Abstract
Background: Pre-existing atrial fibrillation (AF) is common among patients undergoing transcatheter aortic valve replacement (TAVR). However, evidence regarding its impact on the risk of permanent pacemaker (PPM) implantation and other conduction disturbances (CDs) after TAVR remains inconsistent. The aim of this study was to assess the effect of baseline heart rhythm on the risk of conduction abnormalities following TAVR. Methods: This study included patients with severe AS who underwent TAVR using either balloon-expandable (BEVs) or self-expanding valves (SEVs). The primary endpoint was the incidence of PPM implantation and new or worsening left bundle branch block (LBBB) after TAVR according to baseline rhythm (sinus rhythm vs. AF). Secondary endpoints were predictors of PPM implantation, LBBB, the occurrence of periprocedural stroke, and in-hospital mortality. Results: A total of 5195 TAVR patients were included: 3560 with baseline sinus rhythm and 1635 with baseline AF. PPM implantation was more frequent in patients with AF than in those with sinus rhythm (17% vs. 15%, p = 0.033), whereas new or worsening LBBB was less common (11% vs. 14%, p = 0.026). After adjustment with multivariable logistic regression, these associations were no longer statistically significant (PPM implantation: OR 1.156, 95% CI 0.969–1.379, p = 0.108; new or worsening LBBB: OR 0.826, 95% CI 0.676–1.010, p = 0.062). Independent peri-procedural predictors of PPM implantation included baseline first-degree AV block, pre-procedural RBBB, the use of self-expandable valves, implantation of larger valve sizes (≥23 mm), and the need for valve repositioning. Conclusions: In this large cohort, baseline AF was not associated with an increased risk of PPM implantation or new onset LBBB compared with sinus rhythm. These findings suggest that baseline rhythm alone should not be considered an independent predictor of PPM implantation or CDs following TAVR.
1. Introduction
Transcatheter aortic valve replacement (TAVR) has become a life-saving intervention for patients with severe aortic stenosis (AS) [1,2,3]. The number of TAVR procedures has increased substantially over the past decade and continues to expand worldwide as indications broaden and technology advances [4,5]. Atrial fibrillation (AF) is one of the most common cardiac arrhythmias, with a lifetime risk estimated at nearly one in three individuals. The prevalence of AF is expected to double in the next few decades as a result of the ageing population, increasing burden of comorbidities, and new technologies for detection [6,7]. A considerable proportion of patients undergoing TAVR present with pre-existing AF, reflecting the overlap between advanced age, structural heart disease, and atrial arrhythmogenesis [8]. Conduction disturbances (CDs), particularly high-degree atrioventricular block requiring permanent pacemaker (PPM) implantation, remain among the most frequent complications of TAVR [9]. While there are several clinical and procedural predictors of PPM after TAVR have been established, such as baseline right bundle branch block, valve type, implantation depth, and valve oversizing [9,10,11,12], the role of baseline cardiac rhythm is less well defined. Specifically, the impact of AF compared with sinus rhythm on post-TAVR conduction outcomes has been inconsistently reported across studies [13,14,15,16], with some suggesting slightly higher risk and others showing no independent association. Moreover, only a limited number of studies have specifically evaluated the effect of baseline AF versus sinus rhythm on the risk of conduction abnormalities and PPM implantation. The present study aims to clarify the influence of baseline AF on the incidence of new CDs and PPM implantation following TAVR in a large contemporary cohort.
2. Methods
This retrospective, single-center clinical registry was conducted at Clinique Pasteur (Toulouse, France). Data were collected from all patients undergoing TAVR for severe aortic stenosis using balloon (Edwards Sapien 3, Sapien 3 Ultra, Colibri, and MyVal) or self-expandable (Medtronic Evolut R, Evolut Pro, Evolut Pro+, Navitor, and ACURATE neo) valves (SEVs) between 2013 and 2024. Patient selection for TAVI followed the European Society of Cardiology guidelines for valvular heart disease [1] and was determined by a multidisciplinary Heart Team. All consecutive patients with documented baseline rhythm were included, while those with a pre-existing PPM, without available ECG data, or undergoing TAVR for isolated aortic regurgitation were excluded. Missing data were minimal and handled using complete-case analysis. Information on AF subtype (paroxysmal, persistent, or permanent) was not available, and all AF patients were analyzed as a single group. All patients provided informed consent for the use of their medical records for research purposes.
The primary endpoint of this study was the incidence of PPM implantation and new or worsening left bundle branch block (LBBB) after TAVR according to baseline rhythm (sinus rhythm vs. AF). New LBBB was defined as a post-procedural QRS duration ≥ 120 ms with typical LBBB morphology that was not present before the procedure, while worsening LBBB was defined as pre-existing LBBB with a post-procedural QRS duration increase to >150 ms. Decisions regarding permanent pacemaker implantation followed the European Society of Cardiology (ESC) Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy [17], ensuring standardized indications across operators. Secondary endpoints included identification of predictors of PPM implantation and new or worsening LBBB, as well as the occurrence of periprocedural stroke, in-hospital mortality and bleeding complications according to baseline rhythm following TAVR.
3. Statistical Analysis
Categorical and dichotomous variables are presented as frequencies and percentages and were compared using Pearson’s chi-square or Fisher’s exact tests, as appropriate.
The Kruskal–Wallis test was used to assess the distribution of continuous variables. Continuous variables with a normal distribution are reported as mean ± standard deviation and were compared using the unpaired, two-sided Student’s t-test. Non-normally distributed variables are reported as median and interquartile range and were compared using the Mann–Whitney U test.
Univariate and multivariate logistic regression analyses were performed to identify factors associated with PPM implantation and new or worsening LBBB, using a backward stepwise method including variables with p < 0.20 in univariate analysis. Potential collinearity between AF and baseline conduction parameters (first degree AV block, RBBB and LBBB) was assessed using variance inflation factors (VIFs); all VIF values were <5, indicating no significant multicollinearity. All p-values were two-sided, and values < 0.05 were considered statistically significant. Analyses were conducted using SPSS version 29.0.2.0 (IBM Corp., Armonk, NY, USA).
4. Results
A total of 5195 patients who underwent TAVR were included, comprising 3560 with baseline sinus rhythm and 1635 with baseline AF. Baseline characteristics are summarized in Table 1. The median age was 84 years in both groups. The AF group included a higher proportion of males compared with the sinus rhythm group (56% vs. 50%). Cardiovascular comorbidities were prevalent in both groups, including dyslipidemia, hypertension, diabetes mellitus, and coronary artery disease. In the sinus rhythm group, 13% of patients had first-degree AV block. The prevalence of preprocedural RBBB was similar between groups (13% vs. 14%), whereas patients with AF had a higher prevalence of preprocedural LBBB (14% vs. 10%). The mean aortic gradient was slightly higher in the sinus rhythm group (48 mmHg vs. 44 mmHg), whereas patients with baseline AF had a higher prevalence of mitral regurgitation (54% vs. 43%, p < 0.001). Both the Society of Thoracic Surgeons (STS) score and EuroSCORE were higher in the AF group (median 4.1 and 3.9, respectively) compared with the sinus rhythm group (3.5 and 3, respectively).

Table 1.
Baseline characteristics.
Table 2 summarizes the Peri-procedural data and in hospital outcomes. The use of balloon and self-expandable valves was similar between groups. Larger valve sizes (≥23 mm) were more frequently implanted in the AF group (92% vs. 87%, p < 0.001). Procedure duration, fluoroscopy time, and contrast volume were comparable between groups. Pre-dilatation was performed less often in AF patients (14% vs. 18%, p = 0.009), whereas valve repositioning was more frequent (25% vs. 27%, p = 0.015). Rates of post-dilatation (23% vs. 22%, p = 0.377) and the need for a second valve (1.1% vs. 0.95%, p = 0.632) did not differ significantly between groups.

Table 2.
Peri-procedural data and in hospital outcomes.
In terms of in-hospital outcomes (Figure 1), permanent pacemaker implantation was more frequent in patients with AF compared with sinus rhythm (17% vs. 15%, p = 0.033), while new or worsening LBBB was less common (11% vs. 14%, p = 0.026). However, after multivariable logistic regression analysis (Table 3a,b), these associations were no longer statistically significant. Independent peri-procedural predictors of permanent pacemaker implantation included baseline first-degree AV block, pre-procedural RBBB, the use of self-expandable valves compared with balloon-expandable valves, implantation of larger valve sizes (≥23 mm), and the need for valve repositioning. For new or worsening LBBB, the only independent peri-procedural predictor was implantation of a larger valve size (≥23 mm).
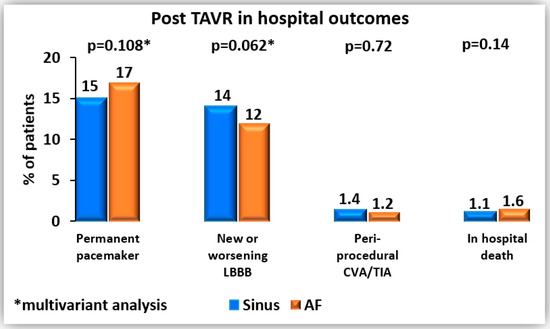
Figure 1.
Post-TAVR in hospital outcomes.

Table 3.
(a) Permanent pacemaker. (b) New or worsening LBBB.
Rates of peri-procedural stroke were low and similar between groups (1.2% vs. 1.4%, p = 0.729). In-hospital mortality was also comparable, at 1.6% in the AF group and 1.1% in the sinus rhythm group (p = 0.142) (Table 2). No statistically significant difference was observed between the groups in life-threatening bleeding complications (1.2% in the sinus rhythm group vs. 1.6% in the AF group, p = 0.232) or major bleeding complications (3.2% vs. 3.3%, p = 0.763).
5. Discussion
In this large study, pre-procedural AF, compared with sinus rhythm, was not associated with an increased risk of permanent pacemaker implantation or new LBBB. Rates of peri-procedural stroke, in-hospital mortality and bleeding complications were low and similar between groups.
New-onset AF after TAVR is associated with worse clinical outcomes, including higher mortality and increased risk of permanent pacemaker implantation [18,19]. In contrast, the effect of baseline rhythm (AF versus sinus rhythm) on pacemaker risk following TAVR remains unclear and inconsistently reported in the literature. Mentias et al. [13] found that pre-existing AF was not independently associated with an increased risk of PPM implantation after TAVR and the study highlights that the role of baseline rhythm in post-TAVR CDs remains unclear and inconsistently reported. Patil et al. [14] evaluated the impact of AF on inpatient outcomes after TAVR and found that AF was not associated with PPM implantation. Khan et al. [15] reported that baseline AF and pre-existing LBBB were independent predictors of late PPM implantation following TAVR. A very large meta-analysis [16] found that pre-existing conduction abnormalities and AF are associated with increased risk of PPM after TAVR. Our study contributes further data suggesting that baseline AF is not associated with an increased incidence of permanent pacemaker implantation or new LBBB after TAVR.
The higher mortality reported in the literature among patients with baseline AF likely reflects the presence of atrial myopathy and global myocardial remodeling, which are markers of advanced cardiac disease and increased vulnerability to adverse outcomes [20,21]. However, these structural and electrophysiological changes do not directly involve the conduction system injured during TAVR, explaining why AF was not independently associated with new CDs.
These findings have important clinical implications, suggesting that AF alone should not be considered a high-risk feature for post-procedural CDs. Procedural planning and patient counseling should therefore focus on other well-established predictors, including baseline first-degree AV block, pre-procedural RBBB, the use of self-expandable valves, implantation of larger valve sizes (≥23 mm), and the need for valve repositioning, factors that were identified as independent predictors of pacemaker implantation in our study, rather than focusing on AF. Consistent with prior evidence, our data confirmed a higher rate of PPM implantation with self-expanding valves compared with balloon-expandable valves. This difference is well recognized in the literature and is primarily attributed to deeper valve positioning and sustained radial force exerted by self-expanding prostheses on the interventricular septum, which increase the risk of conduction system injury [10,22,23]. Moreover, unnecessary heightened monitoring or prophylactic measures based solely on AF may be avoided, optimizing hospital resources and shortening unnecessary prolonged hospital stays.
This study has several limitations that warrant consideration. First, because it is a retrospective observational analysis, there is a risk of confounding factors and bias influencing the results. In particular, potential confounding by unmeasured variables such as medication use (e.g., antiarrhythmics) and procedural factors including valve type or implantation depth cannot be excluded. Second, the study included different generations and types of transcatheter valves, which may have varying impacts on CDs. Third, even though our patient cohort is relatively large, the fact that this was conducted at a single center may limit the generalizability of the findings to other institutions with different patient populations and procedural techniques. Finally, we focused only on in-hospital outcomes and did not capture longer-term events such as late pacemaker implantation.
In conclusion, in this large cohort, baseline atrial fibrillation was not associated with an increased risk of permanent pacemaker implantation or new-onset LBBB, suggesting that baseline rhythm alone is not an independent predictor of post-TAVR conduction disturbances.
Author Contributions
Conceptualization, Z.A. and H.V.-A.; methodology, O.O.; software, L.L.; validation, L.B.; formal analysis, Z.A.; investigation, D.T.; resources, N.D.; data curation, O.O.; writing—original draft preparation, Z.A.; writing—review and editing, N.D.; visualization, A.A. and H.V.-A.; supervision, N.D.; project administration, N.D.; funding acquisition, not applicable (no funding received). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Our study is based on data from the FRANCE TAVI registry, a national registry that has received approval from the French Commission on Informatics and Liberty (CNIL). This approval serves as the ethical authorization required in France for studies using registry-based anonymized data. (protocol: No. 2222005, approval date: 16 December 2021). Internal institution ethical review and approval were waived for this study, due to our institution has agreed to participate in the France TAVI Registry, coordinated and managed by the Société Française de.
Informed Consent Statement
Consent was waived, as the study is retrospective in nature and based on anonymized data.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Dr. Dumonteil—consultancy and proctoring fees from Abbott, Boston Scientific, Edwards, Medtronic.
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. 2022, 75, 524, (In English, Spanish). [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Tuzcu, E.M.; Makkar, R.R.; Svensson, L.G.; Agarwal, S.; Kodali, S.; Fontana, G.P.; Webb, J.G.; Mack, M.; Thourani, V.H.; et al. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation 2014, 130, 1483–1492. [Google Scholar] [CrossRef]
- Goldsweig, A.M.; Thourani, V.H. Decreasing Prices but Increasing Demand for Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2022, 15, e011827. [Google Scholar] [CrossRef]
- Nguyen, V.; Willner, N.; Eltchaninoff, H.; Burwash, I.G.; Michel, M.; Durand, E.; Gilard, M.; Dindorf, C.; Iung, B.; Cribier, A.; et al. Trends in aortic valve replacement for aortic stenosis: A French nationwide study. Eur. Heart J. 2022, 43, 666–679. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Tarantini, G.; Mojoli, M.; Windecker, S.; Wendler, O.; Lefèvre, T.; Saia, F.; Walther, T.; Rubino, P.; Bartorelli, A.L.; Napodano, M.; et al. Prevalence and Impact of Atrial Fibrillation in Patients With Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: An Analysis From the SOURCE XT Prospective Multicenter Registry. JACC Cardiovasc. Interv. 2016, 9, 937–946. [Google Scholar] [CrossRef]
- Sammour, Y.; Krishnaswamy, A.; Kumar, A.; Puri, R.; Tarakji, K.G.; Bazarbashi, N.; Harb, S.; Griffin, B.; Svensson, L.; Wazni, O.; et al. Incidence, Predictors, and Implications of Permanent Pacemaker Requirement After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 115–134. [Google Scholar] [CrossRef]
- Ullah, W.; Zahid, S.; Zaidi, S.R.; Sarvepalli, D.; Haq, S.; Roomi, S.; Mukhtar, M.; Khan, M.A.; Gowda, S.N.; Ruggiero, N.; et al. Predictors of Permanent Pacemaker Implantation in Patients Undergoing Transcatheter Aortic Valve Replacement—A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e020906. [Google Scholar] [CrossRef]
- Maan, A.; Refaat, M.M.; Heist, E.K.; Passeri, J.; Inglessis, I.; Ptaszek, L.; Vlahakes, G.; Ruskin, J.N.; Palacios, I.; Sundt, T.; et al. Incidence and Predictors of Pacemaker Implantation in Patients Undergoing Transcatheter Aortic Valve Replacement. Pacing Clin. Electrophysiol. 2015, 38, 878–886. [Google Scholar] [CrossRef]
- Siontis, G.C.; Jüni, P.; Pilgrim, T.; Stortecky, S.; Büllesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Saad, M.; Girotra, S.; Desai, M.; Elbadawi, A.; Briasoulis, A.; Alvarez, P.; Alqasrawi, M.; Giudici, M.; Panaich, S.; et al. Impact of Pre-Existing and New-Onset Atrial Fibrillation on Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2119–2129. [Google Scholar]
- Patil, N.; Strassle, P.D.; Arora, S.; Patel, C.; Gangani, K.; Vavalle, J.P. Trends and effect of atrial fibrillation on inpatient outcomes after transcatheter aortic valve replacement. Cardiovasc. Diagn. Ther. 2020, 10, 3–11. [Google Scholar] [CrossRef]
- Khan, M.Z.; Gupta, A.; Franklin, S.; Abraham, A.; Jarrar, A.; Patel, K.K.; Ahmad, S.; Kutalek, S. Predictors of Early and Late Atrioventricular Block Requiring Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: A Single-Center Experience. Cardiovasc. Revasc. Med. 2022, 42, 67–71. [Google Scholar] [CrossRef]
- Abu Rmilah, A.A.; Al-Zu’bi, H.; Haq, I.U.; Yagmour, A.H.; Jaber, S.A.; Alkurashi, A.K.; Qaisi, I.; Kowlgi, G.N.; Cha, Y.M.; Mulpuru, S.; et al. Predicting permanent pacemaker implantation following transcatheter aortic valve replacement: A contemporary meta-analysis of 981,168 patients. Heart Rhythm O2 2022, 3, 385–392. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022, 24, 71–164. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.; Grindal, A.; Jinah, R.; Um, K.J.; Vadakken, M.E.; Pandey, A.; Jaffer, I.H.; Healey, J.S.; Belley-Coté, É.P.; McIntyre, W.F. New-Onset Atrial Fibrillation After Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2022, 15, 603–613. [Google Scholar] [CrossRef]
- Reichl, J.J.; Stolte, T.; Boeddinghaus, J.; Wagener, M.; Leibundgut, G.; Badertscher, P.; Sticherling, C.; Kühne, M.; Kaiser, C.; Mahfoud, F.; et al. Prognostic impact of atrial fibrillation in patients undergoing transcatheter aortic valve implantation. Heart Rhythm O2 2025, 6, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Rivera-Caravaca, J.M.; Chiarito, M.; Ehrlinder, H.; Iliakis, P.; Gąsecka, A.; Romiti, G.F.; Parker, W.A.E.; Lip, G.Y.H. Atrial fibrillation versus atrial myopathy in thrombogenesis: Two sides of the same coin? Trends Cardiovasc. Med. 2025, 35, 271–281. [Google Scholar] [CrossRef]
- Masuda, M.; Matsuda, Y.; Uematsu, H.; Sugino, A.; Ooka, H.; Kudo, S.; Fujii, S.; Asai, M.; Okamoto, S.; Ishihara, T.; et al. Clinical impact of left atrial remodeling pattern in patients with atrial fibrillation: Comparison of volumetric, electrical, and combined remodeling. J. Cardiovasc. Electrophysiol. 2024, 35, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Vlastra, W.; Chandrasekhar, J.; Muñoz-Garcia, A.J.; Tchétché, D.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation: From the CENTER-collaboration. Eur. Heart J. 2019, 40, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Tretter, J.T.; Mori, S.; Anderson, R.H.; Taylor, M.D.; Ollberding, N.; Truong, V.; Choo, J.; Kereiakes, D.; Mazur, W. Anatomical predictors of conduction damage after transcatheter implantation of the aortic valve. Open Heart 2019, 6, e000972. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).