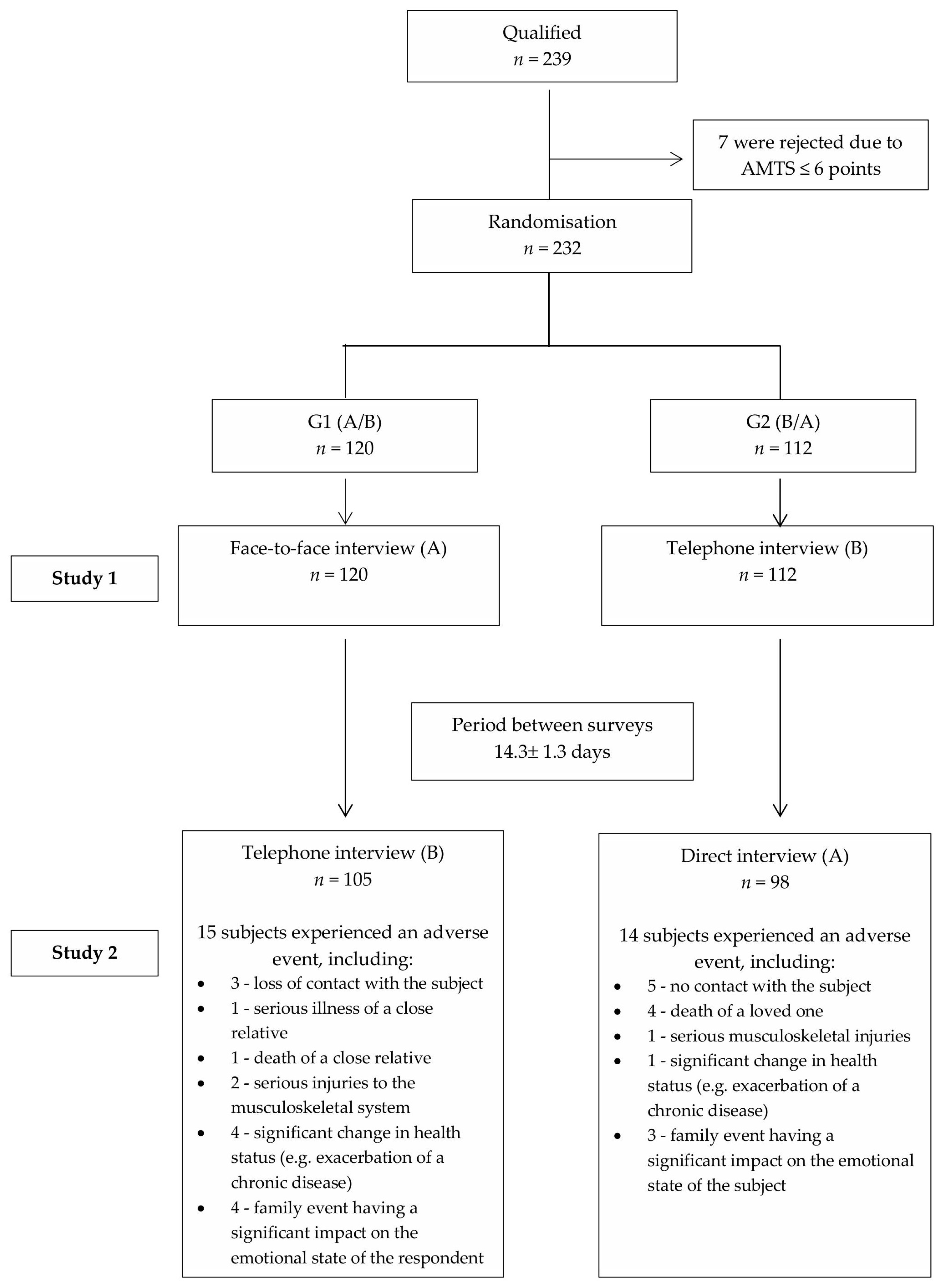
2. Materials and Methods
2.1. Study Design
2.2. Participants and Setting
2.3. Measurement
2.4. Sample Size
2.5. Statistical Analysis
2.6. Ethics Approval
3. Results
Characteristics of the Study Population
4. Discussion
Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- England, K.; Azzopardi-Muscat, N. Demographic trends and public health in Europe. Eur. J. Public Health 2017, 27, 9–13. [Google Scholar] [CrossRef]
- Central Statistical Office. The Situation of Older People in Poland in 2023; GUS: Białystok: Warsaw, Poland, 2024; ISBN 2956-8358. [Google Scholar]
- World Health Organization. World Health Statistics 2024: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Aubert, C.E.; Kabeto, M.; Kumar, N.; Wei, M.Y. Multimorbidity and long-term disability and physical functioning decline in middle-aged and older Americans: An observational study. BMC Geriatr. 2022, 22, 910. [Google Scholar]
- St John, P.D.; Tyas, S.L.; Menec, V.; Tate, R.; Griffith, L. Multimorbidity predicts functional decline in community-dwelling older adults: Prospective cohort study. Can. Fam. Physician 2019, 65, e56–e63. [Google Scholar]
- Zhang, J.; Sun, Y.; Li, A. The prevalence of disability in older adults with multimorbidity: A meta-analysis. Aging Clin. Exp. Res. 2024, 36, 186. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Mehta, K.M.; Boscardin, W.J.; Fortinsky, R.H.; Palmer, R.M.; Kirby, K.A.; Landefeld, C.S. Prediction of recovery, dependence or death in elders who become disabled during hospitalisation. J. Gen. Intern. Med. 2013, 28, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ankuda, C.K.; Ornstein, K.A.; Kelley, A.S. Assessing health care use trajectories after the onset of functional disability: Application of a group-based trajectory model. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77 (Suppl. S1), S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Greysen, S.R.; Cenzer, I.S.; Auerbach, A.D.; Covinsky, K.E. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern. Med. 2015, 175, 559–565. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Chin, K.L.; Curtis, A.J.; Zomer, E.; Zoungas, S.; Liew, D. Recent patterns of multimorbidity among older adults in high-income countries. Popul. Health Manag. 2019, 22, 127–137. [Google Scholar] [CrossRef]
- Jindai, K.; Nielson, C.M.; Vorderstrasse, B.A.; Quiñones, A.R. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005–2012. Prev. Chronic Dis. 2016, 13, E151. [Google Scholar] [CrossRef]
- Pan, C.; Kelifa, M.O.; Liang, J.; Wang, P. Joint trajectories of disability and related factors among older adults in China. Public Health 2021, 199, 96–102. [Google Scholar] [CrossRef]
- Palmer, R.M. Geriatric assessment. Med. Clin. N. Am. 1999, 83, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, E.; Calvani, R.; Marzetti, E.; Cesari, M. Physical functional assessment in older adults. J. Frailty Aging 2021, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Queirós, A.; Cerqueira, M.; Rocha, N.P. Pain intensity is associated with both performance-based disability and self-reported disability in a sample of older adults attending primary health care centres. Disabil. Health J. 2014, 7, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Queirós, A.; Sa-Couto, P.; Rocha, N.P. Self-reported disability: Association with lower extremity performance and other determinants in older adults attending primary care. Phys. Ther. 2015, 95, 1628–1637. [Google Scholar] [CrossRef]
- Federici, S.; Bracalenti, M.; Meloni, F.; Luciano, J.V. World Health Organization Disability Assessment Schedule 2.0: An international systematic review. Disabil. Rehabil. 2017, 39, 2347–2380. [Google Scholar] [CrossRef]
- Lim, C.Y.; In, J. Considerations for crossover design in clinical study. Korean J. Anesthesiol. 2021, 74, 293–299. [Google Scholar] [CrossRef]
- Mills, E.J.; Chan, A.W.; Wu, P.; Vail, A.; Guyatt, G.H.; Altman, D.G. Design, analysis, and presentation of crossover trials. Trials 2009, 10, 27. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Knol, D.L.; Stratford, P.W.; Alonso, J.; Patrick, D.L.; Bouter, L.M.; de Vet, H.C. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: A clarification of its content. BMC Med. Res. Methodol. 2010, 10, 22. [Google Scholar] [CrossRef]
- Üstün, T.B.; Kostanjsek, N.; Chatterji, S.; Rehm, J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Ćwirlej-Sozańska, A.; Wilmowska-Pietruszyńska, A.; Sozański, B. Validation of the Polish version of the World Health Organization Disability Assessment Schedule (WHODAS 2.0) in an elderly population (60–70 years old). Int. J. Occup. Saf. Ergon. 2018, 24, 386–394. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Romanik, W.; Skalska, A.; Gryglewska, B.; Szczerbińska, K.; Derejczyk, J.; Krzyżewski, R.M.; Grodzicki, T.; Gąsowski, J. The comparison of the 1972 Hodkinson’s Abbreviated Mental Test Score (AMTS) and its variants in screening for cognitive impairment. Aging Clin. Exp. Res. 2019, 31, 561–566. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Wądołowska, L. Validation of Methods and Statistical Measures. 2013. Available online: https://www.researchgate.net/publication/271645344_Walidacja_metod_i_mierniki_statystyczne (accessed on 15 October 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 October 2025).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.C.R.; Rocha, R.O.; Araujo, A.K.D.S.; Castro, S.S.; Moreira, M.A.; Nascimento, S.L.D. Reliability and validity of the Brazilian version of the WHO Disability Assessment Schedule (WHODAS 2.0) questionnaire for women with urinary incontinence. Disabil. Rehabil. 2024, 46, 6455–6460. [Google Scholar] [CrossRef]
- Shahedifar, N.; Sadeghi-Bazargani, H.; Asghari-Jafarabadi, M.; Farahbakhsh, M.; Bazargan-Hejazi, S. Psychometric properties of the 12-item WHODAS applied through phone survey: An experience in PERSIAN Traffic Cohort. Health Qual. Life Outcomes 2022, 20, 106. [Google Scholar]
- Cardoso da Silva, A.; Cordeiro de Oliveira, L.; Martins Dos Santos, H.; Caldeira Monteiro, B.; Santos Pereira, G.; Sulyvan de Castro, S.; Silva, S.M. Validation of tele-assessment of disability and health after stroke using WHODAS 2.0 through video call in a middle-income country. Top. Stroke Rehabil. 2025, 32, 428–437. [Google Scholar]
- Martin-Khan, M.; Wootton, R.; Gray, L. A systematic review of the reliability of screening for cognitive impairment in older adults by use of standardised assessment tools administered via the telephone. J. Telemed. Telecare 2010, 16, 422–428. [Google Scholar] [CrossRef]
- Loh, P.K.; Ramesh, P.; Maher, S.; Saligari, J.; Flicker, L.; Goldswain, P. Can patients with dementia be assessed at a distance? The use of telehealth and standardised assessments. Intern. Med. J. 2004, 34, 239–242. [Google Scholar] [CrossRef]
- Dauphinot, V.; Boublay, N.; Moutet, C.; Achi, S.; Bathsavanis, A.; Krolak-Salmon, P. Comparison of instrumental activities of daily living assessment by face-to-face or telephone interviews: A randomised, crossover study. Alzheimers Res. Ther. 2020, 12, 24. [Google Scholar]
- Conwell, Y.; Simning, A.; Driffill, N.; Xia, Y.; Tu, X.; Messing, S.P.; Oslin, D. Validation of telephone-based behavioural assessments in ageing services clients. Int. Psychogeriatr. 2018, 30, 95–102. [Google Scholar] [CrossRef]
- Carlew, A.R.; Fatima, H.; Livingstone, J.R.; Reese, C.; Lacritz, L.; Pendergrass, C.; Bailey, K.C.; Presley, C.; Mokhtari, B.; Cullum, C.M. Cognitive assessment via telephone: A scoping review of instruments. Arch. Clin. Neuropsychol. 2020, 35, 1215–1233. [Google Scholar] [CrossRef]

| Parameter | Total (n = 203) | G1 (n1 = 105) | G2 (n2 = 98) | p-Value | |
|---|---|---|---|---|---|
| Age [years] | Mean (SD) | 73.48 (6.95) | 73.65 (7.18) | 73.31 (6.74) | 0.786 (a) |
| Median (quartiles) | 72 (68–78) | 72 (68–78) | 72 (68–78) | ||
| Range | 62–95 | 62–95 | 65–93 | ||
| Sex | Female | 125 (61.58%) | 65 (61.90%) | 60 (61.22%) | 0.921 (b) |
| Male | 78 (38.42%) | 40 (38.10%) | 38 (38.78%) | ||
| The Abbreviated Mental Test Score | Mean (SD) | 9.28 (0.99) | 9.20 (1.07) | 9.37 (0.89) | 0.446 (a) |
| Median (quartiles) | 10 (9–10) | 10 (9–10) | 10 (9–10) | ||
| Range | 7–10 | 7–10 | 7–10 | ||
| Marital status | In a relationship | 106 (52.22%) | 53 (50.48%) | 53 (54.08%) | 0.607 (b) |
| Single | 97 (47.78%) | 52 (49.52%) | 45 (45.92%) | ||
| Professional activity | Inactive | 185 (91.13%) | 12 (11.43%) | 6 (6.12%) | 0.279 (c) |
| Active | 18 (8.87%) | 93 (88.57%) | 92 (93.88%) | ||
| Place of residence | Urban area | 84 (41.38%) | 59 (56.19%) | 60 (61.22%) | 0.467 (b) |
| Rural area | 119 (58.62%) | 46 (43.81%) | 38 (38.78%) | ||
| Number of people in a household | Mean (SD) | 3.23 (1.60) | 3.17 (1.60) | 3.30 (1.60) | 0.550 (a) |
| Median (quartiles) | 3 (2–4) | 3 (2–4) | 3 (2–4) | ||
| Range | 1–7 | 1–7 | 1–7 | ||
| Education level | Vocational or lower | 97 (47.78%) | 51 (48.57%) | 46 (46.94%) | 0.957 (b) |
| Secondary | 56 (27.59%) | 29 (27.62%) | 27 (27.55%) | ||
| Higher | 50 (24.63%) | 25 (23.81%) | 25 (25.51%) | ||
| Number of chronic diseases | Mean (SD) | 4.94 (2.92) | 5.32 (3.22) | 4.52 (2.52) | 0.102 (a) |
| Median (quartiles) | 5 (3–6) | 5 (3–7) | 4 (3–6) | ||
| Range | 0–18 | 0–18 | 0–15 | ||
| Use of mobility aids | No | 150 (73.89%) | 73 (69.52%) | 77 (78.57%) | 0.142 (b) |
| Yes | 53 (26.11%) | 32 (30.48%) | 21 (21.43%) | ||
| Number of medications taken daily | 4 or more | 96 (47.29%) | 57 (54.29%) | 39 (39.80%) | 0.100 (b) |
| 3 | 45 (22.17%) | 17 (16.19%) | 28 (28.57%) | ||
| 2 | 38 (18.72%) | 17 (16.19%) | 21 (21.43%) | ||
| 1 | 17 (8.37%) | 11 (10.48%) | 6 (6.12%) | ||
| 0 | 7 (3.45%) | 3 (2.86%) | 4 (4.08%) | ||
| Pain intensity in last 30 days | Mean (SD) | 3.76 (2.16) | 3.94 (2.19) | 3.56 (2.11) | 0.264 (a) |
| Median (quartiles) | 4 (2–5) | 4 (3–5) | 3.5 (2–5) | ||
| Range | 0–10 | 0–10 | 0–10 | ||
| Physical activity | Never | 106 (52.22%) | 58 (55.24%) | 48 (48.98%) | 0.616 (b) |
| 1–2 times a week | 70 (34.48%) | 33 (31.43%) | 37 (37.76%) | ||
| 3 or more times a week | 27 (13.30%) | 14 (13.33%) | 13 (13.27%) | ||
| Quality of life in last 14 days self-assessment | Poor | 16 (7.88%) | 10 (9.52%) | 6 (6.12%) | 0.197 (b) |
| Neither good nor bad | 63 (31.03%) | 38 (36.19%) | 25 (25.51%) | ||
| Good | 106 (52.22%) | 50 (47.62%) | 56 (57.14%) | ||
| Very good | 18 (8.87%) | 7 (6.67%) | 11 (11.22%) | ||
| Parameter | Agreement | κ | 95% CI | Agreement (McHugh) | |
|---|---|---|---|---|---|
| D1.1 | 96.55% | 0.947 | 0.908 | 0.986 | Almost perfect |
| D1.2 | 91.13% | 0.873 | 0.817 | 0.929 | Strong |
| D1.3 | 92.61% | 0.888 | 0.834 | 0.943 | Strong |
| D1.4 | 89.16% | 0.849 | 0.788 | 0.909 | Strong |
| D1.5 | 91.63% | 0.795 | 0.706 | 0.884 | Moderate |
| D1.6 | 90.64% | 0.792 | 0.706 | 0.879 | Moderate |
| D2.1 | 92.61% | 0.906 | 0.860 | 0.952 | Almost perfect |
| D2.2 | 92.12% | 0.870 | 0.810 | 0.930 | Strong |
| D2.3 | 92.61% | 0.846 | 0.771 | 0.920 | Strong |
| D2.4 | 89.66% | 0.833 | 0.768 | 0.898 | Strong |
| D2.5 | 88.67% | 0.858 | 0.803 | 0.913 | Strong |
| D3.1 | 93.10% | 0.877 | 0.816 | 0.938 | Strong |
| D3.2 | 94.09% | 0.866 | 0.796 | 0.937 | Strong |
| D3.3 | 98.03% | 0.931 | 0.864 | 0.998 | Almost perfect |
| D3.4 | 89.66% | 0.834 | 0.769 | 0.900 | Strong |
| D4.1 | 95.07% | 0.926 | 0.882 | 0.970 | Almost perfect |
| D4.2 | 94.58% | 0.905 | 0.850 | 0.959 | Almost perfect |
| D4.3 | 94.58% | 0.892 | 0.831 | 0.952 | Strong |
| D4.4 | 91.63% | 0.877 | 0.821 | 0.933 | Strong |
| D4.5 | 86.70% | 0.827 | 0.767 | 0.887 | Strong |
| D5.1 | 93.60% | 0.909 | 0.861 | 0.956 | Almost perfect |
| D5.2 | 93.10% | 0.904 | 0.856 | 0.952 | Almost perfect |
| D5.3 | 88.67% | 0.845 | 0.785 | 0.905 | Strong |
| D5.4 | 91.63% | 0.888 | 0.837 | 0.939 | Strong |
| D6.1 | 94.09% | 0.914 | 0.867 | 0.961 | Almost perfect |
| D6.2 | 94.09% | 0.909 | 0.859 | 0.959 | Almost perfect |
| D6.3 | 91.63% | 0.856 | 0.792 | 0.921 | Strong |
| D6.4 | 85.71% | 0.788 | 0.718 | 0.859 | Moderate |
| D6.5 | 87.68% | 0.824 | 0.759 | 0.888 | Strong |
| D6.6 | 89.16% | 0.842 | 0.780 | 0.904 | Strong |
| D6.7 | 87.19% | 0.794 | 0.722 | 0.865 | Moderate |
| D6.8 | 89.66% | 0.842 | 0.780 | 0.905 | Strong |
| ICC | 95% CI | Agreement (Koo & Li) | |||||
|---|---|---|---|---|---|---|---|
| 0.986 | 0.982 | 0.989 | Excellent | ||||
| Bland–Altman | |||||||
| WHODAS 2.0 total (Mean±SD) | RC | VC | LOA (95% CI) | Bland–Altman index | |||
| Direct | Phone | Difference: Direct-Phone | Lower limit | Upper limit | |||
| 27.3 ± 19.44 | 26.87 ± 19.52 | −0.42 ± 3.26 | 6.52 | 12.03% | −6.81 (−6.99; −6.63) | 5.97 (5.79; 6.14) | 5.42% |
| Subscale | ICC | 95% CI | Agreement (Koo & Li) | |
|---|---|---|---|---|
| Do1 | 0.964 | 0.953 | 0.973 | Excellent |
| Do2 | 0.964 | 0.953 | 0.973 | Excellent |
| Do3 | 0.967 | 0.956 | 0.975 | Excellent |
| Do4 | 0.966 | 0.955 | 0.974 | Excellent |
| Do5.1 | 0.953 | 0.939 | 0.964 | Excellent |
| Do6 | 0.964 | 0.953 | 0.972 | Excellent |
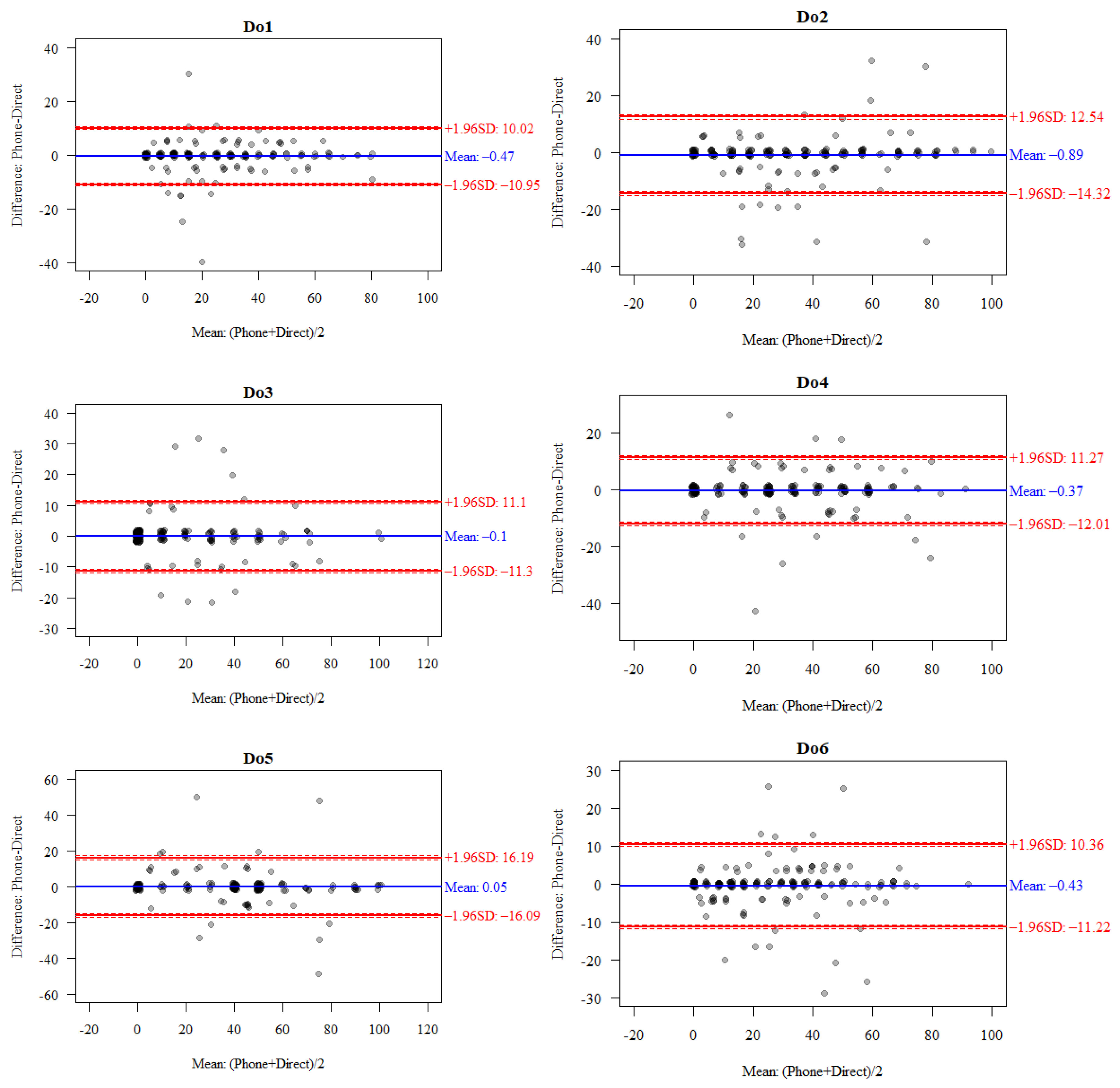
| Subscale | Total (Mean ± SD) | RC | VC | LOA (95% CI) | Bland–Altman Index | |||
|---|---|---|---|---|---|---|---|---|
| Direct | Phone | Difference: Direct-Phone | Lower Limit | Upper Limit | ||||
| Do1 | 23.65 ± 19.88 | 23.18 ± 20.02 | −0.47 ± 5.35 | 10.7 | 22.85 | −10.95 (−11.43; −10.47) | 10.02 (9.54; 10.5) | 3.45% |
| Do2 | 34.27 ± 25.37 | 33.37 ± 25.87 | −0.89 ± 6.85 | 13.71 | 20.26 | −14.32 (−15.11; −13.53) | 12.54 (11.75; 13.33) | 5.42% |
| Do3 | 16.4 ± 22.26 | 16.31 ± 22.02 | −0.1 ± 5.72 | 11.43 | 34.95 | −11.3 (−11.85; −10.75) | 11.1 (10.55; 11.65) | 3.94% |
| Do4 | 28.57 ± 22.89 | 28.2 ± 22.54 | −0.37 ± 5.94 | 11.88 | 20.92 | −12.01 (−12.6; −11.42) | 11.27 (10.68; 11.86) | 4.43% |
| Do5.1 | 36.8 ± 27.31 | 36.85 ± 26.42 | 0.05 ± 8.24 | 16.47 | 22.36 | −16.09 (−17.23; −14.95) | 16.19 (15.05; 17.33) | 4.93% |
| Do6 | 25.64 ± 20.49 | 25.21 ± 20.49 | −0.43 ± 5.51 | 11.01 | 21.66 | −11.22 (−11.73; −10.71) | 10.36 (9.85; 10.87) | 6.40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozańska, A.; Sozański, B.; Wilmowska-Pietruszyńska, A.; Dzięcioł-Anikiej, Z.; Hagner-Derengowska, M.; Wiśniowska-Szurlej, A. Comparison of the 36-Item WHODAS 2.0 Functional Assessment in Older Adults by Face-to-Face or Telephone Interviews: A Randomised Crossover Study. J. Clin. Med. 2025, 14, 7902. https://doi.org/10.3390/jcm14227902
Sozańska A, Sozański B, Wilmowska-Pietruszyńska A, Dzięcioł-Anikiej Z, Hagner-Derengowska M, Wiśniowska-Szurlej A. Comparison of the 36-Item WHODAS 2.0 Functional Assessment in Older Adults by Face-to-Face or Telephone Interviews: A Randomised Crossover Study. Journal of Clinical Medicine. 2025; 14(22):7902. https://doi.org/10.3390/jcm14227902
Chicago/Turabian StyleSozańska, Agnieszka, Bernard Sozański, Anna Wilmowska-Pietruszyńska, Zofia Dzięcioł-Anikiej, Magdalena Hagner-Derengowska, and Agnieszka Wiśniowska-Szurlej. 2025. "Comparison of the 36-Item WHODAS 2.0 Functional Assessment in Older Adults by Face-to-Face or Telephone Interviews: A Randomised Crossover Study" Journal of Clinical Medicine 14, no. 22: 7902. https://doi.org/10.3390/jcm14227902
APA StyleSozańska, A., Sozański, B., Wilmowska-Pietruszyńska, A., Dzięcioł-Anikiej, Z., Hagner-Derengowska, M., & Wiśniowska-Szurlej, A. (2025). Comparison of the 36-Item WHODAS 2.0 Functional Assessment in Older Adults by Face-to-Face or Telephone Interviews: A Randomised Crossover Study. Journal of Clinical Medicine, 14(22), 7902. https://doi.org/10.3390/jcm14227902







