Glenohumeral Instability and Clinical Outcomes Following Proximal Humerus Resection and Megaprosthesis Implantation: A Systematic Review
Abstract
1. Introduction
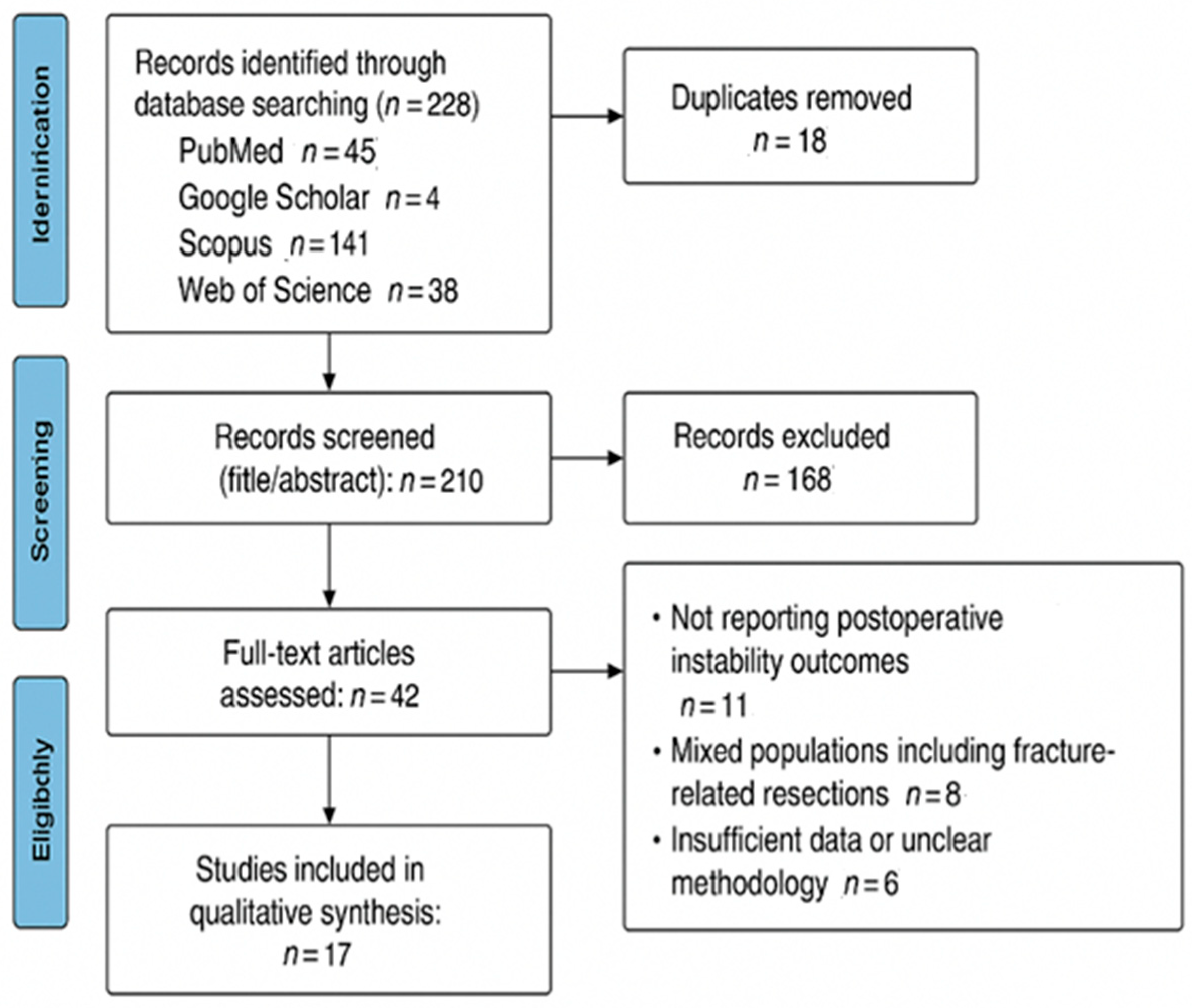
2. Materials and Methods
| Authors | Study Design | Level of Evidence | Number of Patients | Follow-Up (Months) | Mean Age | Gender (M/F) | Primary Histology | MINORS Score/Cochrane Risk of Bias (RoB) | Post Operative Instability | Number of Cases of Postoperative Instability |
|---|---|---|---|---|---|---|---|---|---|---|
| El Beaino et al. [16] | Retrospective | IV | 21 | 97 | 41 | 14/7 | Chondrosarcoma, Osteosarcoma | 14 | Yes | 12 |
| Hartigan et al. [17] | Retrospective | IV | 30 | 76.8 | 43.8 | 14/13 | n/s | 13 | Yes | 7 |
| Rahman et al. [18] | Retrospective | IV | 10 | 61 | 36 | n/s | Chondrosarcoma, Osteosarcoma | 14 | No | 0 |
| Wang et al. [19] | Retrospective | IV | 18 | 56 | 29.8 | 8/10 | Osteosarcoma, Chondrosarcoma | 13 | Yes | 1 |
| Van de Sande et al. [20] | Retrospective | IV | 37 | 120 | 44.8 | 21/16 | Osteosarcoma, Chondrosarcoma | 14 | Yes | 2 |
| Wang et al. [21] | Retrospective | IV | 16 | 27.4 | 45.9 | 7/9 | Metastatic, Chondrosarcoma | 12 | No | 0 |
| Black et al. [22] | Retrospective | IV | 6 | 25.2 | 40.7 | 2/4 | Chondrosarcoma | 14 | Yes | 5 |
| Wang et al. [23] | Retrospective | IV | 25 | 48 | 32 | 10/15 | Chondrosarcoma, Osteosarcoma | 15 | Yes | 10 |
| El Motassime et al. [24] | Retrospective | IV | 20 | 21 | 61.3 | 12/8 | Metastatic tumor | 20 | Yes | 2 |
| Rachbauer AM et al. [25] | Retrospective | IV | 46 | 25 | 52 | 15/31 | Chondrosarcoma, Osteosarcoma, Ewing’s sarcoma, Giant Cell Tumor, Metastatic | 14 | Yes | 8 |
| Errani et al. [26] | Retrospective | IV | 18 | 56.4 | n/s | 9/9 | n/s | 14 | Yes | 4 |
| Vonck et al. [27] | Retrospective | IV | 20 | 18.3 | 55.3 | 8/12 | Liposarcoma, Osteosarcoma, Metastatic | 13 | Yes | 4 |
| Shi et al. [28] | Retrospective | IV | 18 | 29 | 37 | 9/9 | Osteosarcoma, Chondrosarcoma | 13 | Yes | 2 |
| Fucentese et al. [29] | Retrospective | IV | 30 | 24 | 63.3 | 19/11 | n/s | 13 | Yes | 2 |
| Tagliero et al. [30] | Retrospective | IV | 33 | 96 | 67 | 17/16 | Osteosarcoma, Soft tissue sarcoma | 14 | Yes | 5 |
| Hu et al. [31] | Retrospective | IV | 7 | 23.6 | 34.9 | 3/4 | Osteosarcoma, Chondrosarcoma | 12 | No | 0 |
| Raiss et al. [32] | Retrospective | IV | 39 | 38 | 60 | 19/20 | Metastatic + Primary | 15 | yes | 4 |
| Study | Study Design | Level of Evidence | MINORS Total Score (0–16) | Quality Category |
|---|---|---|---|---|
| El Beaino et al. [16] | Retrospective | IV | 14 | Moderate |
| Hartigan et al. [17] | Retrospective | IV | 13 | Moderate |
| Rahman et al. [18] | Retrospective | IV | 14 | Moderate |
| Wang et al. [19] | Retrospective | IV | 13 | Moderate |
| Van de Sande et al. [20] | Retrospective | IV | 14 | Moderate |
| Wang et al. [21] | Retrospective | IV | 12 | Moderate–Low |
| Black et al. [22] | Retrospective | IV | 14 | Moderate |
| Wang et al. [23] | Retrospective | IV | 15 | Moderate–High |
| El Motassime et al. [24] | Retrospective | IV | 20 | High (comparative study) |
| Rachbauer et al. [25] | Retrospective | IV | 14 | Moderate |
| Errani et al. [26] | Retrospective | IV | 14 | Moderate |
| Vonck et al. [27] | Retrospective | IV | 13 | Moderate |
| Shi et al. [28] | Retrospective | IV | 13 | Moderate |
| Fucentese et al. [29] | Retrospective | IV | 13 | Moderate |
| Tagliero et al. [30] | Retrospective | IV | 14 | Moderate |
| Hu et al. [31] | Retrospective | IV | 12 | Moderate–Low |
| Raiss et al. [32] | Retrospective | IV | 15 | Moderate–High |
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinnamaneni, S.; Damron, T.A. Proximal humerus reconstruction in orthopedic oncology. J. Cancer Metastasis Treat. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adam, T. Management of common upper limb fractures in adults. Surgery 2022, 40, 184–191. [Google Scholar]
- Kulkarni, P.G.; Paudel, N.; Magar, S.; Santilli, M.F.; Kashyap, S.; Baranwal, A.K.; Zamboni, P.; Vasavada, P.; Katiyar, A.; Singh, A.V. Overcoming Challenges and Innovations in Orthopedic Prosthesis Design: An Interdisciplinary Perspective. Biomed. Mater. Devices 2023, 2, 58–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theil, C.; Schwarze, J.; Gosheger, G.; Moellenbeck, B.; Schneider, K.N.; Deventer, N.; Klingebiel, S.; Grammatopoulos, G.; Boettner, F.; Schmidt-Braekling, T. Implant Survival, Clinical Outcome and Complications of Megaprosthetic Reconstructions Following Sarcoma Resection. Cancers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labrum Joseph, T.; de Marinis, R.; Atwan, Y.; Marigi, E.M.; Houdek, M.T.; Barlow, J.D.; Morrey, M.E.; Sanchez-Sotelo, J.; Sperling, J.W. Reverse shoulder arthroplasty megaprosthesis for surgical management of severe proximal humeral bone loss. J. Shoulder Elb. Surg. 2024, 33, S64–S73. [Google Scholar] [CrossRef]
- Warby, S.A.; Watson, L.; Ford, J.J.; Hahne, A.J.; Pizzari, T. Multidirectional instability of the glenohumeral joint: Etiology, classification, assessment, and management. J. Hand Ther. 2017, 30, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Moisan, G.; Ma, C.Z.-H. Advances in prosthetics and orthotics. BMC Musculoskelet. Disord. 2024, 25, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiater, J.M.; Fabing, M.H. Shoulder arthroplasty: Prosthetic options and indications. J. Am. Acad. Orthop. Surg. 2009, 17, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Romero, B.A.; Horneff, J.G., 3rd. Soft Tissue Management in Shoulder Arthroplasty. Orthop. Clin. N. Am. 2022, 53, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Gosheger, G.; Hillmann, A.; Lindner, N.; Rödl, R.; Hoffmann, C.; Bürger, H.; Winkelmann, W. Soft tissue reconstruction of megaprostheses using a trevira tube. Clin. Orthop. Relat. Res. 2001, 393, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Paderno, M.; Saccomanno, M.F.; Milano, F.; Milano, G. The Musculoskeletal Tumor Society Scoring system is a valid subjective and objective tool to evaluate outcomes of surgical treatment of patients affected by upper and lower extremity tumors. Musculoskelet. Surg. 2024, 108, 201–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padua, R.; Padua, L.; Ceccarelli, E.; Romanini, E.; Zanoli, G.; Amadio, P.C.; Campi, A. Italian version of the Disability of the Arm, Shoulder and Hand (DASH) questionnaire. Cross-cultural adaptation and validation. J. Hand Surg. Br. 2003, 28, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- El Beaino, M.; Liu, J.; Lewis, V.O.; Lin, P.P. Do Early Results of Proximal Humeral Allograft-Prosthetic Composite Reconstructions Persist at 5-year Followup? Clin. Orthop. Relat. Res. 2019, 477, 758–765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartigan, D.E.; Veillette, C.J.; Sanchez-Sotelo, J.; Sperling, J.W.; Shives, T.C.; Cofield, R.H. Reconstruction of the proximal humerus for bone neoplasm using an anatomic prosthesis-bone graft composite. Acta. Orthop. Belg. 2012, 78, 450–457. [Google Scholar] [PubMed]
- Rahman, M.A.; Bassiony, A.A. Endoprosthetic replacement for tumors of the proximal humerus. Egypt. Orthop. J. 2013, 48, 37–44. [Google Scholar]
- Wang, B.; Wu, Q.; Liu, J.; Yang, S.; Shao, Z. Endoprosthetic reconstruction of the proximal humerus after tumour resection with polypropylene mesh. Int. Orthop. 2015, 39, 501–506. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, M.A.; Dijkstra, P.D.; Taminiau, A.H. Proximal humerus reconstruction after tumour resection: Biological versus endoprosthetic reconstruction. Int. Orthop. 2011, 35, 1375–1380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Luo, Y.; Wang, Y.; Zhang, Y.; Gong, T.; Tu, C.; Zhou, Y. Early functional and therapeutic effect of reversed tumour shoulder prosthesis reconstruction after proximal humerus tumour resection. Front. Surg. 2022, 9, 987161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Black, A.W.; Szabo, R.M.; Titelman, R.M. Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J. Shoulder Elb. Surg. 2007, 16, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Z.; Li, J.; Li, X.D.; Sang, H.X. Functional outcomes and complications of reconstruction of the proximal humerus after intra-articular tumor resection. Orthop. Surg. 2010, 2, 19–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Motassime, A.; Meschini, C.; Di Costa, D.; Rovere, G.; Matrangolo, M.R.; De Maio, F.; Farsetti, P.; Ziranu, A.; Maccauro, G.; Vitiello, R. Functional Outcomes and Shoulder Instability in Reconstruction of Proximal Humerus Metastases. Curr. Oncol. 2023, 30, 3571–3579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rachbauer, A.M.; Schneider, K.N.; Gosheger, G.; Deventer, N. Endoprosthetic Reconstruction of the Proximal Humerus with an Inverse Tumor Prosthesis. Cancers 2023, 15, 5330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Errani, C.; Aiba, H.; Atherley, A.; Palmas, M.; Kimura, H.; Donati, D.M.; Manfrini, M. What is the Revision-free Survival of Resurfaced Allograft-prosthesis Composites for Proximal Humerus Reconstruction in Children with Bone Tumors? Clin. Orthop. Relat. Res. 2024, 482, 979–990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vonck Caroline, E.; Kang, H.P.; Bell, J.A.; Gettleman, B.S.; Sebastian, R.; Trasolini, N.; Christ, A.B.; Menendez, L.R. Functional outcomes of oncologic reverse total shoulder arthroplasty after tumor resection. Semin. Arthroplast. JSES 2023, 33, 321–330. [Google Scholar] [CrossRef]
- Shi, S.F.; Dong, Y.; Zhang, C.L.; Bao, K.; Ma, X.J. Prosthesis replacement of the proximal humerus after the resection of bone tumors. Chin. J. Cancer 2010, 29, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Fucentese, S.F.; Sutter, R.; Wolfensperger, F.; Jost, B.; Gerber, C. Large metaphyseal volume hemiprostheses for complex fractures of the proximal humerus. J. Shoulder Elb. Surg. 2014, 23, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Tagliero, A.J.; Bukowski, B.R.; Rose, P.S.; Morrey, M.E.; Elhassan, B.T.; Barlow, J.D.; Wagner, E.R.; Sanchez-Sotelo, J.; Houdek, M.T. High incidence of complications associated with shoulder girdle reconstruction utilizing a Stryker proximal humerus cap endoprosthesis following Tikhoff-Linberg resections. Int. Orthop. 2020, 44, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, W.; Zeng, Q.; Wang, S.; Zhang, Z.; Liu, J.; Zhang, Y.; Shao, Z.; Wang, B. The Personalized Shoulder Reconstruction Assisted by 3D Printing Technology After Resection of the Proximal Humerus Tumours. Cancer Manag. Res. 2019, 11, 10665–10673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raiss, P.; Kinkel, S.; Sauter, U.; Bruckner, T.; Lehner, B. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur. J. Surg. Oncol. 2010, 36, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Heydari, S.; Hushmandi, K.; Daneshi, S.; Raesi, R. Bone tumors: A systematic review of prevalence, risk determinants, and survival patterns. BMC Cancer 2025, 25, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henrichs, M.P.; Krebs, J.; Gosheger, G.; Streitbuerger, A.; Nottrott, M.; Sauer, T.; Hoell, S.; Singh, G.; Hardes, J. Modular tumor endoprostheses in surgical palliation of long-bone metastases: A reduction in tumor burden and a durable reconstruction. World J. Surg. Oncol. 2014, 12, 330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goetti, P.; Denard, P.J.; Collin, P.; Ibrahim, M.; Mazzolari, A.; Lädermann, A. Biomechanics of anatomic and reverse shoulder arthroplasty. EFORT Open Rev. 2021, 6, 918–931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanchez-Sotelo, J.; Wagner, E.R.; Houdek, M.T. Allograft-Prosthetic Composite Reconstruction for Massive Proximal Humeral Bone Loss in Reverse Shoulder Arthroplasty. JBJS Essent. Surg. Tech. 2018, 8, e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maxwell, M.J.; Glass, E.A.; Bowler, A.R.; Koechling, Z.; Lohre, R.; Diestel, D.R.; McDonald-Stahl, M.; Bartels, W.; Vancleef, S.; Murthi, A.; et al. The effect of reverse shoulder arthroplasty design and surgical indications on deltoid and rotator cuff muscle length. J. Shoulder Elb. Surg. 2025, 34, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Hoogervorst, L.A.; van Schie, P.; Nagels, J.; Nelissen, R.G.H.H.; Marang-van de Mheen, P.J. The reliability of revision rates following primary shoulder arthroplasty as a quality indicator to rank hospital performance: A national registry analysis including 13,104 shoulders and 87 hospitals. J. Shoulder Elb. Surg. 2023, 32, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Scoring System | No. of Studies | Reported Range |
|---|---|---|
| MSTS (Musculoskeletal Tumor Society score) | 12 | 12/30 (40%)–27/30 (90%) |
| DASH (Disabilities of the Arm, Shoulder, and Hand score) | 3 | 20.8 (range 2.5–35.8) to 61.4 in unstable cases |
| DASH (stable vs. unstable shoulders) | 2 (36 pts) | Stable: 26.6; Unstable: 61.4 |
| MSTS (stable vs. unstable shoulders) | 2 | Stable: 75.8%; Unstable: 45.6% |
| Other scores (Constant, ASES, Enneking, ISOLS) | <2 each | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianni, L.; Capece, G.; Fiore, L.; De Fazio, A.; Martellini, S.; Maccauro, G.; Saccomanno, M.F. Glenohumeral Instability and Clinical Outcomes Following Proximal Humerus Resection and Megaprosthesis Implantation: A Systematic Review. J. Clin. Med. 2025, 14, 7850. https://doi.org/10.3390/jcm14217850
Cianni L, Capece G, Fiore L, De Fazio A, Martellini S, Maccauro G, Saccomanno MF. Glenohumeral Instability and Clinical Outcomes Following Proximal Humerus Resection and Megaprosthesis Implantation: A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7850. https://doi.org/10.3390/jcm14217850
Chicago/Turabian StyleCianni, Luigi, Giacomo Capece, Luca Fiore, Andrea De Fazio, Sara Martellini, Giulio Maccauro, and Maristella Francesca Saccomanno. 2025. "Glenohumeral Instability and Clinical Outcomes Following Proximal Humerus Resection and Megaprosthesis Implantation: A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7850. https://doi.org/10.3390/jcm14217850
APA StyleCianni, L., Capece, G., Fiore, L., De Fazio, A., Martellini, S., Maccauro, G., & Saccomanno, M. F. (2025). Glenohumeral Instability and Clinical Outcomes Following Proximal Humerus Resection and Megaprosthesis Implantation: A Systematic Review. Journal of Clinical Medicine, 14(21), 7850. https://doi.org/10.3390/jcm14217850







