Analgesic Efficacy of Acetaminophen/Ibuprofen as an Adjuvant in Patient-Controlled Analgesia for Postoperative Pain After Oncoplastic Breast Surgery: A Randomized Controlled Trial
Abstract
1. Introduction
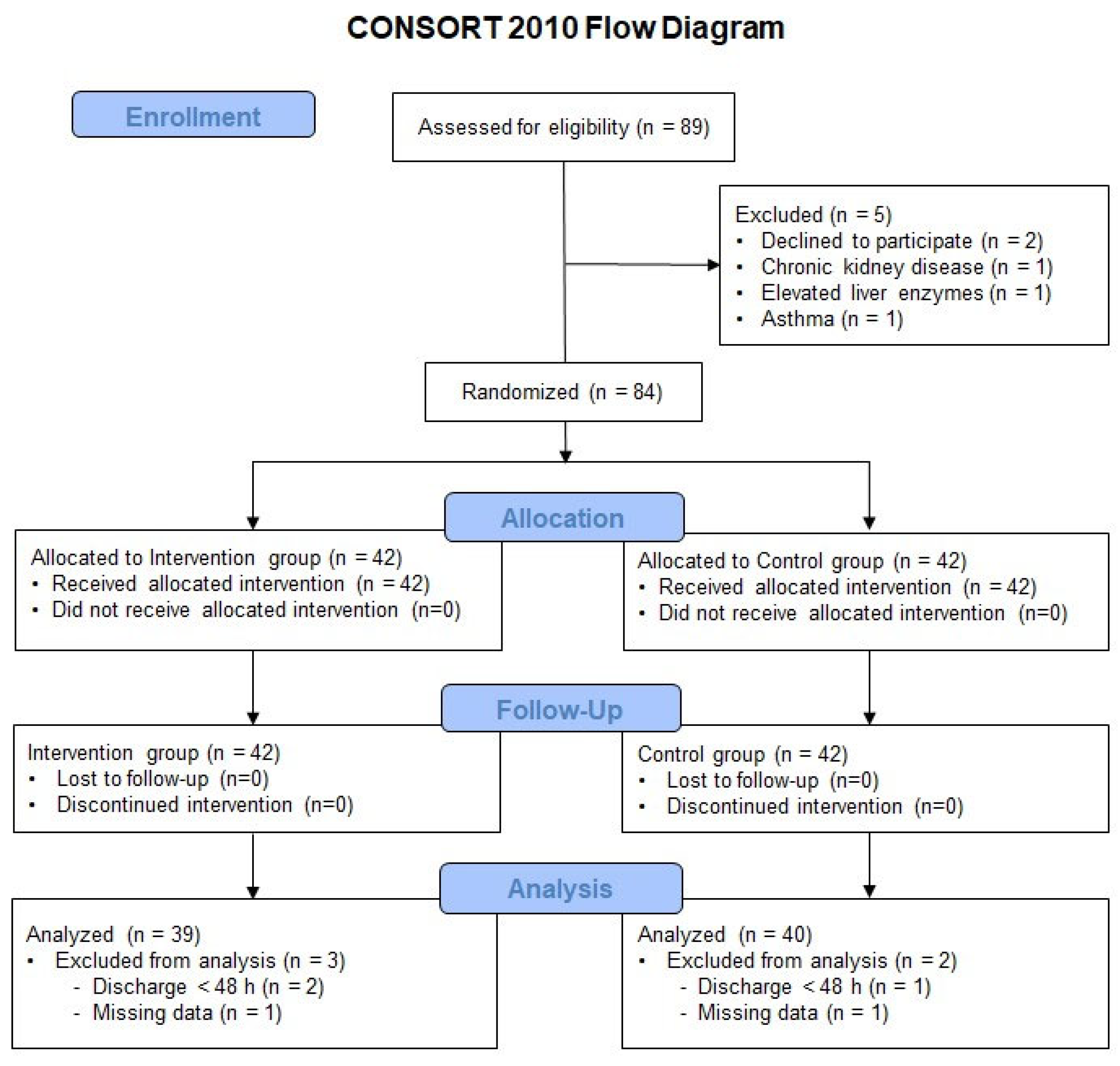
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Interventions
2.4. Perioperative Management
2.5. Outcome Measures
2.6. Statistics
3. Results
3.1. Demographics and Clinical Data
3.2. Postoperative Pain Intensity and Additional Analgesic Requirement
3.3. Analgesic-Related Side Effects and Hospital Stay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCA | Patient-Controlled Analgesia |
| PROSPECT | Procedure-Specific Postoperative Pain Management |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| ERAS | Enhanced Recovery After Surgery |
| ASA | American Society of Anesthesiologists |
| BIS | Bispectral Index |
| NRS | Numerical Rating Scale |
| COX | Cyclooxygenase |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cata, J.P.; Corrales, G.; Speer, B.; Owusu-Agyemang, P. Postoperative acute pain challenges in patients with cancer. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Lemoine, A.; Joshi, G.P.; Van de Velde, M.; Bonnet, F.; the PROSPECT Working Group collaborators. PROSPECT guideline for oncological breast surgery: A systematic review and procedure—Specific postoperative pain management recommendations. Anaesthesia 2020, 75, 664–673. [Google Scholar] [CrossRef]
- Temple-Oberle, C.M.; Shea-Budgell, M.A.M.; Tan, M.; Semple, J.L.M.; Schrag, C.; Barreto, M.; Blondeel, P.M.; Hamming, J.; Dayan, J.; Ljungqvist, O.M.; et al. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast. Reconstr. Surg. 2017, 139, 1056e–1071e. [Google Scholar] [CrossRef]
- Ong, C.K.S.; Seymour, R.A.; Lirk, P.; Merry, A.F. Combining Paracetamol (Acetaminophen) with Nonsteroidal Antiinflammatory Drugs. Anesth. Analg. 2010, 110, 1170–1179. [Google Scholar] [CrossRef]
- Lee, H.-J.; Choi, S.; Yoon, S.; Yoon, S.; Bahk, J.-H. Effect of an intravenous acetaminophen/ibuprofen fixed-dose combination on postoperative opioid consumption and pain after video-assisted thoracic surgery: A double-blind randomized controlled trial. Surg. Endosc. 2024, 38, 3061–3069. [Google Scholar] [CrossRef]
- Retsky, M.; Rogers, R.; Demicheli, R.; Hrushesky, W.J.; Gukas, I.; Vaidya, J.S.; Baum, M.; Forget, P.; DeKock, M.; Pachmann, K. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: Particular relevance to triple negative subgroup. Breast Cancer Res. Treat. 2012, 134, 881–888. [Google Scholar] [CrossRef]
- Shaji, S.; Smith, C.; Forget, P. Perioperative NSAIDs and Long-Term Outcomes After cancer Surgery: A Systematic Review and Meta-analysis. Curr. Oncol. Rep. 2021, 23, 146. [Google Scholar] [CrossRef]
- Horine, S.V.; Rakesh, N.; Nadav, D.; Gulati, A. Perioperative Pain Management in Patients with Cancer-Related Pain: A Narrative Review. Anesth. Analg. 2025, 140, 833–845. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef]
- Neuman, M.D.; Bateman, B.T.; Wunsch, H. Inappropriate opioid prescription after surgery. Lancet 2019, 393, 1547–1557. [Google Scholar] [CrossRef]
- Hoffmann, J.; Wallwiener, D. Classifying breast cancer surgery: A novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. BMC Cancer 2009, 9, 108. [Google Scholar] [CrossRef]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef]
- Clephas, P.R.; Orbach-Zinger, S.; Gosteli-Peter, M.A.; Hoshen, M.; Halpern, S.; Hilber, N.D.; Leo, C.; Heesen, M. Regional analgesia techniques for postoperative pain after breast cancer surgery: A network meta-analysis. Cochrane Database Syst. Rev. 2025, 6, CD014818. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Faist, E. Clinical review: Immunodepression in the surgical patient and increased susceptibility to infection. Crit. Care 2002, 6, 298–305. [Google Scholar] [CrossRef] [PubMed]
- O’nEill, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef]
- Martinez, V.; Beloeil, H.; Marret, E.; Fletcher, D.; Ravaud, P.; Trinquart, L. Non-opioid analgesics in adults after major surgery: Systematic review with network meta-analysis of randomized trials. Br. J. Anaesth. 2016, 118, 22–31. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, M.-H.; Kim, S.I.; Park, S.; Park, H.S.; Oh, E.; Lee, J.H.; Koo, B.-N. The Effects of Perioperative Anesthesia and Analgesia on Immune Function in Patients Undergoing Breast Cancer Resection: A Prospective Randomized Study. Int. J. Med. Sci. 2017, 14, 970–976. [Google Scholar] [CrossRef]
- Boland, J.W.; Pockley, A.G. Influence of opioids on immune function in patients with cancer pain: From bench to bedside. Br. J. Pharmacol. 2017, 175, 2726–2736. [Google Scholar] [CrossRef]
- Tripolt, S.; Neubauer, H.A.; Knab, V.M.; Elmer, D.P.; Aberger, F.; Moriggl, R.; Fux, D.A. Opioids drive breast cancer metastasis through the δ-opioid receptor and oncogenic STAT3. Neoplasia 2021, 23, 270–279. [Google Scholar] [CrossRef]
- Klifto, K.M.; Elhelali, A.; Payne, R.M.; Cooney, C.M.; Manahan, M.A.; Rosson, G.D. Perioperative systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in women undergoing breast surgery. Cochrane Database Syst. Rev. 2021, 11, CD013290. [Google Scholar] [CrossRef]
- Boland, G.P.; Butt, I.S.; Prasad, R.; Knox, W.F.; Bundred, N.J. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br. J. Cancer 2004, 90, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.; Holt, D.; Fulton, A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011, 30, 449–463. [Google Scholar] [CrossRef]
- Moris, D.; Kontos, M.; Spartalis, E.; Fentiman, I.S. The Role of NSAIDs in Breast Cancer Prevention and Relapse: Current Evidence and Future Perspectives. Breast Care 2016, 11, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Chen, P.-T.; Lin, M.-C.; Lin, C.-C.; Wang, S.-H.; Pan, Y.-J. Nonsteroidal Anti-Inflammatory Drugs Reduce Second Cancer Risk in Patients With Breast Cancer: A Nationwide Population-Based Propensity Score-Matched Cohort Study in Taiwan. Front. Oncol. 2021, 11, 756143. [Google Scholar] [CrossRef]
- Forget, P.; Vandenhende, J.; Berliere, M.; Machiels, J.-P.; Nussbaum, B.; Legrand, C.; De Kock, M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 2010, 110, 1630–1635. [Google Scholar] [CrossRef]
- Retsky, M.; Demicheli, R.; Hrushesky, W.J.; Forget, P.; De Kock, M.; Gukas, I.; Rogers, R.A.; Baum, M.; Sukhatme, V.; Vaidya, J.S. Reduction of Breast Cancer Relapses with Perioperative Non-Steroidal Anti-Inflammatory Drugs: New Findings and a Review. Curr. Med. Chem. 2013, 20, 4163–4176. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temperature 2021, 8, 351–371. [Google Scholar] [CrossRef]
- Camu, F.; Borgeat, A.; Heylen, R.J.; Viel, E.J.; Boye, M.E.; Cheung, R.Y. Parecoxib, propacetamol, and their combination for analgesia after total hip arthroplasty: A randomized non-inferiority trial. Acta Anaesthesiol. Scand. 2016, 61, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.E.; Playne, R.; Stanescu, I.; Zhang, J.; Gottlieb, I.J.; Atkinson, H.C. Efficacy and Safety of an Intravenous Acetaminophen/Ibuprofen Fixed-dose Combination After Bunionectomy: A Randomized, Double-blind, Factorial, Placebo-controlled Trial. Clin. Ther. 2019, 41, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, I.J.; Gilchrist, N.; Carson, S.; Stanescu, I.; Atkinson, H. Extending the safety profile of the post-operative administration of an intravenous acetaminophen/ibuprofen fixed dose combination: An open-label, multi-center, single arm, multiple dose study. Biomed. Pharmacother. 2021, 139, 111710. [Google Scholar] [CrossRef]
- Tena-Garitaonaindia, M.; Rubio, J.M.; Martínez-Plata, E.; Martínez-Augustin, O.; de Medina, F.S. Pharmacological bases of combining nonsteroidal antiinflammatory drugs and paracetamol. Biomed. Pharmacother. 2025, 187, 118069. [Google Scholar] [CrossRef]
- Ohnesorge, H.; Bein, B.; Hanss, R.; Francksen, H.; Mayer, L.; Scholz, J.; Tonner, P.H. Paracetamol versus metamizol in the treatment of postoperative pain after breast surgery: A randomized, controlled trial. Eur. J. Anaesthesiol. 2009, 26, 648–653. [Google Scholar] [CrossRef]
- De Oliveira, G.S.; Rodes, M.E.; Bialek, J.; Kendall, M.C.; McCarthy, R.J. Single dose systemic acetaminophen to improve patient reported quality of recovery after ambulatory segmental mastectomy: A prospective, randomized, double-blinded, placebo controlled, clinical trial. Breast J. 2017, 24, 240–244. [Google Scholar] [CrossRef]
- Mitchell, A.; McCrea, P.; Inglis, K.; Porter, G. A Randomized, Controlled Trial Comparing Acetaminophen Plus Ibuprofen versus Acetaminophen Plus Codeine Plus Caffeine (Tylenol 3) after Outpatient Breast Surgery. Ann. Surg. Oncol. 2012, 19, 3792–3800. [Google Scholar] [CrossRef]
- van Helmond, N.; Steegers, M.A.; Moor, G.P.F.-D.; Vissers, K.C.; Wilder-Smith, O.H. Hyperalgesia and Persistent Pain after Breast Cancer Surgery: A Prospective Randomized Controlled Trial with Perioperative COX-2 Inhibition. PLoS ONE 2016, 11, e0166601. [Google Scholar] [CrossRef]
- Graham, L.A.; Illarmo, S.S.; Wren, S.M.; Odden, M.C.; Mudumbai, S.C. Variations in Current Practice and Protocols of Intraoperative Multimodal Analgesia: A Cross-Sectional Study Within a Six-Hospital US Health Care System. Anesth. Analg. 2024, 141, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, K.H.; Jeong, Y.; Lee, K.H.; Yoon, S.; Kim, W.H.; Lee, H. Effect of Fentanyl-Based Intravenous Patient-Controlled Analgesia with and without Basal Infusion on Postoperative Opioid Consumption and Opioid-Related Side Effects: A Retrospective Cohort Study. J. Pain Res. 2020, 13, 3095–3106. [Google Scholar] [CrossRef]
| Variables | Intervention Group (n = 39) | Control Group (n = 40) | p Value |
|---|---|---|---|
| Age (years) | 50.1 ± 10.4 | 48.4 ± 12.3 | 0.515 |
| Body mass index (kg/m2) | 23.2 ± 3.4 | 23.5 ± 3.2 | 0.686 |
| Hypertension | 10 (25.6%) | 6 (15.0%) | 0.239 |
| Diabetes mellitus | 1 (2.6%) | 3 (7.5%) | 0.317 |
| ASA class I/II/III | 25/13/1 | 26/11/3 | 0.556 |
| Preoperative albumin (g/dL) | 4.5 (4.3, 4.6) | 4.5 (4.3, 4.7) | 0.670 |
| Preoperative creatinine (mg/dL) | 0.68 (0.62, 0.79) | 0.68 (0.62, 0.75) | 0.424 |
| Preoperative aspartate transaminase (IU/L) | 24 (20, 29) | 24 (20, 29) | 0.918 |
| Preoperative alanine transferase (IU/L) | 20 (14, 25) | 18 (15, 32) | 0.988 |
| Operation type | |||
| Tissue expander procedures | 4 (10.3%) | 4 (10.0%) | 0.485 |
| Implant procedures | 12 (30.8%) | 9 (22.5%) | |
| Tissue expander removal & implant insertion | 13 (33.3%) | 14 (35.0%) | |
| Implant exchange & capsulectomy | 10 (25.6%) | 10 (25.0%) | |
| Implant procedure & simple mammoplasty | 0 | 3 (7.5%) | |
| Duration of operation (min) | 105.8 ± 46.3 | 110.4 ± 54.4 | 0.697 |
| Duration of anesthesia (min) | 135.7 ± 49.5 | 141.1 ± 56.3 | 0.659 |
| Remifentanil (mcg/kg/min) | 0.06 (0.05, 0.07) | 0.05 (0.05, 0.07) | 0.211 |
| Variables |
Intervention Group
(n = 39) |
Control Group
(n = 40) | p Value |
|---|---|---|---|
| At 1 h after surgery | |||
| NRS at rest | 2 (1, 2) | 2 (1, 2) | 0.789 |
| NRS during movement | 2 (2, 2) | 2 (2, 3) | 0.040 |
| At 6 h after surgery | |||
| NRS at rest | 3 (2, 4) | 3 (2, 4) | 0.319 |
| NRS during movement | 6 (4, 7) | 5 (4, 7) | 0.758 |
| At 24 h after surgery | |||
| NRS at rest | 2 (0, 3) | 2 (1, 2) | 0.630 |
| NRS during movement | 4 (3, 5) | 3 (2, 5) | 0.353 |
| At 48 h after surgery | |||
| NRS at rest | 1 (0, 2) | 1 (0, 1) | 0.398 |
| NRS during movement | 2 (2, 3) | 2 (1, 2) | 0.314 |
| Variables | Intervention Group (n = 39) | Control Group (n = 40) | p Value |
|---|---|---|---|
| Cumulative fentanyl consumption via PCA (mcg) | |||
| During 24 h after surgery | 252.4 (186.7, 289.9) | 299.7 (208.3, 366.6) | <0.001 |
| During 48 h after surgery | 482.4 (283.2, 548.0) | 537.0 (390.9, 586.0) | 0.001 |
| Additional analgesic use beyond PCA, n (%) | |||
| 0–6 h after surgery | |||
| Any rescue analgesic | 22 (56.4%) | 32 (80.0%) | 0.024 |
| Tramadol use | 5 (12.8%) | 9 (22.5%) | 0.260 |
| Acetaminophen | 21 (53.8%) | 28 (70.0%) | 0.139 |
| 6–24 h after surgery | |||
| Any rescue analgesic | 16 (41.0%) | 21 (52.5%) | 0.307 |
| Tramadol use | 6 (15.4%) | 5 (12.5%) | 0.711 |
| Acetaminophen use | 14 (35.9%) | 19 (47.5%) | 0.296 |
| 24–48 h after surgery | |||
| Any rescue analgesic | 11 (28.2%) | 12 (30.0%) | 0.861 |
| Tramadol use | 1 (2.6%) | 1 (2.5%) | 0.986 |
| Acetaminophen use | 10 (25.6%) | 12 (30.0%) | 0.666 |
| Variables |
Intervention Group
(n = 39) |
Control Group
(n = 40) | p Value |
|---|---|---|---|
| Nausea | 17 (43.6%) | 14 (35.0%) | 0.434 |
| Vomiting | 5 (12.8%) | 3 (7.5%) | 0.433 |
| Headache | 2 (5.1%) | 3 (7.5%) | 0.665 |
| Hypotension | 5 (12.8%) | 4 (10.0%) | 0.693 |
| Dizziness | 4 (10.3%) | 10 (25.0%) | 0.086 |
| Pruritus | 0 | 1 (2.5%) | 0.320 |
| Postoperative hospital stay (days) | 3 (2, 3) | 3 (2, 4) | 0.275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Lee, D.W.; Kim, E.J.; Cho, J.S. Analgesic Efficacy of Acetaminophen/Ibuprofen as an Adjuvant in Patient-Controlled Analgesia for Postoperative Pain After Oncoplastic Breast Surgery: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 7901. https://doi.org/10.3390/jcm14227901
Park JH, Lee DW, Kim EJ, Cho JS. Analgesic Efficacy of Acetaminophen/Ibuprofen as an Adjuvant in Patient-Controlled Analgesia for Postoperative Pain After Oncoplastic Breast Surgery: A Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(22):7901. https://doi.org/10.3390/jcm14227901
Chicago/Turabian StylePark, Jin Ha, Dong Won Lee, Eun Jung Kim, and Jin Sun Cho. 2025. "Analgesic Efficacy of Acetaminophen/Ibuprofen as an Adjuvant in Patient-Controlled Analgesia for Postoperative Pain After Oncoplastic Breast Surgery: A Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 22: 7901. https://doi.org/10.3390/jcm14227901
APA StylePark, J. H., Lee, D. W., Kim, E. J., & Cho, J. S. (2025). Analgesic Efficacy of Acetaminophen/Ibuprofen as an Adjuvant in Patient-Controlled Analgesia for Postoperative Pain After Oncoplastic Breast Surgery: A Randomized Controlled Trial. Journal of Clinical Medicine, 14(22), 7901. https://doi.org/10.3390/jcm14227901






