Aortic Valve Annular Features in Acromegaly—A Detailed Three-Dimensional Speckle-Tracking Echocardiographic Analysis from the MAGYAR-Path Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
- -
- If the IGF-1 index is ≤1.0, the value is within the normal range (biochemically controlled/inactive acromegaly).
- -
- If the IGF-1 index is >1.0, the IGF-1 is elevated (active disease).
2.2. Two-Dimensional Doppler Echocardiography
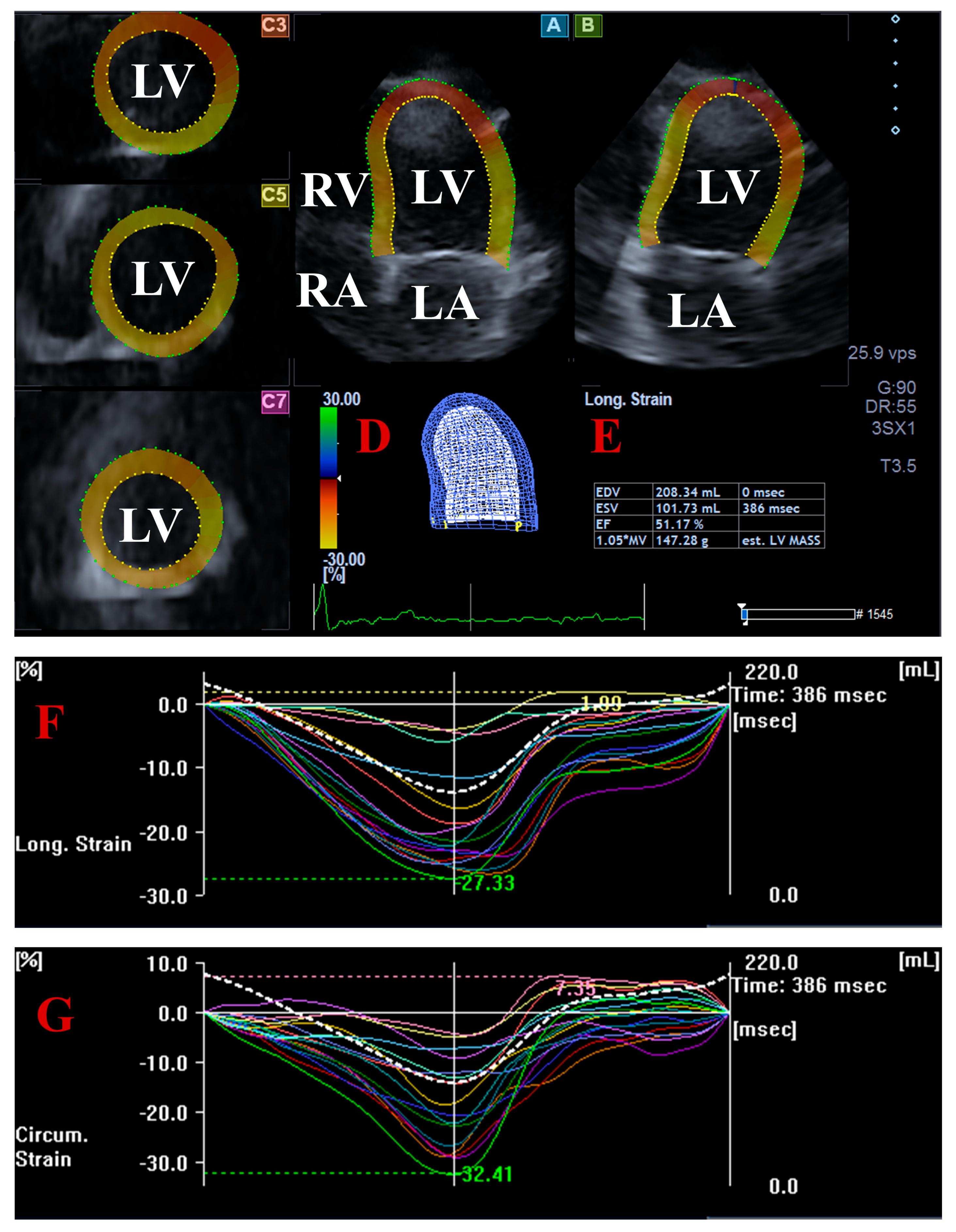
2.3. Three-Dimensional Speckle-Tracking Echocardiography
2.4. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Two-Dimensional Doppler Echocardiography
3.3. Three-Dimensional Speckle-Tracking Echocardiography
3.4. Reproducibility of 3DSTE-Derived AVA Assessments
4. Discussion
5. Limitation Section
- -
- A limited number of patients with acromegaly were analyzed in this study. It should be noted that acromegaly is a rare disease, and the present study is a single-center study. However, in Hungary, with a population of less than 10 million, approximately 300 patients with acromegaly are followed, which means that about 10% of this population was examined in the present study.
- -
- Disease activity was determined based on IGF-1 and IGF-1 index levels only, without considering GH suppression tests, which may have influenced the categorization of active and inactive acromegaly patients [15].
- -
- Acromegaly-associated cardiovascular risk factors could partially explain the findings.
- -
- One of the most important technical issues regarding 3DSTE is its low temporal and spatial resolution. Moreover, the footprint of the transducer for 3DSTE is greater than that of the transducer used for 2D echocardiography. In addition, the fact that more than one subvolume is necessary to be acquired for optimal images may result in stitching and motion artifacts and consequential deterioration of image quality [17,18,19,20].
- -
- -
- Comparison of the determination of AVA dimensions by 2D Doppler echocardiography and 3DSTE was not the aim of this study.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.N.; Tan, M.; Amsterdam, E.A.; Singh, G.D. Acromegalic cardiomyopathy: Epidemiology, diagnosis, and management. Clin. Cardiol. 2018, 41, 419–425. [Google Scholar] [CrossRef]
- Mizera, Ł.; Elbaum, M.; Daroszewski, J.; Bolanowski, M. Cardiovascular complications of acromegaly. Acta Endocrinol. 2018, 14, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.A.; Matkovich, S.J. Cardiomyocytes structure, function and associated pathologies. Int. J. Biochem. Cell Biol. 2005, 37, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Pramstaller, P.P.; Rossini, A.; De Bortoli, M. Myocardial fibrosis in hypertrophic cardiomyopathy: A perspective from fibroblasts. Int. J. Mol. Sci. 2023, 24, 14845. [Google Scholar] [CrossRef]
- Konstam, M.A.; Abboud, F.M. Ejection fraction: Misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A. Pathophysiology of hypertension and endothelial dysfunction in patients with diabetes mellitus. Endocrinol. Metab. Clin. N. Am. 2001, 30, 983–997. [Google Scholar] [CrossRef]
- Nemes, A.; Gavallér, H.; Csajbók, É.; Julesz, J.; Forster, T.; Csanády, M. Aortic stiffness is increased in acromegaly—A transthoracic echocardiographic study. Int. J. Cardiol. 2008, 124, 121–123. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C.; Valkusz, Z. Myocardial, valvular and vascular structural and functional properties in acromegaly. J. Clin. Med. 2023, 12, 6857. [Google Scholar] [CrossRef]
- Colao, A.; Spinelli, L.; Marzullo, P.; Pivonello, R.; Petretta, M.; Di Somma, C.; Vitale, G.; Bonaduce, D.; Lombardi, G. High prevalence of cardiac valve disease in acromegaly: An observational, analytical, case-control study. J. Clin. Endocrinol. Metab. 2003, 88, 3196–3201. [Google Scholar] [CrossRef]
- Pereira, A.M.; van Thiel, S.W.; Lindner, J.R.; Roelfsema, F.; van der Wall, E.E.; Morreau, H.; Smit, J.W.A.; Romijn, J.A.; Bax, J.J. Increased prevalence of regurgitant valvular heart disease in acromegaly. J. Clin. Endocrinol. Metab. 2004, 89, 71–75. [Google Scholar] [CrossRef]
- Huang, R.; Jin, J.; Zhang, P.; Yan, K.; Zhang, H.; Chen, X.; He, W.; Guan, H.; Liao, Z.; Xiao, H.; et al. Use of speckle tracking echocardiography in evaluating cardiac dysfunction in patients with acromegaly: An update. Front. Endocrinol. 2023, 14, 1260842. [Google Scholar] [CrossRef]
- van der Klaauw, A.A.; Bax, J.J.; Smit, J.W.A.; Holman, E.R.; Delgado, V.; Bleeker, G.B.; Biermasz, N.R.; Roelfsema, F.; Romijn, J.A.; Pereira, A.M. Increased aortic root diameters in patients with acromegaly. Eur. J. Endocrinol. 2008, 159, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Yeboah-Kordieh, Y.A.; Arif, W.; Weisman, D.; Salvatori, R. Aortic root dilation in acromegaly. BMJ Case Rep. 2024, 17, e260204. [Google Scholar] [CrossRef]
- Gomaa, M.; El-Shirbiny, H.; Elshaer, O. Massive aortic root dilation in a Young male with previously undiagnosed acromegaly: A case report and review. Oxf. Med. Case Rep. 2025, 2025, omae185. [Google Scholar] [CrossRef] [PubMed]
- Brooke, A.M.; Drake, W.M. Serum IGF-1 levels in the diagnosis and monitoring of acromegaly. Pituitary 2007, 10, 173–179. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical utility of three-dimensional speckle-tracking echocardiography in heart failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Nemes, A.; Ambrus, N.; Lengyel, C. Normal reference values of three-dimensional speckle-tracking echocardiography-derived aortic valve annular dimensions in healthy adults—A detailed analysis from the MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2025, 15, 6776–6786. [Google Scholar] [CrossRef]
- Nemes, A.; Ambrus, N.; Lengyel, C. The dimensions of the aortic valve annulus are not associated with systolic excursion of its plane in the same healthy adults: Detailed insights from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Med. 2025, 14, 5760. [Google Scholar] [CrossRef]
- Sherin, R.P.V.; Vietor, N.O.; Usman, A.; Hoang, T.D.; Shakir, M.K.M. Cardiovascular disorders associated with acromegaly: An update. Endocr. Pract. 2024, 30, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Komuro, I.; Yazaki, Y. Molecular aspects of mechanical stress-induced cardiac hypertrophy. Mol. Cell. Biochem. 1996, 163-164, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.F.; Dohmen, P.M.; Choi, Y.H.; Wahlers, T.; et al. Cardiac hypertrophy: An introduction to molecular and cellular basis. Med. Sci. Monit. Basic Res. 2016, 22, 75–79. [Google Scholar] [CrossRef]
- Lazzeroni, D.; Rimoldi, O.; Camici, P.G. From left ventricular hypertrophy to dysfunction and failure. Circ. J. 2016, 80, 555–564. [Google Scholar] [CrossRef]
- Casini, A.F.; Neto, L.V.; Fontes, R.; França, R.F.; Xavier, S.S.; Gadelha, M.R. Aortic root ectasia in patients with acromegaly: Experience at a single center. Clin. Endocrinol. 2011, 75, 495–500. [Google Scholar] [CrossRef] [PubMed]
- van der Klaauw, A.A.; Bax, J.J.; Roelfsema, F.; Bleeker, G.B.; Holman, E.R.; Corssmit, E.P.M.; van der Wall, E.E.; Smit, J.W.A.; Romijn, J.A.; Pereira, A.M. Uncontrolled acromegaly is associated with progressive mitral valvular regurgitation. Growth Horm. IGF Res. 2006, 16, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Fusini, L.; Muratori, M.; Cefalù, C.; Gripari, P.; Ali, S.G.; Pontone, G.; Andreini, D.; Bartorelli, A.L.; Alamanni, F.; et al. Feasibility and accuracy of three-dimensional transthoracic echocardiography vs. multidetector computed tomography in the evaluation of aortic valve annulus in patient candidates to transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1316–1323. [Google Scholar] [CrossRef]

| Controls | All Acromegaly Patients | Inactive Acromegaly Patients | Active Acromegaly Patients | |
|---|---|---|---|---|
| (n = 31) | (n = 23) | (n = 12) | (n = 11) | |
| Clinical and demographic data | ||||
| Age (years) | 50.0 ± 7.5 | 54.3 ± 14.5 | 46.3 ± 15.0 | 63.2 ± 7.3 *,† |
| Male gender (%) | 9 (29) | 6 (26) | 5 (42) | 1 (9) |
| Hypertension (%) | 0 (0) | 13 (57) * | 6 (50) * | 7 (64) * |
| Diabetes mellitus (%) | 0 (0) | 4 (17) | 2 (17) | 2 (18) |
| Hypercholesterolemia (%) | 0 (0) | 11 (48) * | 5 (42) * | 6 (55) * |
| Laboratory findings | ||||
| Serum IGF-1 (ng/mL) | - | 369.2 ± 324.5 | 345.4 ± 392.1 | 384.2 ± 234.5 |
| Serum IGF-1 index | - | 1.46 ± 1.07 | 1.17 ± 1.05 | 1.71 ± 1.03 † |
| Controls | All Acromegaly Patients | Inactive Acromegaly Patients | Active Acromegaly Patients | |
|---|---|---|---|---|
| (n = 31) | (n = 23) | (n = 12) | (n = 11) | |
| LA and LV dimensions | ||||
| LA diameter (mm) | 38.7 ± 4.7 | 42.4 ± 5.8 * | 41.2 ± 3.9 * | 43.7 ± 7.1 * |
| LV-EDD (mm) | 47.2 ± 3.5 | 51.0 ± 4.2 | 50.7 ± 4.2 | 51.4 ± 4.1 |
| LV-EDV (mL) | 104.1 ± 20.4 | 126.5 ± 23.4 * | 126.2 ± 24.6 | 127.1 ± 22.1 |
| LV-ESD (mm) | 31.4 ± 3.1 | 31.2 ± 4.2 | 31.3 ± 3.4 | 31.1 ± 4.9 |
| LV-ESV (mL) | 35.3 ± 8.4 | 40.1 ± 12.8 | 40.5 ± 10.7 | 39.8 ± 14.6 |
| IVS (mm) | 9.4 ± 1.3 | 10.1 ± 1.4 * | 9.7 ± 1.0 | 10.5 ± 1.6 * |
| LV-PW (mm) | 9.7 ± 1.3 | 10.8 ± 1.6 * | 10.7 ± 1.6 * | 11.0 ± 1.6 * |
| LV-EF (%) | 65.7 ± 3.3 | 68.2 ± 7.2 | 67.6 ± 5.1 | 68.8 ± 9.0 |
| E (cm/s) | 72.8 ± 15.6 | 69.0 ± 14.6 | 74.2 ± 16.6 | 62.8 ± 8.5 |
| A (cm/s) | 68.8 ± 17.8 | 81.6 ± 13.9 * | 79.0 ± 15.3 | 84.8 ± 11.3 * |
| Aortic valve regurgitation | ||||
| grade 0 (%) | 31 (100) | 20 (87) | 10 (83) | 10 (91) |
| grade 1 (%) | 0 (0) | 2 (9) | 2 (17) | 0 (0) |
| grades 2–4 (%) | 0 (0) | 1 (4) | 0 (0) | 1 (9) |
| Mitral regurgitation | ||||
| grade 0 (%) | 31 (100) | 12 (52) * | 8 (67) * | 4 (36) * |
| grade 1 (%) | 0 (0) | 9 (39) * | 4 (33) * | 5 (45) * |
| grades 2–4 (%) | 0 (0) | 2 (9) | 0 (0) | 2 (18) |
| Tricuspid regurgitation | ||||
| grade 0 (%) | 31 (100) | 12 (52) * | 9 (75) * | 3 (27) *,† |
| grade 1 (%) | 0 (0) | 11 (48) * | 3 (25) * | 8 (73) *,† |
| grades 2–4 (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Controls | All Acromegaly Patients | Inactive Acromegaly Patients | Active Acromegaly Patients | |
|---|---|---|---|---|
| (n = 31) | (n = 23) | (n = 12) | (n = 11) | |
| AVA dimensions | ||||
| AVA-Dmax-D (cm) | 2.05 ± 0.24 | 2.21 ± 0.38 | 2.14 ± 0.45 | 2.31 ± 0.22 * |
| AVA-Dmin-D (cm) | 1.84 ± 0.26 | 1.97 ± 0.39 | 1.90 ± 0.44 | 2.07 ± 0.31 |
| AVA-A-D (cm2) | 3.22 ± 0.72 | 3.85 ± 1.36 | 3.88 ± 1.45 | 4.07 ± 1.19 |
| AVA-P-D (cm) | 6.41 ± 0.74 | 6.91 ± 1.22 | 6.73 ± 1.29 | 7.14 ± 1.06 |
| AVA-Dmax-S (cm) | 1.99 ± 0.28 | 2.17 ± 0.34 | 2.19 ± 0.39 | 2.14 ± 0.26 |
| AVA-Dmin-S (cm) | 1.88 ± 0.25 | 1.92 ± 0.29 | 1.92 ± 0.31 | 1.92 ± 0.26 |
| AVA-A-S (cm2) | 3.24 ± 0.81 | 3.60 ± 0.94 | 3.50 ± 1.04 | 3.73 ± 0.76 |
| AVA-P-S (cm) | 6.39 ± 0.87 | 6.77 ± 0.90 | 6.69 ± 0.98 | 6.87 ± 0.75 |
| AAPSE (cm) | 1.14 ± 0.22 † | 1.00 ± 0.28 * | 1.03 ± 0.26 | 0.97 ± 0.29 * |
| LV strains | ||||
| basal LV-LS (%) | −21.2 ± 4.3 | −19.1 ± 5.4 | −20.3 ± 4.2 | −17.9 ± 5.1 |
| global LV-LS (%) | −16.4 ± 2.3 | −15.6 ± 3.3 | −16.9 ± 2.3 | −15.8 ± 3.3 |
| basal LV-CS (%) | −26.3 ± 6.0 | −28.8 ± 5.1 | −27.8 ± 4.6 | −29.8 ± 5.4 |
| global LV-CS (%) | −27.1 ± 6.0 | −28.7 ± 4.5 | −26.8 ± 4.3 | −30.8 ± 3.5 *,† |
| Intraobserver Agreement | Interobserver Agreement | |||
|---|---|---|---|---|
| Mean ± 2SD Difference in Values Obtained by Two Measurements of the Same Observer | Correlation Coefficient Between Measurements of the Same Observer | Mean ± 2SD Difference in Values Obtained by Two Observers | Correlation Coefficient Between Independent Measurements of Two Observers | |
| AVA-Dmax-D (cm) | −0.04 ± 0.19 | 0.87 (p < 0.01) | −0.07 ± 0.19 | 0.89 (p < 0.01) |
| AVA-Dmin-D (cm) | −0.02 ± 0.23 | 0.90 (p < 0.01) | −0.04 ± 0.25 | 0.92 (p < 0.01) |
| AVA-A-D (cm2) | −0.12 ± 0.58 | 0.93 (p < 0.01) | −0.12 ± 0.53 | 0.93 (p < 0.01) |
| AVA-P-D (cm) | −0.07 ± 0.64 | 0.91 (p < 0.01) | −0.12 ± 0.72 | 0.93 (p < 0.01) |
| AVA-Dmax-S (cm) | 0.02 ± 0.30 | 0.91 (p < 0.01) | 0.03 ± 0.34 | 0.93 (p < 0.01) |
| AVA-Dmin-S (cm) | 0.08 ± 0.31 | 0.81 (p < 0.01) | 0.04 ± 0.32 | 0.82 (p < 0.01) |
| AVA-A-S (cm2) | 0.13 ± 0.71 | 0.92 (p < 0.01) | 0.13 ± 0.73 | 0.93 (p < 0.01) |
| AVA-P-S (cm) | −0.02 ± 0.55 | 0.91 (p < 0.01) | 0.02 ± 0.50 | 0.91 (p < 0.01) |
| AAPSE (cm) | −0.02 ± 0.22 | 0.92 (p < 0.01) | −0.02 ± 0.18 | 0.93 (p < 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Lengyel, C.; Várkonyi, T.; Valkusz, Z.; Kupai, K. Aortic Valve Annular Features in Acromegaly—A Detailed Three-Dimensional Speckle-Tracking Echocardiographic Analysis from the MAGYAR-Path Study. J. Clin. Med. 2025, 14, 7899. https://doi.org/10.3390/jcm14227899
Nemes A, Lengyel C, Várkonyi T, Valkusz Z, Kupai K. Aortic Valve Annular Features in Acromegaly—A Detailed Three-Dimensional Speckle-Tracking Echocardiographic Analysis from the MAGYAR-Path Study. Journal of Clinical Medicine. 2025; 14(22):7899. https://doi.org/10.3390/jcm14227899
Chicago/Turabian StyleNemes, Attila, Csaba Lengyel, Tamás Várkonyi, Zsuzsanna Valkusz, and Krisztina Kupai. 2025. "Aortic Valve Annular Features in Acromegaly—A Detailed Three-Dimensional Speckle-Tracking Echocardiographic Analysis from the MAGYAR-Path Study" Journal of Clinical Medicine 14, no. 22: 7899. https://doi.org/10.3390/jcm14227899
APA StyleNemes, A., Lengyel, C., Várkonyi, T., Valkusz, Z., & Kupai, K. (2025). Aortic Valve Annular Features in Acromegaly—A Detailed Three-Dimensional Speckle-Tracking Echocardiographic Analysis from the MAGYAR-Path Study. Journal of Clinical Medicine, 14(22), 7899. https://doi.org/10.3390/jcm14227899







