ORBE II Study: Clinical Characteristics and Outcomes After Treatment with Benralizumab According to Airflow Obstruction Status and Smoking Habit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Classification
2.3. Statistical Analysis
3. Results
3.1. ORBE II Cohort Classification Based on Baseline PAO and/or Smoking Habit
3.2. Baseline Characterization of Patient Subgroups
3.3. Benralizumab Response According to PAO Status or Smoking Habit
3.4. Exploring the Role of Smoking in PAO+ Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Investigators ORBE II Study Group and Site
Abbreviations
| ACT | Asthma Control Test |
| CRSwNP | chronic rhinosinusitis with nasal polyps |
| FeNO, | fraction of exhaled nitric oxide |
| FEV1 | forced expiratory volume in 1 s |
| IQR | interquartile range |
| OCS | oral corticosteroid |
| PAO | persistent airflow obstruction |
| SA | severe asthma |
| SD | standard deviation |
| SEA | severe eosinophilic asthma |
| SMK | smoking habit |
References
- Global Strategy for Asthma Management and Prevention (GINA). Available online: www.ginasthma.org (accessed on 10 April 2024).
- Zhang, L.; He, L.; Gong, J.; Liu, C. Risk Factors Associated with Irreversible Airway Obstruction in Asthma: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 9868704. [Google Scholar] [CrossRef]
- Tamada, T.; Sugiura, H.; Takahashi, T.; Matsunaga, K.; Kimura, K.; Katsumata, U.; Ohta, K.; Ichinose, M. Coexisting COPD in elderly asthma with fixed airflow limitation: Assessment by DLco %predicted and HRCT. J. Asthma 2017, 54, 606–615. [Google Scholar] [CrossRef]
- Sears, M.R. Smoking, asthma, chronic airflow obstruction and COPD. Eur. Respir. J. 2015, 45, 586–588. [Google Scholar] [CrossRef]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Haselkorn, T.; Borish, L.; Rasouliyan, L.; Chipps, B.E.; Wenzel, S.E. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: Insights from the TENOR study. Chest 2007, 132, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef]
- Venegas Garrido, C.; Mukherjee, M.; Svenningsen, S.; Nair, P. Eosinophil-mucus interplay in severe asthma: Implications for treatment with biologicals. Allergol. Int. 2024, 73, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Baraldo, S.; Marku, B.; Casolari, P.; Marwick, J.A.; Turato, G.; Romagnoli, M.; Caramori, G.; Saetta, M.; Fabbri, L.M.; et al. Fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, I.; Alving, K.; Dahlen, S.E.; James, A.; Forsberg, B.; Ono, J.; Ohta, S.; Venge, P.; Borres, M.P.; Izuhara, K.; et al. Fixed airflow obstruction relates to eosinophil activation in asthmatics. Clin. Exp. Allergy 2019, 49, 155–162. [Google Scholar] [CrossRef]
- Konstantellou, E.; Papaioannou, A.I.; Loukides, S.; Patentalakis, G.; Papaporfyriou, A.; Hillas, G.; Papiris, S.; Koulouris, N.; Bakakos, P.; Kostikas, K. Persistent airflow obstruction in patients with asthma: Characteristics of a distinct clinical phenotype. Respir. Med. 2015, 109, 1404–1409. [Google Scholar] [CrossRef]
- Polosa, R.; Thomson, N.C. Smoking and asthma: Dangerous liaisons. Eur. Respir. J. 2012, 41, 716–726. [Google Scholar] [CrossRef]
- Bellou, V.; Gogali, A.; Kostikas, K. Asthma and Tobacco Smoking. J. Pers. Med. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Santos, V.; Moreira, M.A.F.; Rosa, A.V.D.; Sobragi, S.M.; Silva, C.; Dalcin, P.T.R. Association of quality of life and disease control with cigarette smoking in patients with severe asthma. Braz. J. Med. Biol. Res. 2022, 55, e11149. [Google Scholar] [CrossRef]
- Baastrup Soendergaard, M.; Hansen, S.; Bjerrum, A.S.; von Bulow, A.; Haakansson, K.E.J.; Hilberg, O.; Ingebrigtsen, T.S.; Johnsen, C.R.; Lock-Johansson, S.; Makowska Rasmussen, L.; et al. Tobacco Exposure and Efficacy of Biologic Therapy in Patients with Severe Asthma: A Nationwide Study From the Danish Severe Asthma Register. J. Allergy Clin. Immunol. Pract. 2024, 12, 146–155.e5. [Google Scholar] [CrossRef]
- Chatkin, J.M.; Dullius, C.R. The management of asthmatic smokers. Asthma Res. Pract. 2016, 2, 10. [Google Scholar] [CrossRef]
- Principe, S.; Richards, L.B.; Hashimoto, S.; Kroes, J.A.; Van Bragt, J.; Vijverberg, S.J.; Sont, J.K.; Scichilone, N.; Bieksiene, K.; Ten Brinke, A.; et al. Characteristics of severe asthma patients on biologics: A real-life European registry study. ERJ Open Res. 2023, 9, 00586-2022. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M.; et al. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkstrom, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta(2)-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Padilla-Galo, A.; Moya Carmona, I.; Ausín, P.; Carazo Fernández, L.; García-Moguel, I.; Velasco-Garrido, J.L.; Andújar-Espinosa, R.; Casas-Maldonado, F.; Martínez-Moragón, E.; Martínez Rivera, C.; et al. Achieving clinical outcomes with benralizumab in severe eosinophilic asthma patients in a real-world setting: Orbe II study. Respir. Res. 2023, 24, 235. [Google Scholar] [CrossRef]
- Chipps, B.E.; Hirsch, I.; Trudo, F.; Alacqua, M.; Zangrilli, J.G. Benralizumab efficacy for patients with fixed airflow obstruction and severe, uncontrolled eosinophilic asthma. Ann. Allergy Asthma Immunol. 2020, 124, 79–86. [Google Scholar] [CrossRef]
- Dunican, E.M.; Elicker, B.M.; Gierada, D.S.; Nagle, S.K.; Schiebler, M.L.; Newell, J.D.; Raymond, W.W.; Lachowicz-Scroggins, M.E.; Di Maio, S.; Hoffman, E.A.; et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Investig. 2018, 128, 997–1009. [Google Scholar] [CrossRef]
- Bumbacea, D.; Campbell, D.; Nguyen, L.; Carr, D.; Barnes, P.J.; Robinson, D.; Chung, K.F. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur. Respir. J. 2004, 24, 122–128. [Google Scholar] [CrossRef]
- Cianchetti, S.; Cardini, C.; Puxeddu, I.; Latorre, M.; Bartoli, M.L.; Bradicich, M.; Dente, F.; Bacci, E.; Celi, A.; Paggiaro, P. Distinct profile of inflammatory and remodelling biomarkers in sputum of severe asthmatic patients with or without persistent airway obstruction. World Allergy Organ. J. 2019, 12, 100078. [Google Scholar] [CrossRef]
- Rogliani, P.; Ora, J.; Puxeddu, E.; Cazzola, M. Airflow obstruction: Is it asthma or is it COPD? Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Madeira Gerardo, A.; da Silva Alves, C.; Gomes, M.; Pardal, C.; Sokolova, A.; Liberato, H.; Mendes, A.; Tonin, F.S.; Duarte-Ramos, F.; Lopes, C. The Effects of Benralizumab on Lung Volumes and Airway Resistance in Severe Eosinophilic Asthma: A Real-World Study. Cureus 2024, 16, e52452. [Google Scholar] [CrossRef]
- Maniscalco, M.; Candia, C.; Calabrese, C.; D’Amato, M.; Matera, M.G.; Molino, A.; Cazzola, M. Impact of biologics on lung hyperinflation in patients with severe asthma. Respir. Med. 2024, 225, 107578. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; RuiWen Kuo, C.; Jabbal, S.; Lipworth, B.J. Eosinophil depletion with benralizumab is associated with attenuated mannitol airway hyperresponsiveness in severe uncontrolled eosinophilic asthma. J. Allergy Clin. Immunol. 2023, 151, 700–705.e10. [Google Scholar] [CrossRef]
- McIntosh, M.J.; Kooner, H.K.; Eddy, R.L.; Jeimy, S.; Licskai, C.; Mackenzie, C.A.; Svenningsen, S.; Nair, P.; Yamashita, C.; Parraga, G. Asthma Control, Airway Mucus, and 129Xe MRI Ventilation After a Single Benralizumab Dose. Chest 2022, 162, 520–533. [Google Scholar] [CrossRef]
- McIntosh, M.J.; Kooner, H.K.; Eddy, R.L.; Wilson, A.; Serajeddini, H.; Bhalla, A.; Licskai, C.; Mackenzie, C.A.; Yamashita, C.; Parraga, G. CT Mucus Score and 129Xe MRI Ventilation Defects After 2.5 Years’ Anti-IL-5Rα in Eosinophilic Asthma. Chest 2023, 164, 27–38. [Google Scholar] [CrossRef]
- Brembach, T.C.; Sabat, R.; Witte, K.; Schwerdtle, T.; Wolk, K. Molecular and functional changes in neutrophilic granulocytes induced by nicotine: A systematic review and critical evaluation. Front. Immunol. 2023, 14, 1281685. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.K.; Silberbrandt, A.; Frøssing, L.; Hvidtfeldt, M.; von Bülow, A.; Nair, P.; Mukherjee, M.; Porsbjerg, C. Impact of former smoking exposure on airway eosinophilic activation and autoimmunity in patients with severe asthma. Eur. Respir. J. 2022, 60, 2102446. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Casale, T.B.; Cardet, J.C. Biological therapies for eosinophilic asthma. Expert Opin. Biol. Ther. 2018, 18, 747–754. [Google Scholar] [CrossRef] [PubMed]

| Variables | PAO− n = 22 | PAO+ n = 68 | SMK− n = 128 | SMK+ n = 75 |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female Male | 15 (68.2%) 7 (31.8%) | 42 (61.8%) 26 (38.2%) | 89 (69.5%) 39 (30.5%) | 37 (49.3%) 38 (50.7%) |
| Age (years) | ||||
| Mean (SD) | 52.5 (11.4) | 55.3 (13.3) | 56.7 (13.3) | 56.3 (10.2) |
| BMI (Kg/m2); N a | 22 | 68 | 119 | 68 |
| Mean (SD) | 27.5 (6.7) | 28.2 (6.5) | 27.6 (6.2) | 29.1 (6.5) |
| Obese b, n (%) | 6 (27.3%) | 22 (32.4%) | 29 (24.4%) | 25 (36.8%) |
| Age at asthma onset (years); N a | 17 | 51 | 91 | 54 |
| Mean (SD) | 32.3 (15.8) | 32.2 (18.1) | 34.6 (16.5) | 34.5 (16.2) |
| Asthma duration (years) c; N a | 17 | 51 | 35 | 31 |
| Mean (SD) | 19.5 (14.0) | 20.6 (14.2) | 24.5 (13.3) | 18.9 (13.6) |
| Allergic asthma, n (%) | 6 (27.3%) | 23 (33.8%) | 41 (32.0%) | 27 (36.0%) |
| Smoking history, n (%); N a | 21 | 68 | 52 | 36 |
| Never smoker | 12 (57.1%) | 41 (60.3%) | 128 (100.0%) | 0 (0.0%) |
| Former smoker | 9 (42.9%) | 24 (35.3%) | 0 (0.0%) | 69 (92.0%) |
| Smoker | 0 (0.0%) | 3 (4.4%) | 0 (0.0%) | 6 (8.0%) |
| Cigarette pack-year, n; N a Median (IQR) | 7 20 (15.6–26.3) | 19 10 (5.8–14.3) | 0 NA | 57 10 (7.0, 24.5) |
| Comorbidities, n (%) d | 22 (100.0%) | 60 (88.2%) | 120 (93.8%) | 70 (93.3%) |
| CRSwNP COPD Bronchiectasis | 10 (45.5%) 0 (0.0%) 0 (0.0%) | 22 (32.4%) 4 (5.9%) 4 (5.9%) | 50 (39.1%) 1 (0.8%) 1 (0.8%) | 25 (33.3%) 13 (17.3%) 13 (17.3%) |
| GERD | 3 (13.6%) | 14 (20.6%) | 25 (19.5%) | 17 (22.7%) |
| Osteoporosis | 3 (13.6%) | 7 (10.3%) | 25 (19.5%) | 7 (9.3%) |
| OSAS | 5 (22.7%) | 8 (11.8%) | 11 (8.6%) | 16 (21.3%) |
| HBP | 4 (18.2%) | 12 (17.6%) | 23 (18.0%) | 9 (12.0%) |
| Diabetes | 1 (4.5%) | 7 (10.3%) | 11 (8.6%) | 7 (9.3%) |
| Depression | 0 (0.0%) | 6 (8.8%) | 14 (10.9%) | 3 (4.0%) |
| Cataracts | 0 (0.0%) | 2 (2.9%) | 3 (2.3%) | 3 (4.0%) |
| Patients with prior biologic treatment, n (%) d | ||||
| Omalizumab | 4 (18.2%) | 9 (13.2%) | 18 (14.1%) | 14 (18.7%) |
| Mepolizumab d | 2 (9.1%) | 12 (17.6%) | 21 (16.4%) | 12 (16.2%) |
| Reslizumab | 0 (0.0%) | 2 (2.9%) | 3 (2.4%) | 2 (2.7%) |
| Patients with no prior biologic treatment | 16 (72.7%) | 48 (70.6%) | 87 (68.0%) | 51 (68.0%) |
| Peripheral BEC (cell/µL); N a | 20 | 65 | 124 | 72 |
| Median (IQR) | 550 (275.0, 902.5) | 500 (200.0, 730.0) | 530 (200.0, 800.0) | 440 (240.0, 685.0) |
| Total serum IgE concentration, (IU/mL); N a | 18 | 52 | 97 | 46 |
| Median (IQR) | 169 (55.7, 434.5) | 241 (89.2, 456.3) | 128 (44.3, 417.0) | 283 (92.8, 465.8) |
| FeNO (ppb); N a | 14 | 48 | 52 | 36 |
| Median (IQR) | 37 (21.8, 81.2) | 39 (19.3, 66.0) | 36 (19.9, 61.5) | 38 (18.8, 66.3) |
| OCS dependency | ||||
| OCS-dependent patients, n/N a (%) | 8/19 (42.1%) | 12/62 (19.4%) | 35/115 (30.4%) | 17/66 (25.8%) |
| Daily dose of OCS (mg); N a | 8 | 12 | 35 | 17 |
| Median (IQR) | 29 (14.2, 39.0) | 13 (9.4, 20.2) | 12 (5.0, 24.4) | 20 (10.0, 30.0) |
| Patients with daily OCS dose ≥ 5 mg, n (%) | 8 (100.0%) | 12 (100.0%) | 32 (91.4%) | 17 (100.0%) |
| Severe exacerbations; N a | 22 | 68 | 128 | 75 |
| Patients with severe exacerbations, n (%) | 21 (95.5%) | 55 (80.9%) | 107 (83.6%) | 65 (86.7%) |
| Severe exacerbations, mean (SD) | 3.4 (2.1) | 2.5 (2.3) | 2.5 (2.5) | 2.5 (1.9) |
| ED visits; N a | 22 | 68 | 128 | 75 |
| Patients with ED visits, n (%) | 9 (40.9%) | 21 (30.9%) | 43 (33.6%) | 28 (37.3%) |
| ED visits, mean (SD) | 0.7 (1.0) | 0.6 (1.4) | 0.6 (1.8) | 0.8 (1.4) |
| Hospitalizations; N a | 22 | 68 | 128 | 75 |
| Patients with hospitalizations, n (%) | 9 (40.9%) | 12 (17.6%) | 28 (21.9%) | 15 (20.0%) |
| Hospitalizations, mean (SD) | 0.6 (0.8) | 0.3 (0.7) | 0.3 (0.8) | 0.4 (0.9) |
| Asthma control; N a | 17 | 61 | 47 | 27 |
| ACT score, mean (SD) | 14.4 (4.9) | 14.5 (5.5) | 14.2 (4.9) | 14.1 (5.5) |
| Patients with ACT score < 20, n (%) | 14 (82.4%) | 48 (78.7%) | 77 (83.7%) | 45 (83.3%) |
| Lung function; N a | 22 | 67 | 98 | 55 |
| Pre-BD FEV1 (mL), mean (SD) | 2251.4 (847.9) | 1625.4 (624.3) | 1836.1 (801.3) | 2029.6 (765.1) |
| Pre-BD FEV1 (% predicted), mean (SD) | 77.4 (20.2) | 56.5 (15.3) | 68.6 (21.5) | 65.0 (20.4) |
| Patients with pre-BD FEV1 < 80%, n/N a (%) | 12/22 (54.5%) | 61/67 (91.0%) | 74/106 (69.8%) | 45/63 (71.4%) |
| Variables | PAO− n = 22 | PAO+ n = 68 | SMK− n = 128 | SMK+ n = 75 |
|---|---|---|---|---|
| Severe exacerbations; N a | 22 | 68 | 128 | 75 |
| Baseline | ||||
| Severe exacerbations, mean (SD) | 3.4 (2.1) | 2.5 (2.3) | 2.5 (2.5) | 2.5 (1.9) |
| Patients with zero exacerbations, n (%) | 1 (4.5%) | 13 (19.1%) | 21 (16.4%) | 10 (13.3%) |
| 1-year FUP | ||||
| Severe exacerbations, mean (SD) | 0.5 (0.9) | 0.3 (0.8) | 0.2 (0.7) | 0.4 (0.9) |
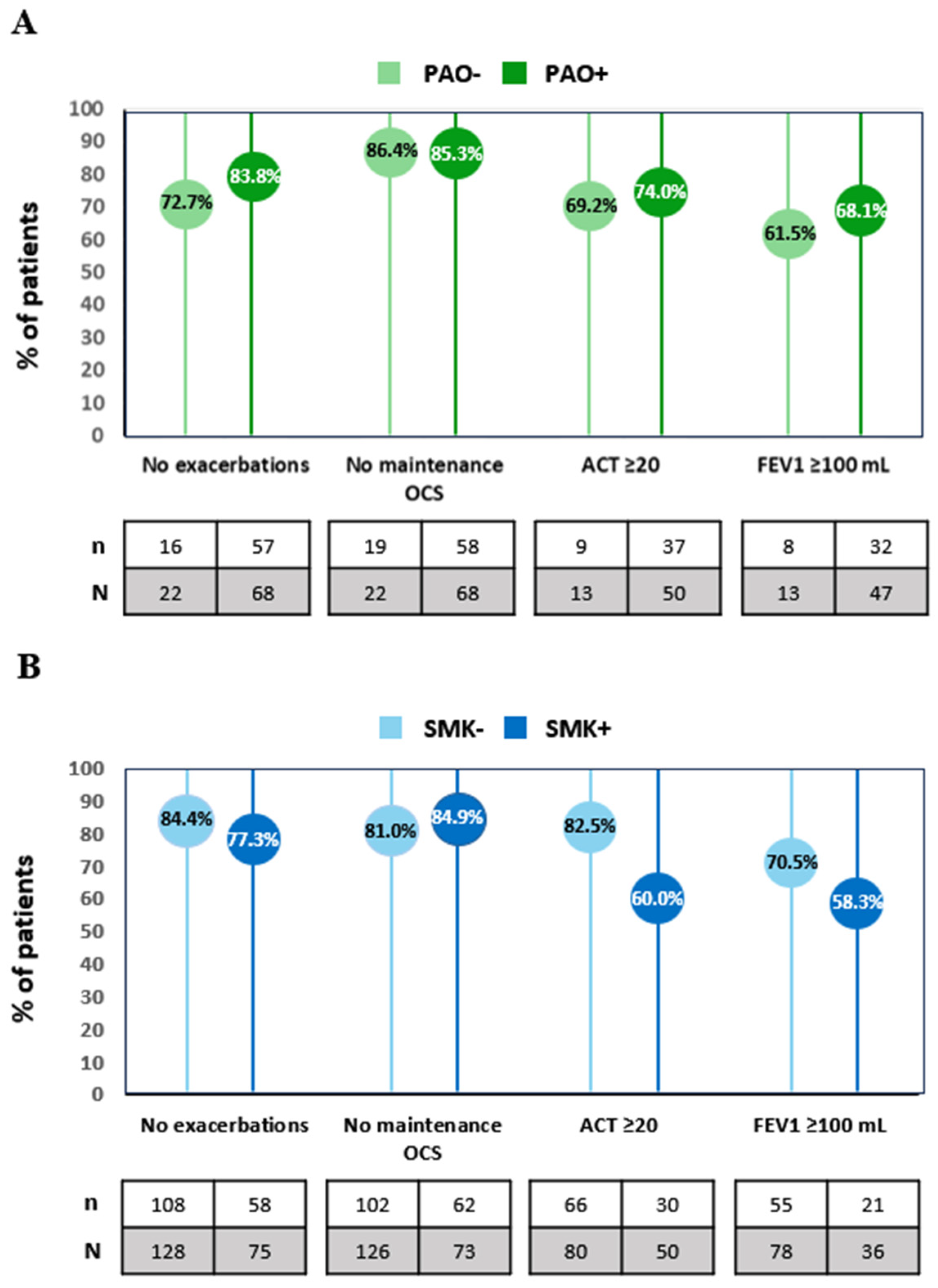
| Patients with zero exacerbations, n (%) | 16 (72.7%) | 57 (83.8%) | 108 (84.4%) | 58 (77.3%) |
| Patients with severe exacerbations reduction, n (%) b | 19 (90.5%) | 52 (94.5%) | 104 (97.2%) | 57 (87.7%) |
| Percentage reduction in severe exacerbations | 85.3% | 88.0% | 92.0% | 84.0% |
| ED visits; N a | 22 | 68 | 128 | 75 |
| Baseline | ||||
| ED visits, mean (SD) | 0.7 (1.0) | 0.6 (1.4) | 0.6 (1.8) | 0.8 (1.4) |
| Patients with zero ED visits, n (%) | 13 (59.1%) | 47 (69.1%) | 85 (66.4%) | 47 (62.7%) |
| 1-year FUP | ||||
| ED visits, mean (SD) | 0.1 (0.3) | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.5) |
| Patients with zero ED visits, n (%) | 20 (90.9%) | 65 (95.6%) | 122 (95.3%) | 68 (90.7%) |
| Patients with reduction in ED visits, n (%) c | 9 (100.0%) | 20 (95.2%) | 42 (97.7%) | 24 (85.7%) |
| Percentage reduction in ED visits | 85.7% | 83.3% | 83.3% | 87.5% |
| Hospitalizations; N a | 22 | 68 | 128 | 75 |
| Baseline | ||||
| Hospitalizations, mean (SD) | 0.6 (0.8) | 0.3 (0.7) | 0.3 (0.8) | 0.4 (0.9) |
| Patients with zero hospitalizations, n (%) | 13 (59.1%) | 56 (82.4%) | 100 (78.1%) | 60 (80.0%) |
| 1-year FUP | ||||
| Hospitalizations, mean (SD) | 0.0 (0.2) | 0.0 (0.2) | 0.1 (0.3) | 0.1 (0.3) |
| Patients with zero hospitalizations, n (%) | 21 (95.5%) | 66 (97.1%) | 123 (96.1%) | 72 (96.0%) |
| Patients with reduction in hospitalizations, n (%) d | 8 (88.9%) | 11 (91.7%) | 26 (92.9%) | 14 (93.3%) |
| Percentage reduction in hospitalizations | 100.0% | 100.0% | 66.7% | 75.0% |
| OCS dependency | ||||
| Baseline | ||||
| OCS-dependent patients, n/N a (%) | 8/19 (42.1%) | 12/62 (19.4%) | 35/115 (30.4%) | 17/66 (25.8%) |
| 1-year FUP | ||||
| OCS-dependent patients, n/N a (%) | 3/22 (13.6%) | 10/68 (14.7%) | 24/126 (19.0%) | 11/73 (15.1%) |
| Daily dose of OCS (mg); N a | 8 | 12 | 35 | 17 |
| Baseline | ||||
| Median (IQR) | 29 (14.2, 39.0) | 13 (9.4, 20.2) | 12 (5.0, 24.4) | 20 (10.0, 30.0) |
| 1-year FUP | ||||
| Median (IQR) | 0 (0.0, 1.3) | 3 (0.0, 6.9) | 0 (0.0, 6.9) | 0 (0.0, 11.5) |
| Patients achieving OCS dose reduction ≥ 50%, n (%) b | 7 (87.5%) | 8 (66.7%) | 22 (62.9%) | 10 (58.8%) |
| Patients achieving complete OCS withdrawal, n (%) | 6 (75.0%) | 6 (50.0%) | 18 (51.4%) | 10 (58.8%) |
| ACT score | ||||
| Baseline | ||||
| ACT score, mean (SD) | 14.4 (4.9) | 14.5 (5.5) | 14.2 (4.9) | 14.1 (5.5) |
| Patients with ACT score < 20, n/N a (%) | 14/17 (82.4%) | 48/61 (78.7%) | 77/92 (83.7%) | 45/54 (83.3%) |
| 1-year FUP | ||||
| ACT score, mean (SD) | 20.5 (5.5) | 21.4 (4.1) | 21.9 (4.0) | 19.4 (5.5) |
| Patients with ACT score <20, n/N a (%) | 4 (30.8%) | 13 (26.0%) | 14 (17.5%) | 20 (40.0%) |
| Increase in ACT score, mean (SD) | 5.7 (5.5) | 6.3 (6.2) | 7.3 (6.1) | 5.5 (5.8) |
| Patients with ACT increase ≥ 3, n/N a (%) | 6/10 (60.0%) | 32/45 (71.1%) | 48/64 (75.0%) | 27/40 (67.5%) |
| Lung function | ||||
| Baseline; N a | 22 | 67 | 106 | 63 |
| Pre-BD FEV1 (% predicted), mean (SD) | 77.4 (20.2) | 56.5 (15.3) | 68.6 (21.5) | 65.0 (20.4) |
| Patients with pre-BD FEV1 < 80%, n (%) | 12 (54.5%) | 61 (91.0%) | 74 (69.8%) | 45 (71.4%) |
| 1-year FUP; N a | 18 | 5 | 36 | 27 |
| Pre-BD FEV1 (% predicted), mean (SD) | 88.1 (17.0) | 72.2 (18.0) | 81.9 (22.4) | 72.5 (20.7) |
| Patients with pre-BD FEV1 < 80%, n (%) | 3 (23.1%) | 32 (68.1%) | 43 (48.3%) | 29 (64.4%) |
| Baseline; N a | 22 | 67 | 98 | 55 |
| Pre-BD FEV1 (mL), mean (SD) | 2251.4 (847.9) | 1625.4 (624.3) | 1836.1 (801.3) | 2029.6 (765.1) |
| 1-year FUP; N a | 18 | 5 | 85 | 42 |
| Pre-BD FEV1 (mL), mean (SD) | 2360.8 (705.3) | 2079.8 (769.8) | 2195.8 (851.5) | 2167.6 (724.2) |
| Increase in pre-BD FEV1 (mL), mean (SD) | 126.9 (292.9) | 402.6 (427.9) | 355.3 (426.8) | 278.3 (380.6) |
| Patients with pre-BD FEV1 increment ≥ 100 mL, n (%) | 8/13 (61.5%) | 32/47 (68.1%) | 55/78 (70.5%) | 21/36 (58.3%) |
| Patients with pre-BD FEV1 increment ≥ 230 mL, n (%) | 5/13 (38.5%) | 26/47 (55.3%) | 39/78 (50.0%) | 15/36 (41.7%) |
| Patients with pre-BD FEV1 increment ≥ 500 mL, n (%) | 0/13 (0.0%) | 18/47 (38.3%) | 27/78 (34.6%) | 12/36 (33.3%) |
| FeNO (ppb); N a | 14 | 48 | 76 | 44 |
| Baseline | ||||
| Median (IQR) | 37 (21.8, 81.2) | 39 (19.3, 66.0) | 36 (19.9, 61.5) | 38 (18.8, 66.3) |
| 1-year FUP | ||||
| Median (IQR) | 25 (19.0, 52.9) | 44 (18.0, 68.0) | 23 (15.4, 59.0) | 34 (17.0, 54.0) |
| Variables | PAO+ | |
|---|---|---|
| SMK− n = 41 | SMK+ n = 27 | |
| Severe exacerbations; N a | 41 | 27 |
| Baseline | ||
| Severe exacerbations, mean (SD) | 2.9 (2.8) | 2.0 (1.4) |
| Patients with zero exacerbations, n (%) | 8 (19.5%) | 5 (18.5%) |
| 1-year FUP | ||
| Severe exacerbations, mean (SD) | 0.3 (0.8) | 0.3 (0.7) |
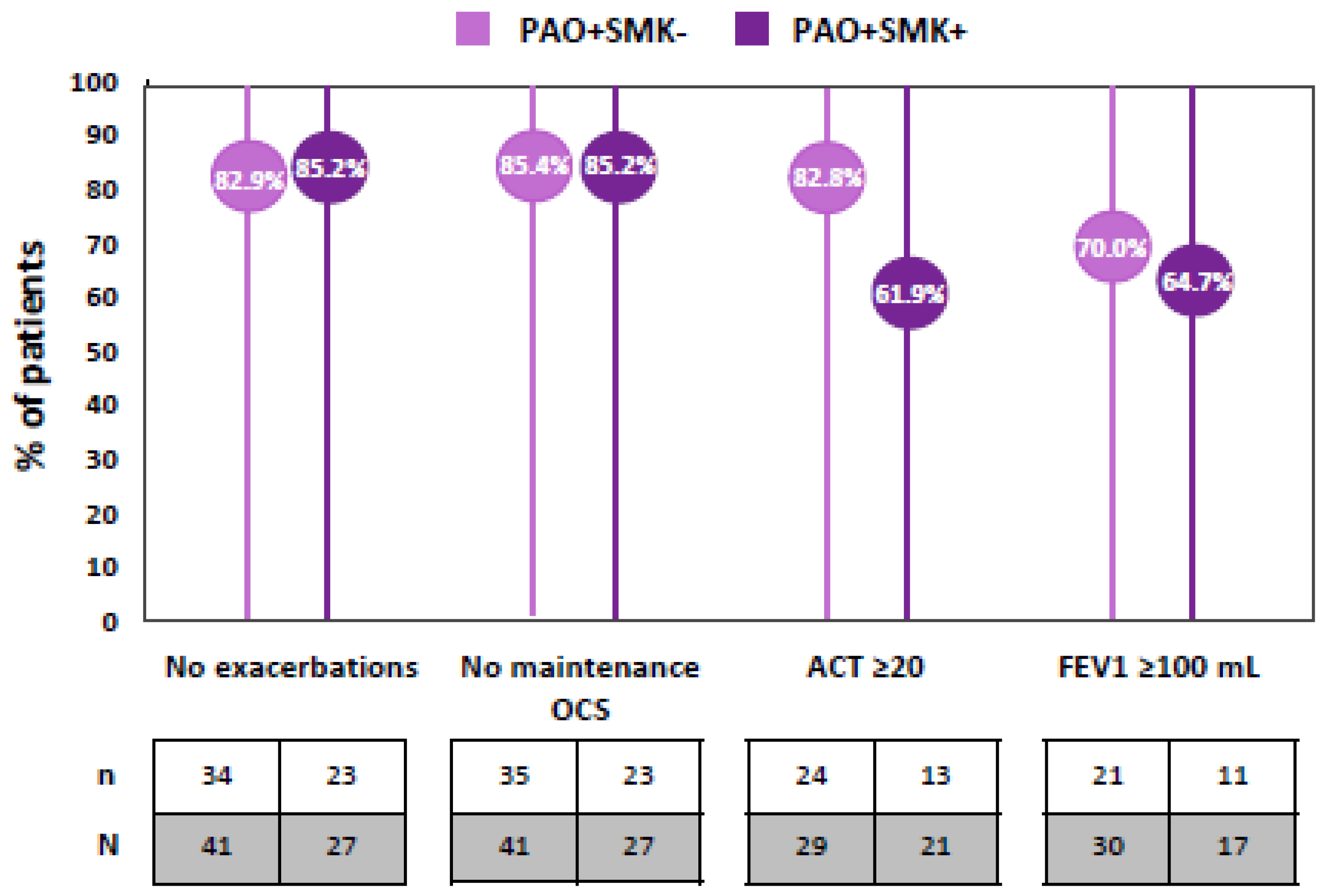
| Patients with zero exacerbations, n (%) | 34 (82.9%) | 23 (85.2%) |
| Patients with severe exacerbations reduction, n (%) b | 32 (97.0%) | 20 (90.9%) |
| Percentage reduction in severe exacerbations | 89.7% | 85.0% |
| ED visits; N a | 41 | 27 |
| Baseline | ||
| ED visits, mean (SD) | 0.7 (1.7) | 0.4 (0.8) |
| Patients with zero ED visits, n (%) | 27 (65.9%) | 20 (74.1%) |
| 1-year FUP | ||
| ED visits, mean (SD) | 0.1 (0.3) | 0.1 (0.6) |
| Patients with zero ED visits, n (%) | 39 (95.1%) | 26 (96.3%) |
| Patients with reduction in ED visits, n (%) c | 14 (100.0%) | 6 (85.7%) |
| Percentage reduction in ED visits | 85.7% | 75.0% |
| Hospitalizations; N a | 128 | 75 |
| Baseline | ||
| Hospitalizations, mean (SD) | 0.4 (0.9) | 0.1 (0.3) |
| Patients with zero hospitalizations, n (%) | 32 (78.0%) | 24 (88.9%) |
| 1-year FUP | ||
| Hospitalizations, mean (SD) | 0.0 (0.2) | 0.0 (0.2) |
| Patients with zero hospitalizations, n (%) | 40 (97.6%) | 26 (96.3%) |
| Patients with reduction in hospitalizations, n (%) d | 9 (100.0%) | 2 (66.7%) |
| Percentage reduction in hospitalizations | 100.0% | 100.0% |
| OCS dependency | ||
| Baseline | ||
| OCS-dependent patients, n/N a (%) | 7/38 (18.4%) | 5/24 (20.8%) |
| 1-year FUP | ||
| OCS-dependent patients, n/N a (%) | 6/41 (14.6%) | 4/27 (14.8%) |
| Daily dose of OCS (mg); N a | 7 | 5 |
| Baseline | ||
| Median (IQR) | 20 (7.5, 30.3) | 10 (10.0, 15.0) |
| 1-year FUP | ||
| Median (IQR) | 5 (0.0, 7.5) | 0 (0.0, 5.8) |
| Patients achieving OCS dose reduction ≥ 50%, n (%) b | 5 (71.4%) | 3 (60.0%) |
| Patients achieving complete OCS withdrawal, n (%) | 3 (42.9%) | 3 (60.0%) |
| ACT score | ||
| Baseline | ||
| ACT score, mean (SD) | 14.1 (4.8) | 15.1 (6.3) |
| Patients with ACT score < 20, n/N a (%) | 30/35 (85.7%) | 18/26 (69.2%) |
| 1-year FUP | ||
| ACT score, mean (SD) | 22.2 (3.8) | 20.4 (4.3) |
| Patients with ACT score < 20, n/N a (%) | 5/29 (17.2%) | 8/21 (38.1%) |
| Increase in ACT score, mean (SD) | 7.5 (6.6) | 4.9 (5.5) |
| Patients with ACT increase ≥ 3, n/N a (%) | 19/25 (76.0%) | 13/20 (65.0%) |
| Lung function | ||
| Baseline; N a | 41 | 26 |
| Pre-BD FEV1 (% predicted), mean (SD) | 54.3 (14.2) | 60.0 (16.6) |
| Patients with pre-BD FEV1 < 80%, n (%) | 38 (92.7%) | 23 (88.5%) |
| 1-year FUP; N a | 30 | 17 |
| Pre-BD FEV1 (% predicted), mean (SD) | 71.5 (16.9) | 73.6 (20.1) |
| Patients with pre-BD FEV1 < 80%, n (%) | 21 (70.0%) | 11 (64.7%) |
| Baseline; N a | 41 | 26 |
| Pre-BD FEV1 (mL), mean (SD) | 1439.0 (452.8) | 1919.2 (744.4) |
| 1-year FUP; N a | 30 | 17 |
| Pre-BD FEV1 (mL), mean (SD) | 1943.7 (722.9) | 2320.0 (812.5) |
| Increase in pre-BD FEV1 (mL), mean (SD) | 454.0 (462.5) | 311.8 (353.6) |
| Patients with pre-BD FEV1 increment ≥ 100 mL, n (%) | 21/30 (70.0%) | 11/17 (64.7%) |
| Patients with pre-BD FEV1 increment ≥ 230 mL, n (%) | 18/30 (60.0%) | 8/17 (47.1%) |
| Patients with pre-BD FEV1 increment ≥ 500 mL, n (%) | 12/30 (40.0%) | 6/17 (35.3%) |
| FeNO (ppb); N a | 29 | 19 |
| Baseline | ||
| Median (IQR) | 38 (19.4, 67.0) | 39 (19.4, 63.9) |
| 1-year FUP | ||
| Median (IQR) | 37 (16.8, 76.3) | 52 (35.8, 63.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rivera, C.; Blanco-Aparicio, M.; Casas-Maldonado, F.; Sánchez-Toril López, F.; Palop-Cervera, M.; Cassini, L.F.; Sanchez-Trincado, J.L.; Luzon, E.; Nuevo, J.; Secall, L.; et al. ORBE II Study: Clinical Characteristics and Outcomes After Treatment with Benralizumab According to Airflow Obstruction Status and Smoking Habit. J. Clin. Med. 2025, 14, 7900. https://doi.org/10.3390/jcm14227900
Martínez-Rivera C, Blanco-Aparicio M, Casas-Maldonado F, Sánchez-Toril López F, Palop-Cervera M, Cassini LF, Sanchez-Trincado JL, Luzon E, Nuevo J, Secall L, et al. ORBE II Study: Clinical Characteristics and Outcomes After Treatment with Benralizumab According to Airflow Obstruction Status and Smoking Habit. Journal of Clinical Medicine. 2025; 14(22):7900. https://doi.org/10.3390/jcm14227900
Chicago/Turabian StyleMartínez-Rivera, Carlos, Marina Blanco-Aparicio, Francisco Casas-Maldonado, Fernando Sánchez-Toril López, Marta Palop-Cervera, Luis F. Cassini, Jose Luis Sanchez-Trincado, Elisa Luzon, Javier Nuevo, Laia Secall, and et al. 2025. "ORBE II Study: Clinical Characteristics and Outcomes After Treatment with Benralizumab According to Airflow Obstruction Status and Smoking Habit" Journal of Clinical Medicine 14, no. 22: 7900. https://doi.org/10.3390/jcm14227900
APA StyleMartínez-Rivera, C., Blanco-Aparicio, M., Casas-Maldonado, F., Sánchez-Toril López, F., Palop-Cervera, M., Cassini, L. F., Sanchez-Trincado, J. L., Luzon, E., Nuevo, J., Secall, L., González-Sierra, M., Barragán, C. P., & Padilla-Galo, A. (2025). ORBE II Study: Clinical Characteristics and Outcomes After Treatment with Benralizumab According to Airflow Obstruction Status and Smoking Habit. Journal of Clinical Medicine, 14(22), 7900. https://doi.org/10.3390/jcm14227900





