Comparison of Immune Checkpoint Inhibitor (ICI) Myocarditis and Non-ICI Myocarditis Using Cardiovascular Magnetic Resonance: A Single-Centre Retrospective Observational Study
Abstract
1. Introduction
2. Methods
2.1. Study Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Cardiovascular Magnetic Resonance (CMR)
2.4. CMR Image Analysis
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Clinical Characteristics of ICI vs. Non-ICI Myocarditis
3.2. Characteristics of Patients with ICI Myocarditis
3.3. CMR Parameters of Patients
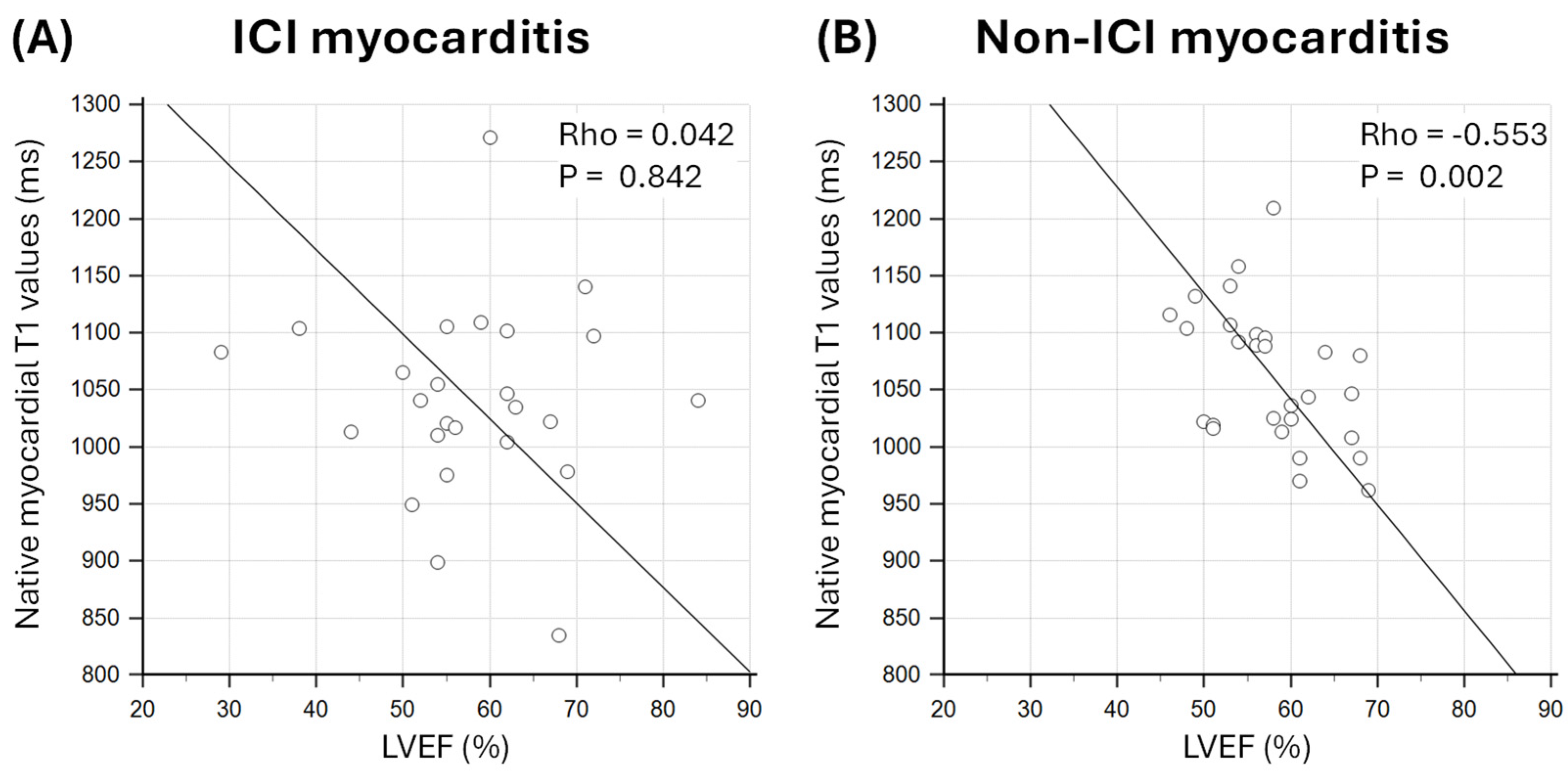
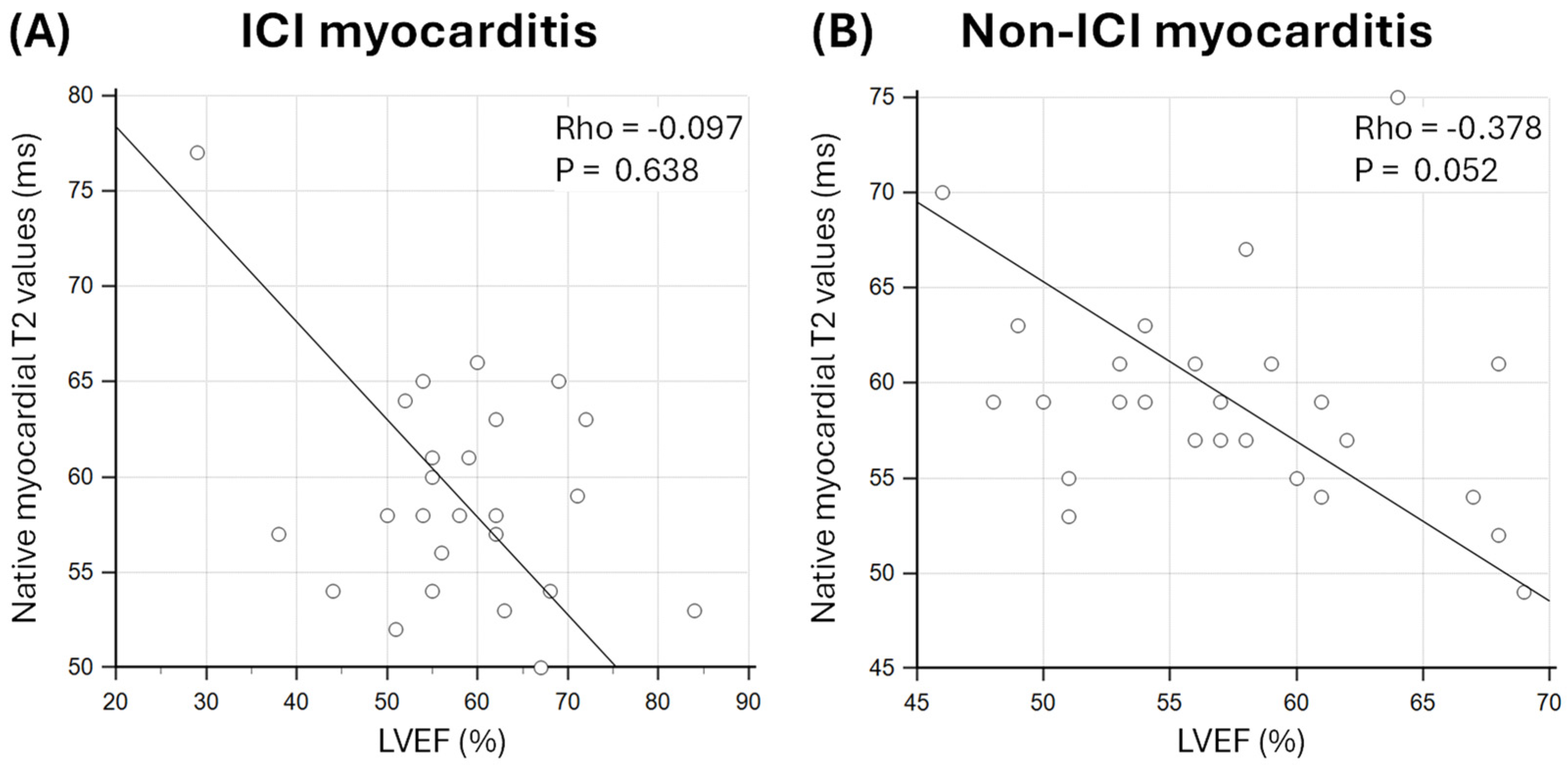
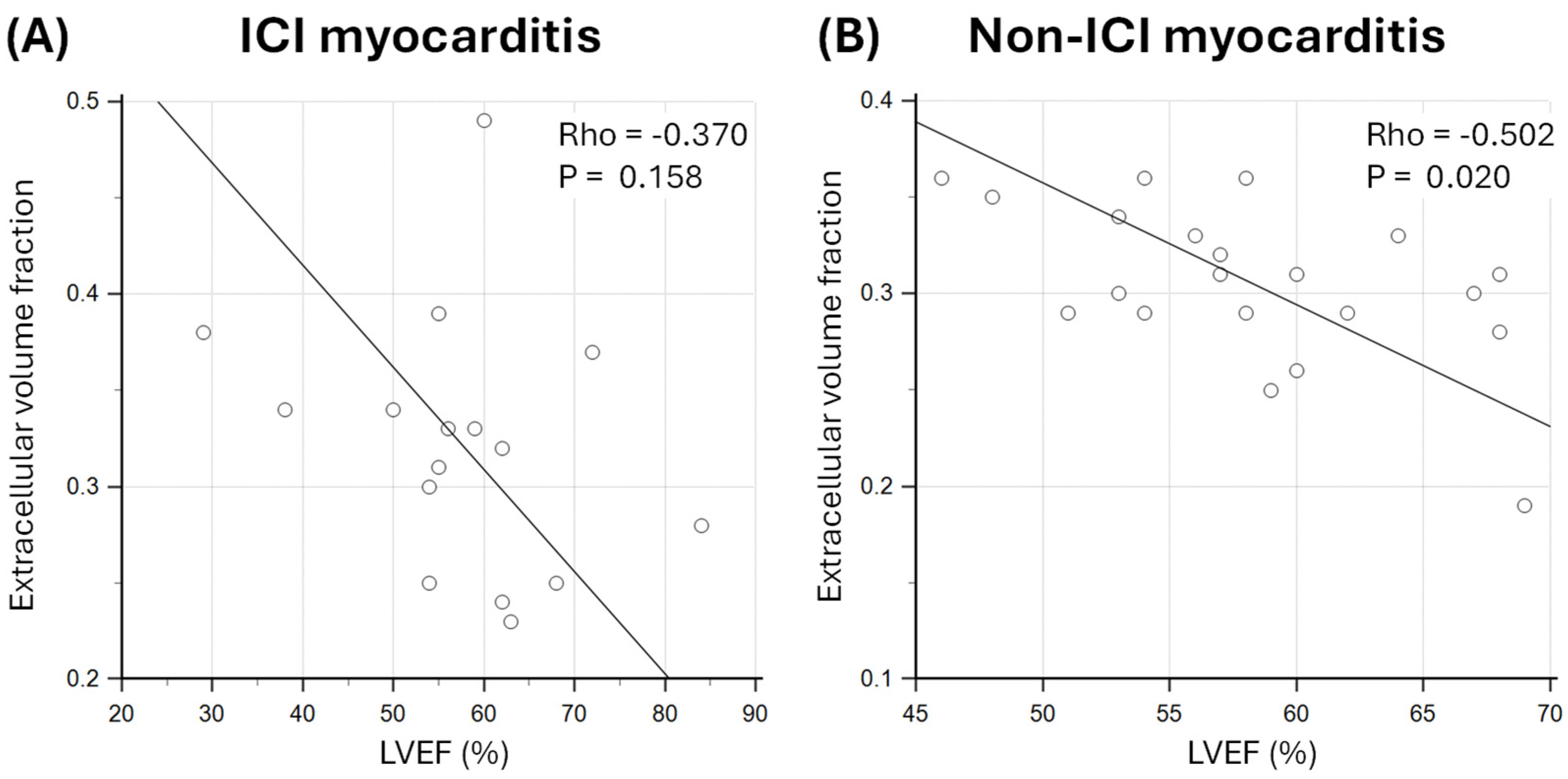
3.4. Relationship Between Markers of Oedema and Diffuse Fibrosis and LVEF
4. Discussion
4.1. Myocardial Oedema and Inflammation in Myocarditis
4.2. Effect of Myocardial Oedema and Fibrosis on LV Function
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef]
- Brociek, E.; Tymińska, A.; Giordani, A.S.; Caforio, A.L.P.; Wojnicz, R.; Grabowski, M.; Ozierański, K. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Biology 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Viadero, M.T.; Caldeiro, M.J.; Fernández-Suarez, N.; Garde, J.; Cabero, M.J.; González-Lamuño, D. Molecular Mechanisms and Pathophysiology of Myocardial Disease: Insights from Pediatric Inflammatory Multisystem Syndrome (PIMS) Associated with SARS-CoV-2. Int. J. Mol. Sci. 2025, 26, 3580. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Baumeier, C.; Aleshcheva, G.; Bock, C.-T.; Escher, F. Viral Myocarditis—From Pathophysiology to Treatment. J. Clin. Med. 2021, 10, 5240. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Frascaro, F.; Bianchi, N.; Sanguettoli, F.; Marchini, F.; Meossi, S.; Zanarelli, L.; Tonet, E.; Serenelli, M.; Guardigli, G.; Campo, G.; et al. Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. J. Clin. Med. 2023, 12, 7737. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-K.; Lam, T.-H.; Liao, S.-Y.; Lau, Y.-M.; Tse, H.-F.; So, B.Y.F. Immunopathogenesis of Immune Checkpoint Inhibitor Induced Myocarditis: Insights from Experimental Models and Treatment Implications. Biomedicines 2023, 11, 107. [Google Scholar] [CrossRef]
- Younis, A.; Gribben, J. Immune Checkpoint Inhibitors: Fundamental Mechanisms, Current Status and Future Directions. Immuno 2024, 4, 186–210. [Google Scholar] [CrossRef]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2023, 13, 22–40. [Google Scholar] [CrossRef]
- Cadour, F.; Cautela, J.; Rapacchi, S.; Varoquaux, A.; Habert, P.; Arnaud, F.; Jacquier, A.; Meilhac, A.; Paganelli, F.; Lalevée, N.; et al. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor–induced Myocarditis. Radiology 2022, 303, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Bluemke, D.A.; Bogaert, J.; Flamm, S.D.; Fontana, M.; Friedrich, M.G.; Grosse-Wortmann, L.; Karamitsos, T.D.; Kramer, C.M.; Kwong, R.Y.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidelines for reporting cardiovascular magnetic resonance examinations. J. Cardiovasc. Magn. Reson. 2022, 24, 29. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2016, 19, 75. [Google Scholar] [CrossRef]
- Malomo, S.; Oswald, T.; Stephenson, E.; Yip, A.; Alway, T.; Hadjivassilev, S.; Coombs, S.; Ellery, S.; Lee, J.; James, R.; et al. Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance. Diagnostics 2025, 15, 997. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.W.; Alexander, M.; Dib, Y.; Lau, P.K.H.; Weppler, A.M.; Au-Yeung, G.; Lee, B.; Khoo, C.; Mooney, D.; Joshi, S.B.; et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur. J. Cancer 2020, 124, 15–24. [Google Scholar] [CrossRef]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Fent, G.J.; Garg, P.; Foley, J.R.J.; Swoboda, P.P.; Dobson, L.E.; Erhayiem, B.; Treibel, T.A.; Moon, J.C.; Greenwood, J.P.; Plein, S. Synthetic Myocardial Extracellular Volume Fraction. JACC Cardiovasc. Imaging 2017, 10, 1402–1404. [Google Scholar] [CrossRef]
- Fouda, S.; Godfrey, R.; Pavitt, C.; Alway, T.; Coombs, S.; Ellery, S.M.; Parish, V.; Silberbauer, J.; Liu, A. Cardiac Sarcoidosis and Inherited Cardiomyopathies: Clinical Masquerade or Overlap? J. Clin. Med. 2025, 14, 1609. [Google Scholar] [CrossRef]
- Benz, D.C.; Gräni, C.; Antiochos, P.; Heydari, B.; Gissler, M.C.; Ge, Y.; Cuddy, S.A.M.; Dorbala, S.; Kwong, R.Y. Cardiac magnetic resonance biomarkers as surrogate endpoints in cardiovascular trials for myocardial diseases. Eur. Heart J. 2023, 44, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.; Hammond, R.; Donnelly, P.D.; Coates, A.R.M.; Liu, A. COVID-19 Pathophysiology: Inflammation to Cardiac Injury. Hearts 2024, 5, 628–644. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Circ. Hear. Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Ammirati, E.; Cartella, I.; Varrenti, M.; Selimi, A.; Sormani, P.; Garascia, A.; Palazzini, M. Acute myocarditis: 2024 state of the art. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2025, 27, i56–i60. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O.; et al. Recognition and Initial Management of Fulminant Myocarditis. Circulation 2020, 141, e69–e92. [Google Scholar] [CrossRef]
- Abumayyaleh, M.; Schupp, T.; Behnes, M.; El-Battrawy, I.; Hamdani, N.; Akin, I. COVID-19 and Myocarditis: Trends, Clinical Characteristics, and Future Directions. J. Clin. Med. 2025, 14, 4560. [Google Scholar] [CrossRef]
- Valevičienė, N.; Petrulionienė, Ž.; Petrauskienė, B.; Lauraitis, G.; Glaveckaitė, S.; Palionis, D.; Tamošiūnas, A.; Laucevičius, A. Differentiation of Acute Myocarditis and Acute Myocardial Infarction by the Regional Distribution of Myocardial Irreversible Injury Using Cardiovascular Magnetic Resonance Imaging. Medicina 2012, 48, 18. [Google Scholar] [CrossRef]
- Liu, A.; Hammond, R.; Donnelly, P.D.; Kaski, J.C.; Coates, A.R.M. Effective prognostic and clinical risk stratification in COVID-19 using multimodality biomarkers. J. Intern. Med. 2023, 294, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hammond, R.; Chan, K.; Chukwuenweniwe, C.; Johnson, R.; Khair, D.; Duck, E.; Olubodun, O.; Barwick, K.; Banya, W.; et al. Normal high-sensitivity cardiac troponin for ruling-out inpatient mortality in acute COVID-19. PLoS ONE 2023, 18, e0284523. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Hear. Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alejandre, R.; Ruiz-Fernández, I.; Martín, P. Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers 2022, 14, 4494. [Google Scholar] [CrossRef]
- Wang, D.; Bauersachs, J.; Berliner, D. Immune Checkpoint Inhibitor Associated Myocarditis and Cardiomyopathy: A Translational Review. Biology 2023, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, X.; Zhu, L.; Xu, J.; Zhou, D.; Yang, W.; Jiang, M.; Zhang, H.; Sirajuddin, A.; Arai, A.E.; et al. Prognostic Value of Myocardial Parametric Mapping in Patients with Acute Myocarditis: A Retrospective Study. Radiol. Cardiothorac. Imaging 2025, 7, e240125. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, L.; Jia, Y.; Liu, J.; Zhao, H.; Huo, L.; Zheng, B. Detection of acute myocarditis using T1 and T2 mapping cardiovascular magnetic resonance: A systematic review and meta-analysis. J. Appl. Clin. Med. Phys. 2021, 22, 239–248. [Google Scholar] [CrossRef]
- Chen, W.; Jeudy, J. Assessment of Myocarditis: Cardiac MR, PET/CT, or PET/MR? Curr. Cardiol. Rep. 2019, 21, 76. [Google Scholar] [CrossRef]
- Matusik, P.S.; Bryll, A.; Pac, A.; Popiela, T.J.; Matusik, P.T. Clinical Data, Chest Radiograph and Electrocardiography in the Screening for Left Ventricular Hypertrophy: The CAR(2)E(2) Score. J. Clin. Med. 2022, 11, 3585. [Google Scholar] [CrossRef]
- Matusik, P.S.; Matusik, P.T.; Stein, P.K. Cardiovascular reflex tests in patients with systemic lupus erythematosus: Clinical performance and utility. Lupus 2018, 27, 1759–1768. [Google Scholar] [CrossRef]
- Yasuda, N.; Kato, S.; Horita, N.; Sekii, R.; Sawamura, S.; Nagase, H.; Utsunomiya, D. Synthetic extracellular volume fraction as an imaging biomarker of the myocardial interstitium without blood sampling: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. 2025, 27, 101889. [Google Scholar] [CrossRef] [PubMed]

| ICI Myocarditis (n = 26) | Non-ICI Myocarditis (n = 28) | p-Value | |
|---|---|---|---|
| Age, years | 75 (71–78) | 39 (30–64) | <0.001 |
| Male | 15 (58) | 18 (64) | 0.781 |
| Body mass index, kg/m2 | 22 (22–26) | 25 (22–27) | 0.135 |
| Body surface area, m2 | 1.8 ± 0.3 | 2.0 ± 0.2 | 0.081 |
| Cardiac symptoms | |||
| Chest pain | 1 (4) | 19 (68) | <0.001 |
| Dyspnoea | 4 (15) | 11 (39) | 0.070 |
| Palpitations | 1 (4) | 2 (7) | 1.000 |
| Syncope/Presyncope | 0 (0) | 3 (11) | 0.237 |
| Co-morbidities | |||
| Diabetes mellitus | 3 (12) | 2 (7) | 0.663 |
| Hypertension | 7 (27) | 4 (14) | 0.320 |
| Hypercholesterolaemia | 5 (19) | 2 (7) | 0.243 |
| Chronic kidney disease | 2 (8) | 2 (7) | 1.000 |
| Atrial fibrillation | 6 (23) | 3 (11) | 0.286 |
| Ischaemic heart disease | 5 (19) | 1 (4) | 0.095 |
| COPD/Asthma | 3 (12) | 1 (4) | 0.342 |
| Stroke/TIA | 1 (4) | 0 (0) | 0.481 |
| Medications | |||
| ACE-I/ARB/ARNI | 16 (62) | 8 (29) | 0.027 |
| Beta-blocker | 19 (73) | 11 (39) | 0.016 |
| MRA | 8 (31) | 1 (4) | 0.010 |
| SGLT-2 inhibitor | 14 (54) | 5 (18) | 0.010 |
| Loop diuretics | 7 (27) | 3 (11) | 0.169 |
| Anti-platelet drugs | 7 (27) | 4 (14) | 0.320 |
| Statins | 5 (19) | 4 (14) | 0.724 |
| Anticoagulation | 4 (15) | 3 (11) | 0.699 |
| Serum biomarkers | |||
| Peak CRP, mg/L | 113 (37–148) (n = 25) | 47 (24–136) (n = 22) | 0.371 |
| Peak Hs-cTnT, ng/L | 77 (51–169) | 294 (57–1018) (n = 25) | 0.039 |
| Peak NT-pro BNP, ng/L | 7838 (2412–13,116) | 1631 (612–4147) (n = 11) | 0.015 |
| ICI Myocarditis (n = 26) | |
|---|---|
| Primary malignancy site | |
| Lung | 10 (38) |
| Skin | 7 (27) |
| Tongue | 3 (12) |
| Bladder | 2 (8) |
| Thyroid | 1 (4) |
| Breast | 1 (4) |
| Cholangiocarcinoma | 1 (4) |
| Renal | 1 (4) |
| ICI agent | |
| Pembrolizumab | 8 (31) |
| Ipilimumab/Nivolumab | 5 (19) |
| Atezolizumab | 3 (12) |
| Cemiplimab | 3 (12) |
| Nivolumab alone | 2 (8) |
| Durvalumab | 2 (8) |
| Avelumab | 1 (4) |
| Denosumab | 1 (4) |
| Lenvatinib | 1 (4) |
| Immunosuppressive therapy | |
| IV methylprednisolone | 21 (81) |
| Oral prednisolone | 24 (92) |
| Mycophenolate mofetil | 15 (58) |
| Tacrolimus | 5 (19) |
| Infliximab | 5 (19) |
| Azathioprine | 3 (12) |
| Tocilizumab | 2 (8) |
| Methotrexate | 1 (4) |
| ICI Myocarditis (n = 26) | Non-ICI Myocarditis (n = 28) | p-Value | |
|---|---|---|---|
| CMR volumes and function | |||
| LV EDVi, mL/m2 | 74 ± 20 | 84 ± 21 | 0.072 |
| LV ESVi, mL/m2 | 33 ± 15 | 36 ± 13 | 0.325 |
| LV SVi, mL/m2 | 42 ± 9 | 48 ± 10 | 0.022 |
| LV EF, % | 58 ± 11 | 58 ± 6 | 0.970 |
| RV EDVi, mL/m2 | 69 (61–89) | 74 (63–100) | 0.229 |
| RV ESVi, mL/m2 | 31 (23–37) | 32 (22–45) | 0.788 |
| RV SVi, mL/m2 | 42 ± 12 | 47 ± 10 | 0.075 |
| RV EF, % | 57 ± 6 | 59 ±10 | 0.341 |
| LV mass index, g/m2 | 52 (44–57) | 58 (48–67) | 0.051 |
| LGE data | |||
| LV LGE present | 12 (52) (n = 23) | 24 (89) (n = 27) | 0.005 |
| Subepicardial/mid-wall | 0 (0) | 11 (41) | <0.001 |
| Mid-wall only | 9 (39) | 9 (33) | 0.771 |
| Subepicardial only | 0 (0) | 2 (7) | 0.493 |
| Transmural/Subendocardial | 3 (13) | 2 (7) | 0.651 |
| RV LGE present | 0 (0) | 0 (0) | - |
| Native myocardial T1 values (ms) | 1041 ± 84 (n = 25) | 1063 ± 60 | 0.281 |
| Native myocardial T2 values (ms) | 59 ± 6 | 59 ± 6 (27) | 0.943 |
| Synthetic ECV | 0.32 ± 0.07 (n = 16) | 0.31 ± 0.04 (21) | 0.403 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, E.; Yip, A.; Hodge, A.; McLean, D.; Lee, J.; Parish, V.; Ellery, S.; Olsson-Brown, A.; Liu, A. Comparison of Immune Checkpoint Inhibitor (ICI) Myocarditis and Non-ICI Myocarditis Using Cardiovascular Magnetic Resonance: A Single-Centre Retrospective Observational Study. J. Clin. Med. 2025, 14, 7809. https://doi.org/10.3390/jcm14217809
Jacobs E, Yip A, Hodge A, McLean D, Lee J, Parish V, Ellery S, Olsson-Brown A, Liu A. Comparison of Immune Checkpoint Inhibitor (ICI) Myocarditis and Non-ICI Myocarditis Using Cardiovascular Magnetic Resonance: A Single-Centre Retrospective Observational Study. Journal of Clinical Medicine. 2025; 14(21):7809. https://doi.org/10.3390/jcm14217809
Chicago/Turabian StyleJacobs, Ella, Anthony Yip, Alison Hodge, Denise McLean, Joon Lee, Victoria Parish, Susan Ellery, Anna Olsson-Brown, and Alexander Liu. 2025. "Comparison of Immune Checkpoint Inhibitor (ICI) Myocarditis and Non-ICI Myocarditis Using Cardiovascular Magnetic Resonance: A Single-Centre Retrospective Observational Study" Journal of Clinical Medicine 14, no. 21: 7809. https://doi.org/10.3390/jcm14217809
APA StyleJacobs, E., Yip, A., Hodge, A., McLean, D., Lee, J., Parish, V., Ellery, S., Olsson-Brown, A., & Liu, A. (2025). Comparison of Immune Checkpoint Inhibitor (ICI) Myocarditis and Non-ICI Myocarditis Using Cardiovascular Magnetic Resonance: A Single-Centre Retrospective Observational Study. Journal of Clinical Medicine, 14(21), 7809. https://doi.org/10.3390/jcm14217809






