TAVI for Bicuspid Aortic Valve: Addressing Technical Challenges and Optimizing Outcomes
Abstract
1. Introduction
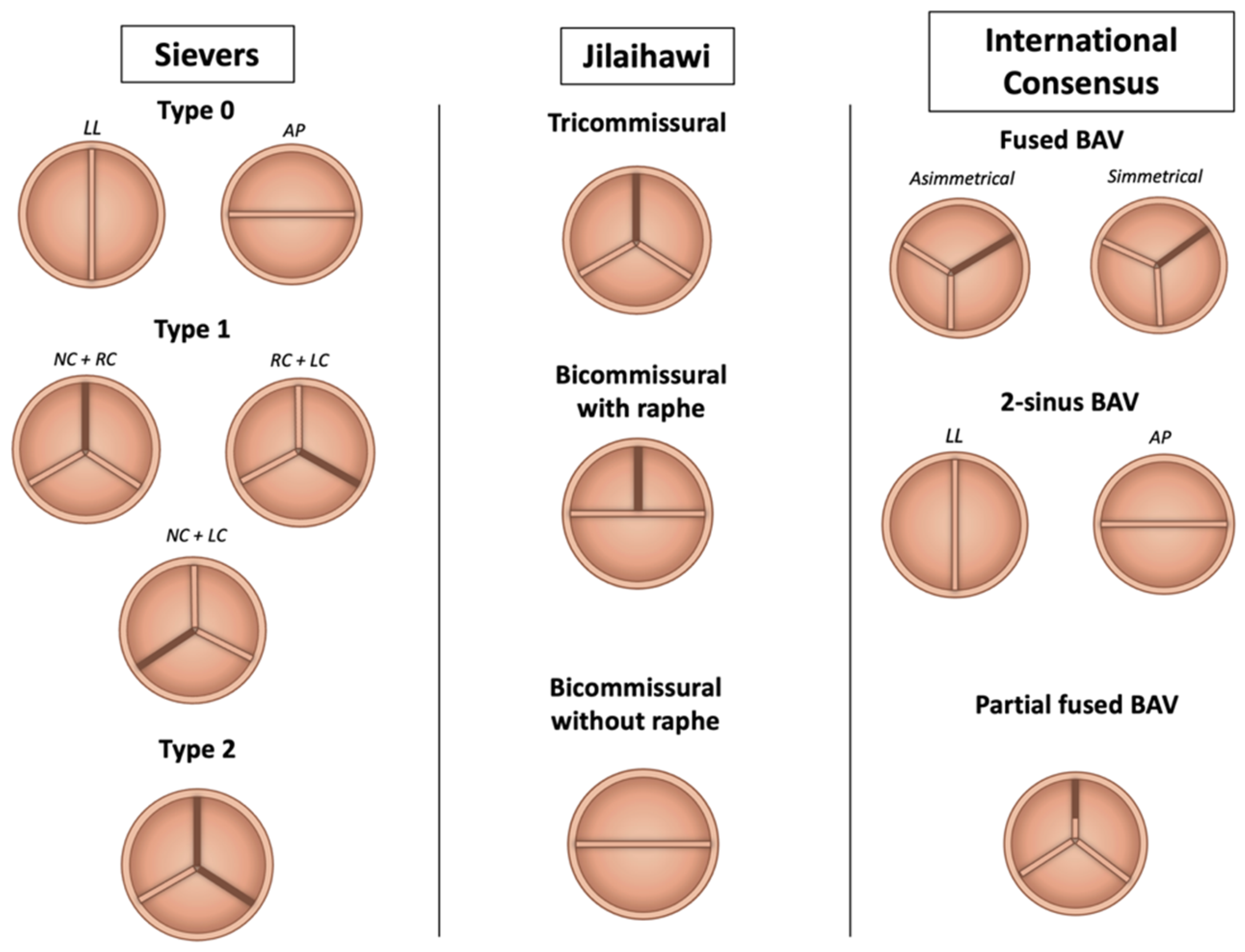
2. Anatomical Classification
3. Current Guidelines and the Unmet Need in Bicuspid Aortic Valve Stenosis Management
4. Technical Challenges
4.1. THV Sizing and Implantation Depth
4.2. THV Post-Dilatation
4.3. Tortuous or Horizontal Aortas
4.4. Valve Crossing
4.5. Very Large Annuli
4.6. Aortic Root and Ascending Aorta Dilatation in BAV Patients Undergoing TAVI
5. TAVI Outcomes in Bicuspid Anatomy
5.1. TAVI vs. SAVR
5.2. TAVI in BAV vs. TAVI in TAV
5.3. SEV vs. BEV
6. Valve Durability After TAVI in BAV Patients
6.1. Short-Term
6.2. Long-Term
7. Limitation of Current Evidence
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; Writing Committee Members; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- Hørsted Thyregod, H.G.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or Surgical Aortic Valve Implantation: 10-Year Outcomes of the NOTION Trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Janning, K.G.; Ko, J.M.; Filardo, G.; Matter, G.J. Frequency of Congenitally Bicuspid Aortic Valves in Patients ≥80 Years of Age Undergoing Aortic Valve Replacement for Aortic Stenosis (with or without Aortic Regurgitation) and Implications for Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2012, 109, 1632–1636. [Google Scholar] [CrossRef]
- Mehta, C.K.; Liu, T.X.; Bonnell, L.; Habib, R.H.; Kaneko, T.; Flaherty, J.D.; Davidson, C.J.; Thomas, J.D.; Rigolin, V.H.; Bonow, R.O.; et al. Age-Stratified Surgical Aortic Valve Replacement for Aortic Stenosis. Ann. Thorac. Surg. 2024, 118, 430–438. [Google Scholar] [CrossRef]
- Sievers, H.H.; Schmidtke, C. A Classification System for the Bicuspid Aortic Valve from 304 Surgical Specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Chen, M.; Webb, J.; Himbert, D.; Ruiz, C.E.; Rodés-Cabau, J.; Pache, G.; Colombo, A.; Nickenig, G.; Lee, M.; et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc. Imaging 2016, 9, 1145–1158. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International Consensus Statement on Nomenclature and Classification of the Congenital Bicuspid Aortic Valve and Its Aortopathy, for Clinical, Surgical, Interventional and Research Purposes. Eur. J. Cardiothorac. Surg. 2021, 60, 448–476. [Google Scholar] [CrossRef]
- Sabet, H.Y.; Edwards, W.D.; Tazelaar, H.D.; Daly, R.C. Congenitally Bicuspid Aortic Valves: A Surgical Pathology Study of 542 Cases (1991 through 1996) and a Literature Review of 2715 Additional Cases. Mayo Clin. Proc. 1999, 74, 14–26. [Google Scholar] [CrossRef]
- Sperling, J.S.; Lubat, E. Forme Fruste or “Incomplete” Bicuspid Aortic Valves with Very Small Raphes: The Prevalence of Bicuspid Valve and Its Significance May Be Underestimated. Int. J. Cardiol. 2015, 184, 1–5. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, W.K.; Dhoble, A.; Milhorini Pio, S.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Ajmone Marsan, N.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2025, 67, ezaf276. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Li, H.; Kobari, Y.; Li, J.; Feng, Y.; Peng, Y.; Wei, J.; Zhao, Z.; Xiong, T.; et al. Long-Term Outcomes of BAV-0 Patients Compared With BAV-1 and TAV Patients After TAVR. JACC Cardiovasc. Interv. 2025, 18, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Costa, G.; Windecker, S.; Maisano, F.; Laterra, G.; Leipsic, J.; Blanke, P.; Bapat, V.N.; Leon, M.B.; Webb, J.G. Bicuspid Aortic Valve Disease: Advancements and Challenges of Transcatheter Aortic Valve Implantation. Eur. Heart J. 2025, 46, 2760–2775. [Google Scholar] [CrossRef] [PubMed]
- Tchetche, D.; De Biase, C.; Van Gils, L.; Parma, R.; Ochala, A.; Lefevre, T.; Hovasse, T.; De Backer, O.; Sondergaard, L.; Bleiziffer, S.; et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ. Cardiovasc. Interv. 2019, 12, e007107. [Google Scholar] [CrossRef]
- Tchétché, D.; Ziviello, F.; De Biase, C.; De Backer, O.; Hovasse, T.; Leroux, L.; Petronio, A.S.; Saint-Etienne, C.; Teles, R.C.; Modine, T.; et al. Transcatheter Aortic Valve Implantation with the Evolut Platform for Bicuspid Aortic Valve Stenosis: The International, Multicentre, Prospective BIVOLUTX Registry. EuroIntervention 2023, 19, 502–511. [Google Scholar] [CrossRef]
- Buono, A.; De Biase, C.; Fabris, T.; Bellamoli, M.; Kim, W.K.; Montarello, N.; Costa, G.; Zito, A.; Alfadhel, M.; Koren, O.; et al. CharActeristics, Sizing anD Outcomes of Stenotic, Tapered, RapHe-Type Bicuspid AOrtic Valves Treated with Trans-Catheter Device Implantation: Insights the AD HOC Registry. Int. J. Cardiol. 2024, 417, 132569. [Google Scholar] [CrossRef]
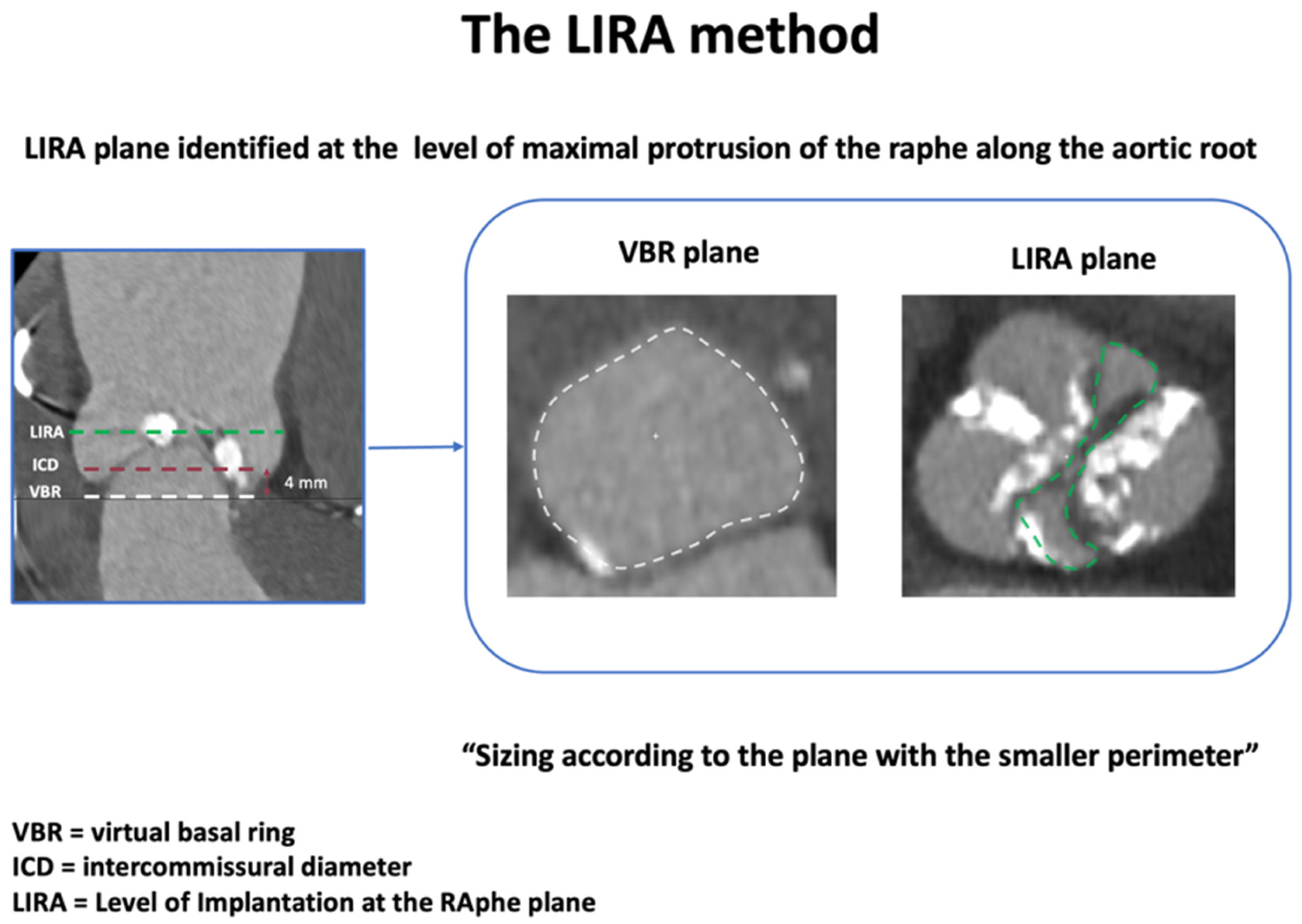
- Iannopollo, G.; Romano, V.; Buzzatti, N.; Ancona, M.; Ferri, L.; Russo, F.; Bellini, B.; Granada, J.F.; Chieffo, A.; Montorfano, M. Supra-Annular Sizing of Transcatheter Aortic Valve Prostheses in Raphe-Type Bicuspid Aortic Valve Disease: The LIRA Method. Int. J. Cardiol. 2020, 317, 144–151. [Google Scholar] [CrossRef]
- Petronio, A.S.; Angelillis, M.; De Backer, O.; Giannini, C.; Costa, G.; Fiorina, C.; Castriota, F.; Bedogni, F.; Laborde, J.C.; Søndergaard, L. Bicuspid Aortic Valve Sizing for Transcatheter Aortic Valve Implantation: Development and Validation of an Algorithm Based on Multi-Slice Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2020, 14, 452–461. [Google Scholar] [CrossRef]
- Blackman, D.; Gabbieri, D.; Del Blanco, B.G.; Kempfert, J.; Laine, M.; Mascherbauer, J.; Parma, R.; Tchétché, D. Expert Consensus on Sizing and Positioning of SAPIEN 3/Ultra in Bicuspid Aortic Valves. Cardiol. Ther. 2021, 10, 277–288. [Google Scholar] [CrossRef]
- Chen, M.; Michel, J.; Kasel, A.M. Application of Balloon-Expandable Transcatheter Heart Valve in Bicuspid Aortic Valve. JACC Asia 2021, 1, 147. [Google Scholar] [CrossRef]
- Elbadawi, A.; Saad, M.; Elgendy, I.Y.; Barssoum, K.; Omer, M.A.; Soliman, A.; Almahmoud, M.F.; Ogunbayo, G.O.; Mentias, A.; Gilani, S.; et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2019, 12, 1811–1822. [Google Scholar] [CrossRef]
- Mentias, A.; Sarrazin, M.V.; Desai, M.Y.; Saad, M.; Horwitz, P.A.; Kapadia, S.; Girotra, S. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2020, 75, 2518–2519. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, M.; Kumar, A.; Doshi, R.; Shariff, M.; Krishnaswamy, A.; Reed, G.W.; Brockett, J.; Lahorra, J.A.; Svensson, L.G.; Puri, R.; et al. Early Outcomes of Transcatheter versus Surgical Aortic Valve Implantation in Patients with Bicuspid Aortic Valve Stenosis. EuroIntervention 2022, 18, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.H.; Thyregod, H.G.H.; Savontaus, M.; Willemen, Y.; Bleie, Ø.; Tang, M.; Niemela, M.; Angerås, O.; Gudmundsdóttir, I.J.; Sartipy, U.; et al. Transcatheter Aortic Valve Implantation in Low-Risk Tricuspid or Bicuspid Aortic Stenosis: The NOTION-2 Trial. Eur. Heart J. 2024, 45, 3804–3814. [Google Scholar] [CrossRef]
- Makkar, R.R.; Yoon, S.H.; Chakravarty, T.; Kapadia, S.R.; Krishnaswamy, A.; Shah, P.B.; Kaneko, T.; Skipper, E.R.; Rinaldi, M.; Babaliaros, V.; et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs. Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk. JAMA 2021, 326, 1034–1044. [Google Scholar] [CrossRef]
- Forrest, J.K.; Kaple, R.K.; Ramlawi, B.; Gleason, T.G.; Meduri, C.U.; Yakubov, S.J.; Jilaihawi, H.; Liu, F.; Reardon, M.J. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valves From the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2020, 13, 1749–1759. [Google Scholar] [CrossRef]
- Yoon, S.H.; Bleiziffer, S.; De Backer, O.; Delgado, V.; Arai, T.; Ziegelmueller, J.; Barbanti, M.; Sharma, R.; Perlman, G.Y.; Khalique, O.K.; et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2017, 69, 2579–2589. [Google Scholar] [CrossRef]
- Montalto, C.; Sticchi, A.; Crimi, G.; Laricchia, A.; Khokhar, A.A.; Giannini, F.; Reimers, B.; Colombo, A.; Latib, A.; Waksman, R.; et al. Outcomes After Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Anatomy: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 2144–2155. [Google Scholar] [CrossRef]
- Viceré, A.; Kim, W.K.; Zito, A.; Fabris, T.; De Biase, C.; Restivo, A.; Montarello, N.; Costa, G.; Colucci, M.; Koren, O.; et al. Comparison between Severe R-L and R-NC Raphe-Type Bicuspid Aortic Valve Stenosis Treated with TAVI: Insights from the International AD HOC Registry. Cardiovasc. Revasc. Med. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Williams, M.R.; Jilaihawi, H.; Makkar, R.; O’Neill, W.W.; Guyton, R.; Malaisrie, S.C.; Brown, D.L.; Blanke, P.; Leipsic, J.A.; Pibarot, P.; et al. The PARTNER 3 Bicuspid Registry for Transcatheter Aortic Valve Replacement in Low-Surgical-Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 523–532. [Google Scholar] [CrossRef]
- Zito, A.; Buono, A.; Scotti, A.; Kim, W.K.; Fabris, T.; de Biase, C.; Bellamoli, M.; Montarello, N.; Costa, G.; Alfadhel, M.; et al. Incidence, Predictors, and Outcomes of Paravalvular Regurgitation After TAVR in Sievers Type 1 Bicuspid Aortic Valves. Cardiovasc. Interv. 2024, 17, 1652–1663. [Google Scholar] [CrossRef]
- Mangieri, A.; Tchetchè, D.; Kim, W.K.; Pagnesi, M.; Sinning, J.M.; Landes, U.; Kornowski, R.; De Backer, O.; Nickenig, G.; Ielasi, A.; et al. Balloon Versus Self-Expandable Valve for the Treatment of Bicuspid Aortic Valve Stenosis: Insights from the BEAT International Collaborative Registrys. Circ. Cardiovasc. Interv. 2020, 13, e008714. [Google Scholar] [CrossRef]
- Xiong, T.Y.; Ben Ali, W.; Feng, Y.; Hayashida, K.; Jilaihawi, H.; Latib, A.; Lee, M.K.Y.; Leon, M.B.; Makkar, R.R.; Modine, T.; et al. Transcatheter Aortic Valve Implantation in Patients with Bicuspid Valve Morphology: A Roadmap towards Standardization. Nat. Rev. Cardiol. 2023, 20, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Ramlawi, B.; Deeb, G.M.; Zahr, F.; Song, H.K.; Kleiman, N.S.; Chetcuti, S.J.; Michelena, H.I.; Mangi, A.A.; Skiles, J.A.; et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Bicuspid Aortic Valve Stenosis. JAMA Cardiol. 2021, 6, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe Tarantini, M.P. Breaking Bicuspid: When Less Is More and 0 Beats 1 (and 3). Cardiovasc. Interv. 2025, 18, 1893–1895. [Google Scholar] [CrossRef]
- Andrea Buono, M.; Andrea Zito, M.; Won-Keun Kim, M.; Tommaso Fabris, M.; Chiara De Biase, M.; Michele Bellamoli, M.; Nicholas Montarello, M.; Giuliano Costa, M.; Mesfer Alfadhel, M.; Ofir Koren, M.; et al. Balloon-Expandable vs. Self-Expanding Valves for Transcatheter Treatment of Sievers Type 1 Bicuspid Aortic Stenosis. Cardiovasc. Interv. 2024, 17, 2596–2608. [Google Scholar] [CrossRef]
- Waksman, R.; Craig, P.E.; Torguson, R.; Asch, F.M.; Weissman, G.; Ruiz, D.; Gordon, P.; Ehsan, A.; Parikh, P.; Bilfinger, T.; et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2020, 13, 1019–1027. [Google Scholar] [CrossRef]
- Li, H.D.; Li, W.Y.; Li, J.L.; Peng, S.Q.; Feng, Y.; Peng, Y.; Wei, J.F.; Zhao, Z.G.; Xiong, T.Y.; Ou, Y.W.X.; et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses in Patients With Bicuspid Versus Tricuspid Aortic Valve. J. Am. Heart Assoc. 2024, 13, e035772. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized Definitions of Structural Deterioration and Valve Failure in Assessing Long-Term Durability of Transcatheter and Surgical Aortic Bioprosthetic Valves: A Consensus Statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef]
- Fiorina, C.; Massussi, M.; Ancona, M.; Montorfano, M.; Petronio, A.S.; Tarantini, G.; Castriota, F.; Chizzola, G.; Costa, G.; Tamburino, C.; et al. Mid-Term Outcomes and Hemodynamic Performance of Transcatheter Aortic Valve Implantation in Bicuspid Aortic Valve Stenosis: Insights from the BicuSpid TAvi DuraBILITY (STABILITY) Registry. Catheter. Cardiovasc. Interv. 2023, 102, 1132–1139. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]

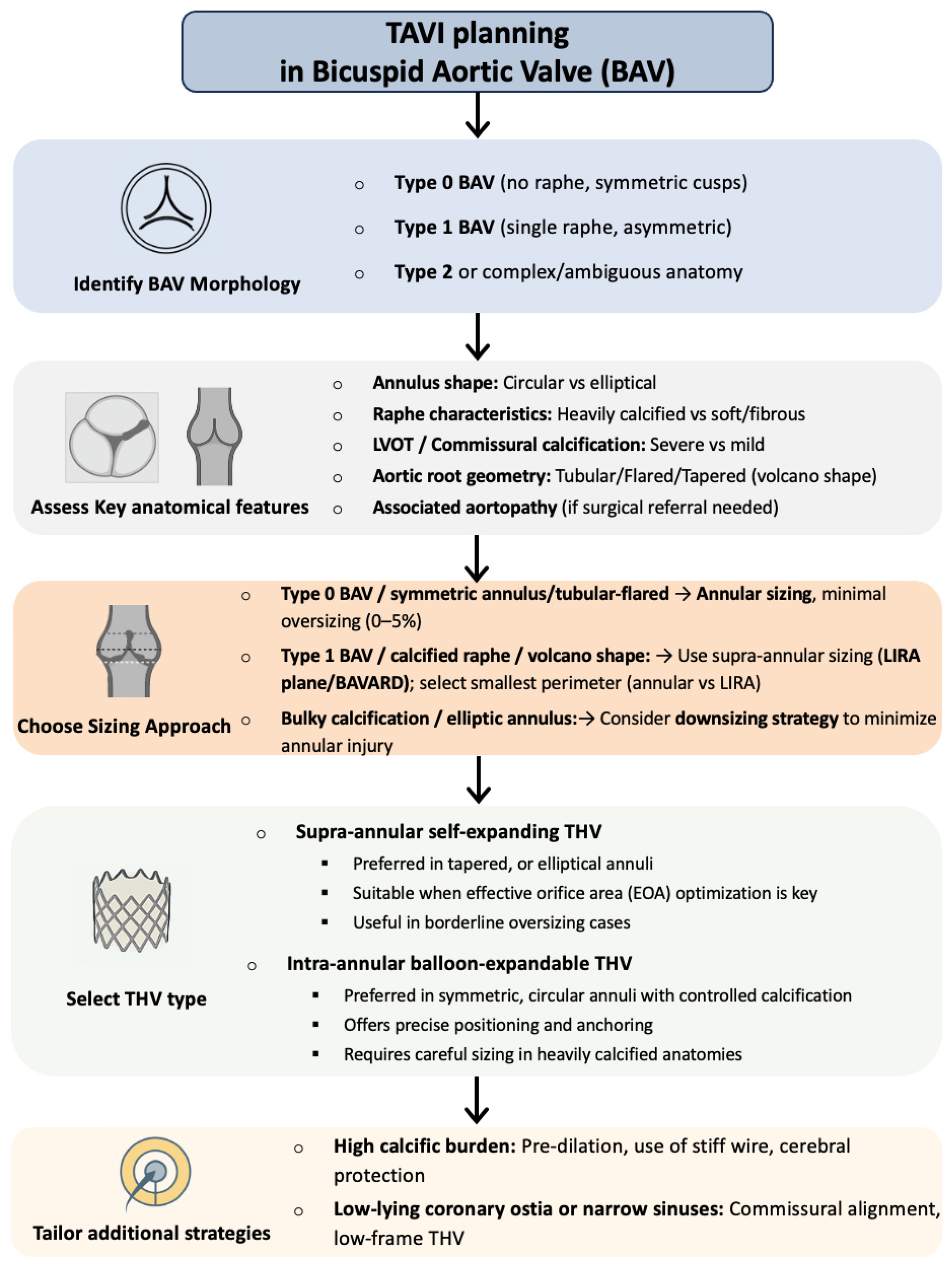
| Method | Measurement Plane/Rule | Core Concept and Workflow | Main Advantages | Main Drawbacks |
|---|---|---|---|---|
| BAVARD | Inter-commissural distance (ICD) 4 mm above annulus; Classify tapered/tubular/flared configurations | If root is tapered, sizing follows ICD (smaller value); in the other cases (88%), annulus guides sizing | Avoids oversizing in tapered roots; validated in both SE and BE THV; suitable for type 0 BAV | ICD measurement at a fixed level (4 mm), independently from interindividual variability; based on mono-dimensional parameter (diameter vs. perimeter/area) |
| LIRA | Maximal raphe protrusion plane (~10 mm supra-annular), choose smaller of LIRA vs. VBR perimeter | Compare supra-annular perimeter (LIRA) with VBR; THV size = smaller perimeter-derived Ø | Accurate THV waist prediction; high device success; low PVL | Applicable only to raphe-type BAV and for SE THVs; limited cohort size |
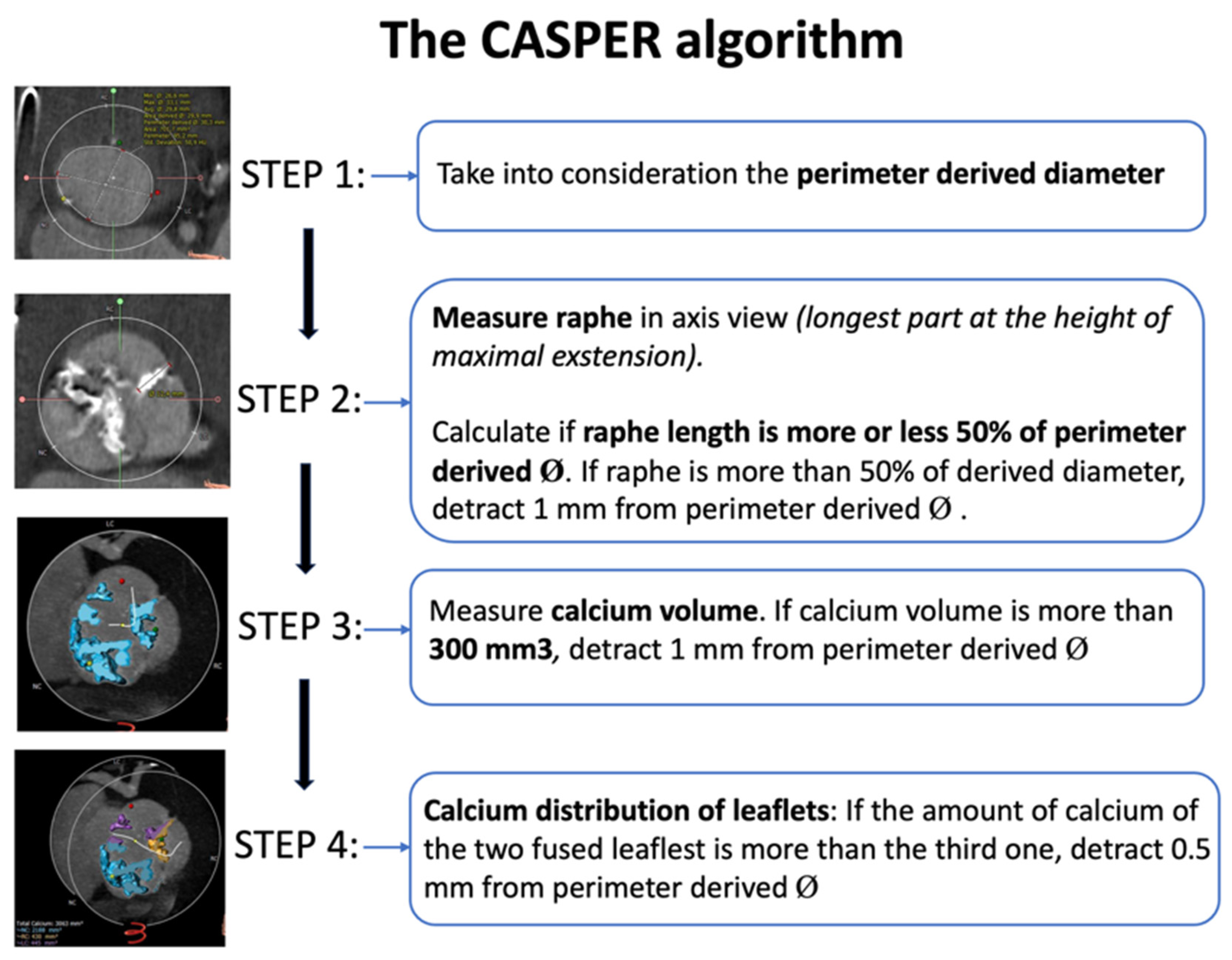
| CASPER | Start from annulus Ø, subtract 0–2.5 mm based on: calcium >300 mm3, raphe length >50%, calcium on raphe | Algorithmic downsizing of annulus Ø according to calcium burden and distribution on raphe | Personalized sizing; low PVL/embolization in pilot studies | Requires calcium-scoring software; not validated for BE THVs and applicable only to raphe-type BAV |
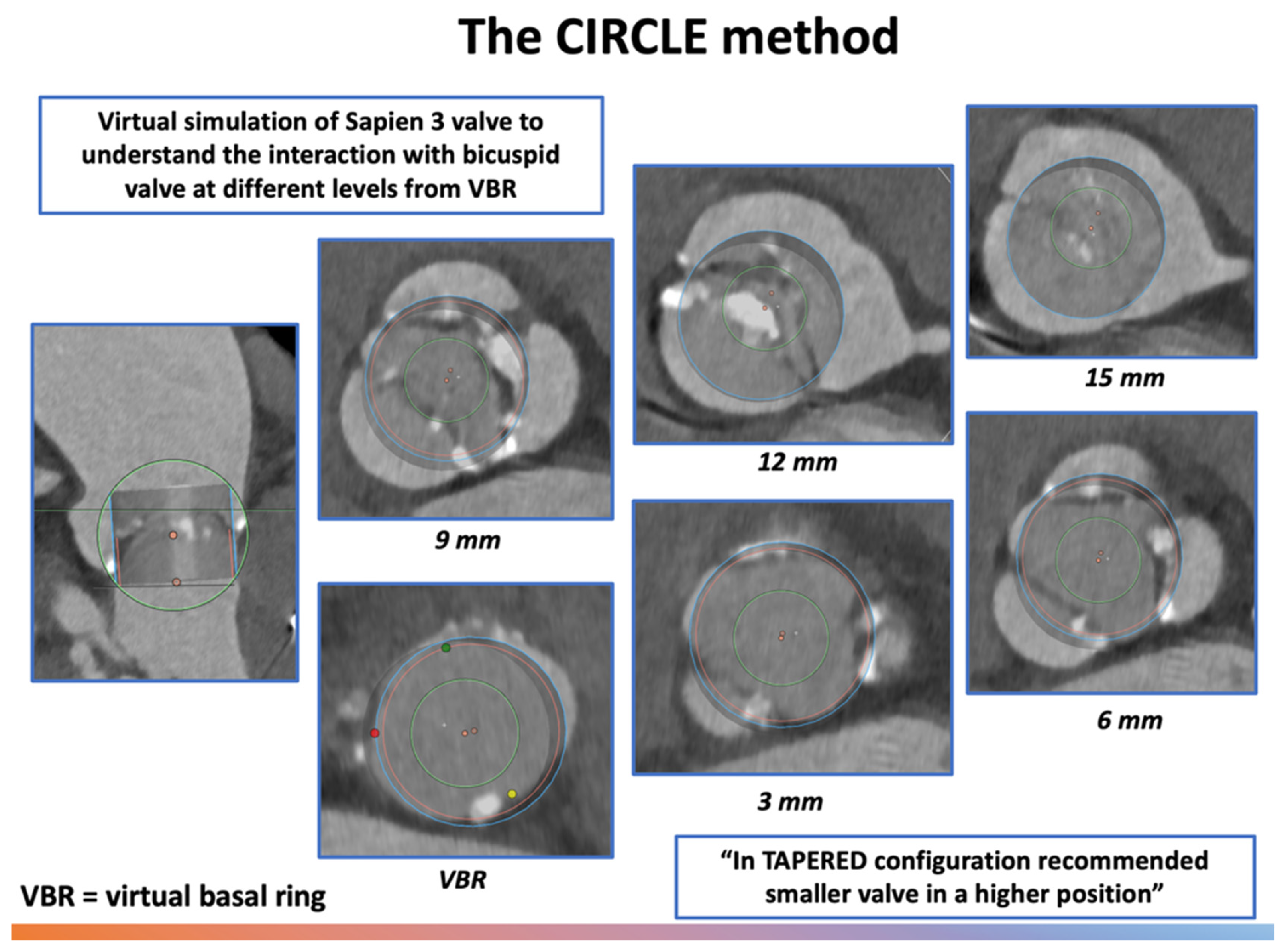
| CIRCLE | Overlay device-sized circles every 3 mm above annulus; first full-contact circle defines size | Visual sizing tool (balloon-expandable THVs) and assessment of coronary/LVOT clearance | Fast, intuitive; highlights anatomic conflicts; suitable for type 0 BAV | Empirical; not validated for self-expanding THVs |
| Features/Scenario | Sizing and Device Selection Tips |
|---|---|
| Sievers Type-0 BAV (no raphe) | Symmetric oval annulus; standard annular sizing with minimal oversizing (≈0–5%). |
| Sievers Type-1 BAV (one raphe) | Asymmetric anatomy with calcified raphe; use supra-annular (LIRA) measurements for sizing. Consider self-expanding valve to achieve larger EOA. Avoid excessive oversizing (>5%). |
| Bulky commissural calcification | Very high-risk for underexpansion. Favor downsizing (e.g., 0–5% oversize) to prevent rupture. |
| Elliptical (oval) annulus | High ellipticity; lean toward the smaller size to avoid distortion. Supra-annular THV can help accommodate shape. |
| Supra-annular valve (e.g., Evolut/Accurate) | Provides larger EOA and lower gradients. Good choice for small annuli, severe gradients or when preserving coronary access. Anchor depth should be planned at raphe level in BAV. |
| Intra-annular valve (balloon-expandable) | Offers precise deployment but requires caution in heavy calcification (risk of rupture). Use minimal oversizing and careful valve positioning. |
| Study (Year) | Study Design | N (Patients) | BAV Type (Sievers) | THV(s) | Follow-Up | Key Results | |
|---|---|---|---|---|---|---|---|
| TAVI vs. SAVR | Elbadawi et al., 2019 [22] | Propensity-matched registry | 975 pairs | NR | Mixed early-gen BEV and SEV | In-hospital | Mortality 3.1% vs. 3.1%. ↑ conduction disorders (14.9% vs. 6.2%) and pacemaker (13.8% vs. 4.6%) with TAVI |
| Mentias et al., 2020 [23] | Propensity-matched registry | 699 pairs | NR | Mixed BEV and SEV | 30 day and 1 y | No Δ mortality/stroke and HF hospitalization at 1 y; ↑ pacemaker (12.2% vs. 7.6%) with TAVI | |
| Majmundar et al., 2022 [24] | Propensity-matched registry | 1393 pairs | NR | Current-gen BEV and SEV | In-hospital, 30-day, 6 months | ↓ in-hospital mortality (0.7% vs. 1.8%) in TAVI. Similar 30-d and 6-mo MACE; | |
| NOTION-2 Trial, 2024 [25] | Randomized clinical trial (low-risk) | 100 BAV (49 TAVI vs. 51 SAVR) | Predominantly Type 1 | Mixed new-gen BEV and SEV | 1 y | Trend to ↑ composite death/stroke/rehospitalization (14.3% vs. 3.9%); ↑ disabling stroke, ↑ moderate/severe PVL but less PPM with TAVI; | |
| TAVI (BAV vs. TAV) | NOTION-2 2024 (sub-analysis) [25] | Randomized clinical trial (subgroup) | 100 BAV vs. ~270 TAV | Predominantly Type 1 | Mixed BEV and SEV | 1 y | ≥Moderate PVL 9.1% vs. 3.1%; Pacemaker similar; Composite endpoint numerically higher in BAV |
| Makkar et al., 2021 [26] | Propensity-matched registry | 3168 pairs | Mixed Type 0/1 (~80% Type 1) | BEV (Sapien 3) | 30 day and 1 y | Similar 30-d and 1-y mortality; ↑ stroke (2.5% vs. 1.6%) at 30-d for BAV; ↑ moderate/severe PVL 2.4% vs. 2.0% for BAV | |
| Forrest et al., 2020 [27] | Propensity-matched registry | 929 pairs | Predominantly Type 1 | SEV (Evolut R/PRO) | 30 day and 1 y | Mortality/stroke similar; ↑ moderate/severe AR 5.6% vs. 2.1% at 30 d (mitigated with PRO) | |
| Yoon et al., 2017 [28] | Propensity-matched multicenter | 546 pairs | 73% Type 1; 27% Type 0 | Early and new-gen BEV and SEV | 30 day | Similar 30-d outcomes; Lower device success and ↑ conversion to surgery in BAV (gap closed with new-gen THVs) | |
| Montalto et al., 2021 [29] | Systematic review and meta-analysis | 7071 (3434 BAV, 3637 TAV) | Mixed (0, 1, 2) | Mixed | 1 y | No differences of 1-y mortality/device success; ↑ PVL, stroke/TIA and annular rupture in BAV | |
| Vicerè et al., 2025 [30] | Propensity-matched registry | 382 pairs among raphe type BAV (251 R-L, 131 R-NC BAV) | Type 1 only | Mixed | In-hospital, 30-day, mid-term | No differences of 30-day VARC-3 technical/device success; Comparable short- and mid-term outcomes. ↑ PM implantation in R-L phenotype. | |
| Forrest et al., 2020 (Low-Risk BAV) [27] | Prospective single-arm | 150 patients (Single-arm (no control) | Type 1 ≈91% | SEV (Evolut/Evolut PRO) | 30 day | Death/disabling stroke 1.3%; device success 95.3%; pacemaker 15.1%; no >mild PVL | |
| Williams et al., 2022 (PARTNER 3 BAV) [31] | Prospective registry, matched | 169 BAV (148 matched) | Type 1 | BAV vs. matched TAV (BE THV) | 1 y | Primary endpoint 10.9% vs. 10.2% (ns); death 0.7% vs. 1.4%; stroke 2.1% vs. 2.0%; rehosp 9.6% vs. 9.5% | |
| Li et al., 2025 [13] | Multinational retrospective cohort | 2553 (134 BAV-0; 305 BAV-1; 2114 TAV) | Type 0 versus Type 1 and TAV | Mixed BEV and SEV | Median ~3.2 y (5-year endpoints) | BAV-0 ↓ 5-year all-cause mortality compared with BAV-1 (adjusted HR ~2.4) and TAV (adjusted HR ~3.0) | |
| SEV vs. BEV | Yoon et al., 2017 (device sub-analysis) [28] | Registry sub-analysis | 546 | 73% Type 1; 27% Type 0 | Early-gen Sapien XT (BEV) vs. CoreValve (SEV) | 30 d | Early BEV: ↑ root injury; Early SEV: ↑ second valve and PVL; gaps reduced with new-gen devices |
| Buono et al., 2024 (AD-HOC registry) [17] | Propensity-matched registry | 301 pairs | Type 1 only | BEV (Sapien 3) vs. SEV (Evolut R/PRO) | Median 1.3 y | BEV: ↓ pacemaker (OR 0.42) and PVL (OR 0.16) but ↑ severe PPM mismatch (OR 3.03); Similar mortality | |
| Zito et al., 2024 (AD-HOC registry) [32] | Registry analysis | 955 | Type 1 only | Predominantly current-gen THVs | 1 y | SEV + large raphe and heavy calcification → independent predictor of ≥moderate PVL; subgroup with significant PVL >> ↑ MAE | |
| Mangieri et al., 2020 (The BEAT registry) [33] | Matched registry | 77 pairs | Predominantly Type 1 | SEV (Evolut R/PRO) vs. BEV (Sapien 3) | 1 y | Device success 85% both; ↑ PVL with SEV; ↑ annular rupture with BEV; SEV better hemodynamics; outcomes similar | |
| Li et al., 2025 [13] | Multinational retrospective cohort | 2553 (134 BAV-0; 305 BAV-1; 2114 TAV) | Type 0 versus Type 1 and TAV | Mixed BEV and SEV | Median ~3.2 y (5-year endpoints) | BEVs in BAV ↑ mortality vs. SEVs (41.7% vs. 23.6%, HR ~1.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paglianiti, D.A.; Aurigemma, C.; Busco, M.; Cappannoli, L.; Bianchini, F.; Romagnoli, E.; Lunardi, M.; Fracassi, F.; Paraggio, L.; Buffon, A.; et al. TAVI for Bicuspid Aortic Valve: Addressing Technical Challenges and Optimizing Outcomes. J. Clin. Med. 2025, 14, 7860. https://doi.org/10.3390/jcm14217860
Paglianiti DA, Aurigemma C, Busco M, Cappannoli L, Bianchini F, Romagnoli E, Lunardi M, Fracassi F, Paraggio L, Buffon A, et al. TAVI for Bicuspid Aortic Valve: Addressing Technical Challenges and Optimizing Outcomes. Journal of Clinical Medicine. 2025; 14(21):7860. https://doi.org/10.3390/jcm14217860
Chicago/Turabian StylePaglianiti, Donato Antonio, Cristina Aurigemma, Marco Busco, Luigi Cappannoli, Francesco Bianchini, Enrico Romagnoli, Mattia Lunardi, Francesco Fracassi, Lazzaro Paraggio, Antonino Buffon, and et al. 2025. "TAVI for Bicuspid Aortic Valve: Addressing Technical Challenges and Optimizing Outcomes" Journal of Clinical Medicine 14, no. 21: 7860. https://doi.org/10.3390/jcm14217860
APA StylePaglianiti, D. A., Aurigemma, C., Busco, M., Cappannoli, L., Bianchini, F., Romagnoli, E., Lunardi, M., Fracassi, F., Paraggio, L., Buffon, A., Montone, R. A., Leone, A. M., Trani, C., & Burzotta, F. (2025). TAVI for Bicuspid Aortic Valve: Addressing Technical Challenges and Optimizing Outcomes. Journal of Clinical Medicine, 14(21), 7860. https://doi.org/10.3390/jcm14217860







