Radiolabeled LHRH and FSH Analogues as Cancer Theranostic Agents: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
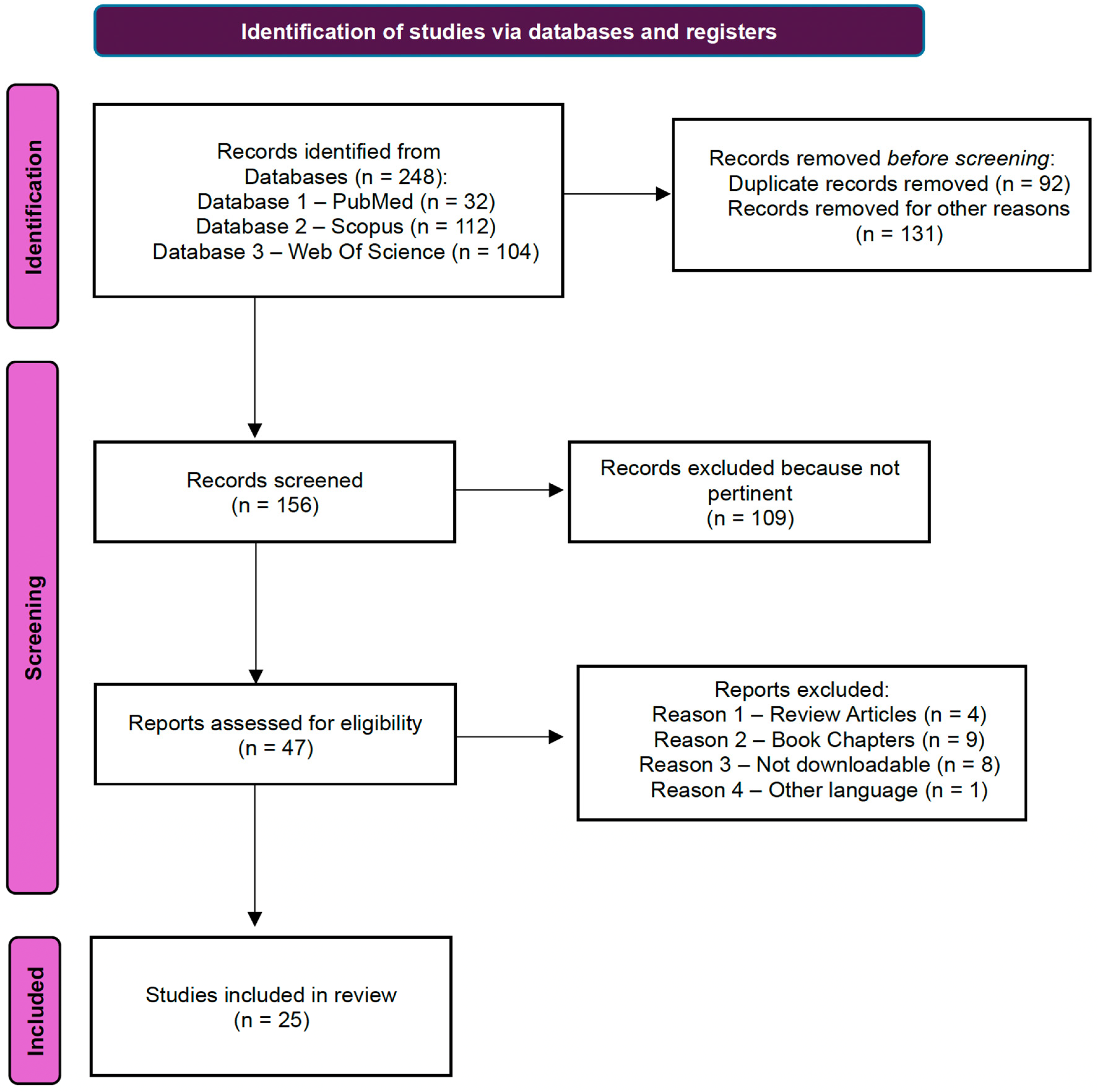
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Management
2.4. Assessment of Risk Bias in Included Studies
3. Results and Discussion
3.1. Data Synthesis
3.2. Radiolabeled Biomolecule Targeting FSH-R
3.2.1. Radiolabeled Monoclonal Antibodies Against FSH-R
3.2.2. Radiolabeled FSHβ-Derivative Peptides in Cancer Imaging
3.3. Radiolabeled LHRH Derivative Peptides
3.3.1. Radiolabeled LHRH in SPECT Imaging Applications
3.3.2. Radiolabeled LHRH in PET Imaging Applications
3.3.3. Nanoradiopharmaceuticals: Applications in Nuclear Medicine Imaging
3.3.4. Radiolabeled LHRH for Therapeutic Applications
3.3.5. Study of an LHRH-Derived Vaccine by SPECT
3.4. Final Summary of Results
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Domain | Animal Selection | Index Test | Reference Standard | Flow and Timing |
|---|---|---|---|---|
| Signaling question (yes, no or unclear) | Does the origin of animals come from company? | (1) Is the origin of bacterial cells certified ATCC? (2) Can the chelator-peptide synthesis be a source of bias? (3) Can the radiolabelling be a source of bias? (4) Were additional in vitro, in vivo, and ex vivo tests performed to support the main results? | Is the reference standard used appropriate for the study? | Is the imaging time appropriated for the study? |
| Risk of bias (high, low or unclear) | Could the selection of animals have introduced bias? | Could the methodology of experiments have introduced bias? | Could the reference standard or its interpretation have introduced bias? | Could the Radiopharmaceuticals injection administration time be a source of bias? |
| Concerns about applicability (high, low or unclear) | Are there concerns that the included animals do not match the review questions? | Are there concerns that the index test or its interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? | Could the study flow have introduced bias? |
References
- Chaudhary, P.K.; Kim, S. An Insight into GPCR and G-Proteins as Cancer Drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.M.; Huckle, W.R.; Andrews, W.V.; Mcardle, C.A. The Molecular Mechanism of Action of Gonadotropin Releasing Hormone (GnRH) in the Pituitary. In Proceedings of the 1986 Laurentian Hormone Conference; Clark, J.H., Ed.; Recent Progress in Hormone Research; Academic Press: Boston, MA, USA, 1987; Volume 43, pp. 29–68. ISBN 978-0-12-571143-2. [Google Scholar]
- Conn, P.M.; Crowley, W.F., Jr. Gonadotropin-releasing hormone and its analogs. Annu. Rev. Med. 1994, 45, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, N.; Hawkins, R.; Atwood, C.; Butler, T. The Roles of GnRH in the Human Central Nervous System. Horm. Behav. 2022, 145, 105230. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Weiss, S.; Ortmann, O.; Grundker, C.; Schulz, K.D. LHRH Might Act as a Negative Autocrine Regulator of Proliferation of Human Ovarian Cancer. Eur. J. Endocrinol. 2000, 142, 665–670. [Google Scholar] [CrossRef][Green Version]
- Dharap, S.S.; Wang, Y.; Chandna, P.; Khandare, J.J.; Qiu, B.; Gunaseelan, S.; Sinko, P.J.; Stein, S.; Farmanfarmaian, A.; Minko, T. Tumor-Specific Targeting of an Anticancer Drug Delivery System by LHRH Peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 12962–12967. [Google Scholar] [CrossRef]
- Buchholz, S.; Seitz, S.; Schally, A.V.; Engel, J.B.; Rick, F.G.; Szalontay, L.; Hohla, F.; Krishan, A.; Papadia, A.; Gaiser, T.; et al. Triple-Negative Breast Cancers Express Receptors for Luteinizing Hormone-Releasing Hormone (LHRH) and Respond to LHRH Antagonist Cetrorelix with Growth Inhibition. Int. J. Oncol. 2009, 35, 789–796. [Google Scholar] [CrossRef][Green Version]
- Völker, P.; Gründker, C.; Schmidt, O.; Schulz, K.-D.; Emons, G. Expression of Receptors for Luteinizing Hormone-Releasing Hormone in Human Ovarian and Endometrial Cancers: Frequency, Autoregulation, and Correlation with Direct Antiproliferative Activity of Luteinizing Hormone-Releasing Hormone Analogues. Am. J. Obstet. Gynecol. 2002, 186, 171–179. [Google Scholar] [CrossRef]
- Gründker, C.; Emons, G. Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer. Cells 2021, 10, 437. [Google Scholar] [CrossRef]
- Halmos, G.; Arencibia, J.M.; Schally, A.V.; Davis, R.; Bostwick, D.G. High Incidence of Receptors for Luteinizing Hormone-Releasing Hormone (LHRH) and LHRH Receptor Gene Expression in Human Prostate Cancers. J. Urol. 2000, 163, 623–629. [Google Scholar] [CrossRef]
- Friess, H.; Büchler, M.; Kiesel, L.; Krüger, M.; Beger, H.G. LH-RH Receptors in the Human Pancreas. Int. J. Pancreatol. 1991, 10, 151–159. [Google Scholar] [CrossRef]
- Keller, G.; Schally, A.V.; Gaiser, T.; Nagy, A.; Baker, B.; Halmos, G.; Engel, J.B. Receptors for Luteinizing Hormone Releasing Hormone Expressed on Human Renal Cell Carcinomas Can Be Used for Targeted Chemotherapy with Cytotoxic Luteinizing Hormone Releasing Hormone Analogues. Clin. Cancer Res. 2005, 11, 5549–5557. [Google Scholar] [CrossRef][Green Version]
- Koushik, K.; Bandi, N.; Sundaram, S.; Kompella, U.B. Evidence for LHRH-Receptor Expression in Human Airway Epithelial (Calu-3) Cells and Its Role in the Transport of an LHRH Agonist. Pharm. Res. 2004, 21, 1034–1046. [Google Scholar] [CrossRef]
- Moretti, R.M.; Montagnani Marelli, M.; Van Groeninghen, J.C.; Limonta, P. Locally Expressed LHRH Receptors Mediate the Oncostatic and Antimetastatic Activity of LHRH Agonists on Melanoma Cells. J. Clin. Endocrinol. Metab. 2002, 87, 3791–3797. [Google Scholar] [CrossRef][Green Version]
- Marelli, M.M.; Moretti, R.M.; Mai, S.; Müller, O.; Van Groeninghen, J.C.; Limonta, P. Novel Insights into GnRH Receptor Activity: Role in the Control of Human Glioblastoma Cell Proliferation. Oncol. Rep. 2009, 21, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Schally, A.V.; Hawes, D.; Xiong, S.; Fazli, L.; Gleave, M.; Cai, J.; Groshen, S.; Brands, F.; Engel, J.; et al. Expression of Receptors for Luteinizing Hormone-Releasing Hormone (LH-RH) in Prostate Cancers Following Therapy with LH-RH Agonists. Clin. Cancer Res. 2010, 16, 4675–4680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Taratula, O.; Taratula, O.; Schumann, C.; Minko, T. LHRH-Targeted Drug Delivery Systems for Cancer Therapy. Mini Rev. Med. Chem. 2017, 17, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Schally, A.V. Targeting of Cytotoxic Luteinizing Hormone-Releasing Hormone Analogs to Breast, Ovarian, Endometrial, and Prostate Cancers. Biol. Reprod. 2005, 73, 851–859. [Google Scholar] [CrossRef]
- Engel, J.; Emons, G.; Pinski, J.; Schally, A.V. AEZS-108: A Targeted Cytotoxic Analog of LHRH for the Treatment of Cancers Positive for LHRH Receptors. Expert. Opin. Investig. Drugs 2012, 21, 891–899. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Zhang, W.-D.; Yuan, B.; Zhang, J.-B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Sardella, C.; Russo, D.; Raggi, F.; Lombardi, M.; Urbani, C.; Brogioni, S.; Boggi, U.; Funel, N.; Chifenti, B.; Campani, D.; et al. Ectopic Expression of FSH Receptor Isoforms in Neoplastic but Not in Endothelial Cells from Pancreatic Neuroendocrine Tumors. J. Endocrinol. Invest. 2013, 36, 174–179. [Google Scholar] [CrossRef]
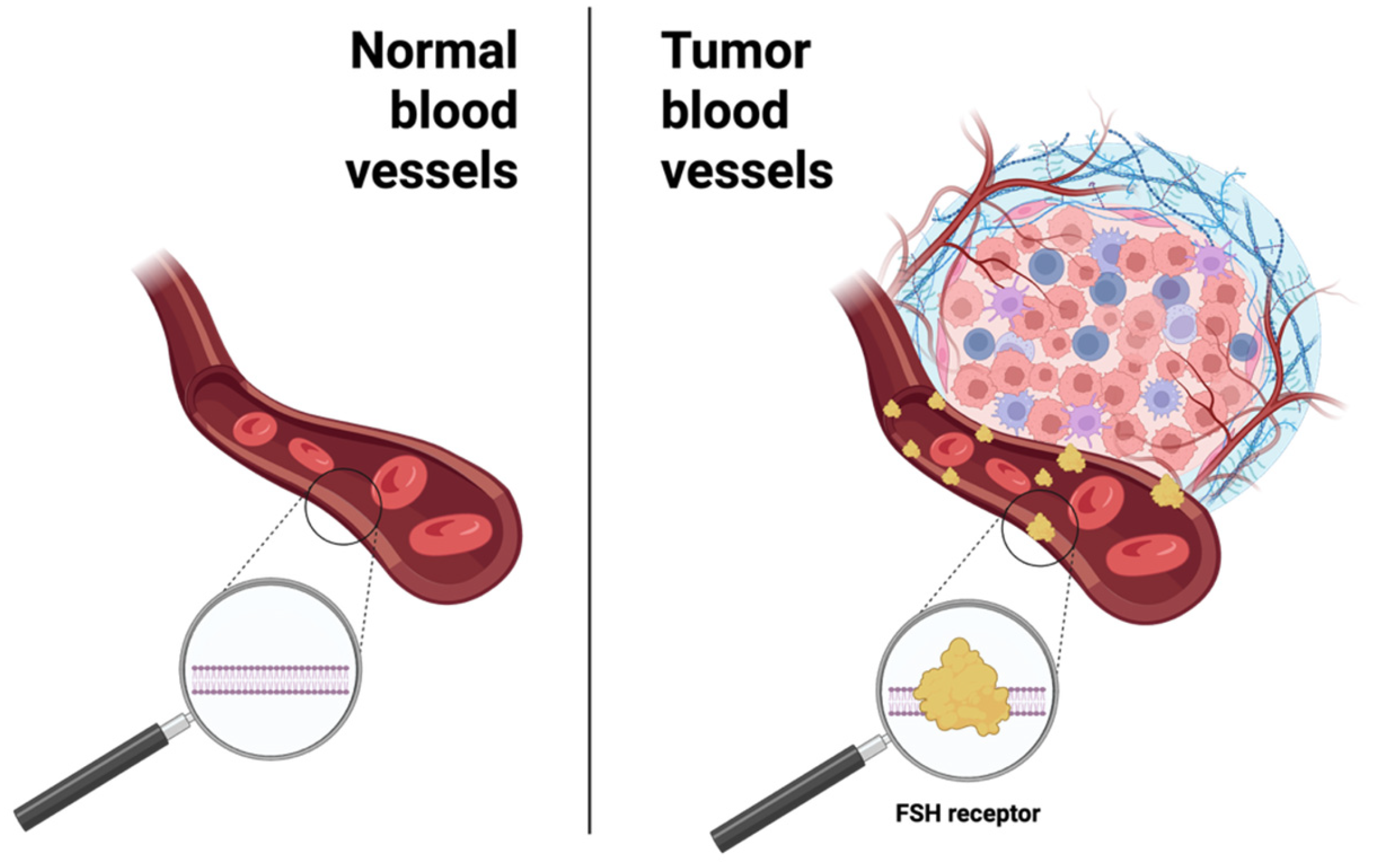
- Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; Fromont, G.; Hai, M.T.V.; Ghinea, N. Expression of Follicle-Stimulating Hormone Receptor in Tumor Blood Vessels. N. Engl. J. Med. 2010, 363, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.; Desestret, V.; Antoine, M.; Fromont, G.; Huerre, M.; Sanson, M.; Camparo, P.; Pichon, C.; Planeix, F.; Gonin, J.; et al. Expression of Follicle-Stimulating Hormone Receptor by the Vascular Endothelium in Tumor Metastases. BMC Cancer 2013, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Kountourakis, P.; Kottorou, A.E.; Antonacopoulou, A.G.; Rolfo, C.; Peeters, M.; Kalofonos, H.P. Follicle-Stimulating Hormone Receptor (FSHR): A Promising Tool in Oncology? Mol. Diagn. Ther. 2016, 20, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jia, L.; Feng, Y.; Zheng, W. Overexpression of Follicle-Stimulating Hormone Receptor Facilitates the Development of Ovarian Epithelial Cancer. Cancer Lett. 2009, 278, 56–64. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Hong, H.; Yan, Y.; Shi, S.; Graves, S.A.; Krasteva, L.K.; Nickles, R.J.; Yang, M.; Cai, W. PET of Follicle-Stimulating Hormone Receptor: Broad Applicability to Cancer Imaging. Mol. Pharm. 2015, 12, 403–410. [Google Scholar] [CrossRef]
- Yang, D.; Feng, L.; Dougherty, C.A.; Luker, K.E.; Chen, D.; Cauble, M.A.; Banaszak Holl, M.M.; Luker, G.D.; Ross, B.D.; Liu, Z.; et al. In Vivo Targeting of Metastatic Breast Cancer via Tumor Vasculature-Specific Nano-Graphene Oxide. Biomaterials 2016, 104, 361–371. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, D.; Zhu, C.; Xu, Q.; Wang, L.; Chen, F.; Yang, R.; Luo, S.; Yang, M.; Yan, Y. Pilot Study of a Novel 18F-Labeled FSHR Probe for Tumor Imaging. Mol. Imaging Biol. 2014, 16, 578–585. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Q.; Pan, D.; Xu, Y.; Liu, P.; Yang, R.; Wang, L.; Sun, X.; Luo, S.; Yang, M. Prostate Cancer Imaging of FSHR Antagonist Modified with a Hydrophilic Linker. Contrast Media Mol. 2016, 11, 99–105. [Google Scholar] [CrossRef]
- Pan, D.; Liu, G.; Xu, Y.; Wang, Y.; Yue, Y.; Wang, L.; Yan, J.; Wang, X.; Yang, R.; Yang, M. PET Imaging of FSHR Expression in Tumors with 68Ga-Labeled FSH1 Peptide. Contrast Media Mol. Imaging 2017, 2017, 2674502. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, L.; Wang, X.; Yan, J.; Xu, Y.; Yang, M. Optimizing the Performance of 68 Ga Labeled FSHR Ligand in Prostate Cancer Model by Co-Administration of Aprotinin. Int. J. Radiat. Biol. 2022, 98, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, M.; Attar Nosrati, S.; Rogni, F.; Mahdiyani, B. Evaluation of 111In-Labeled GnRH-I Tracer for SPECT Tumor Imaging. Radiochemistry 2019, 61, 226–232. [Google Scholar] [CrossRef]
- Guo, H.; Lu, J.; Hathaway, H.; Royce, M.E.; Prossnitz, E.R.; Miao, Y. Synthesis and Evaluation of Novel Gonadotropin-Releasing Hormone Receptor-Targeting Peptides. Bioconjug. Chem. 2011, 22, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gallazzi, F.; Sklar, L.A.; Miao, Y. A Novel Indium-111-Labeled Gonadotropin-Releasing Hormone Peptide for Human Prostate Cancer Imaging. Bioorg. Med. Chem. Lett. 2011, 21, 5184–5187. [Google Scholar] [CrossRef]
- Xu, J.; Feng, C.; Miao, Y. Evaluation of Novel 111In-Labeled Gonadotropin-Releasing Hormone Peptides for Human Prostate Cancer Imaging. Bioorg. Med. Chem. Lett. 2017, 27, 4647–4651. [Google Scholar] [CrossRef]
- Farahani, A.M.; Maleki, F.; Sadeghzadeh, N.; Abediankenari, S.; Abedi, S.M.; Erfani, M. Evaluation of a New 99mTc-Labeled GnRH Analogue as a Possible Imaging Agent for Prostate Cancer Detection. ACAMC 2020, 20, 1695–1703. [Google Scholar] [CrossRef]
- Masteri Farahani, A.; Maleki, F.; Sadeghzadeh, N.; Abediankenari, S.; Abedi, S.M.; Erfani, M. 99mTc-(EDDA/Tricine)-HYNIC-GnRH Analogue as a Potential Imaging Probe for Diagnosis of Prostate Cancer. Chem. Biol. Drug Des. 2020, 96, 850–860. [Google Scholar] [CrossRef]
- Hao, D.; Sun, L.; Hu, X.; Hao, X. 99mTc-LHRH in Tumor Receptor Imaging. Oncol. Lett. 2017, 14, 569–578. [Google Scholar] [CrossRef][Green Version]
- Calderon, L.E.; Black, C.A.; Rollins, J.D.; Overbay, B.; Shiferawe, S.; Elliott, A.; Reitz, S.; Liu, S.; Li, J.; Ng, C.K.; et al. Synthesis of Radiolabeled Technetium- and Rhenium-Luteinizing Hormone-Releasing Hormone (99mTc/Re-Acdien-LHRH) Conjugates for Targeted Detection of Breast Cancer Cells Overexpressing the LHRH Receptor. ACS Omega 2021, 6, 1846–1856. [Google Scholar] [CrossRef]
- Zoghi, M.; Niazi, A.; Jalilian, A.R.; Johari-daha, F.; Alirezapour, B.; Ramezanpour, S. Development of a Ga-68 Labeled Triptorelin Analog for GnRH Receptor Imaging. Radiochim. Acta 2016, 104, 247–255. [Google Scholar] [CrossRef]
- Zoghi, M.; Jalilian, A.R.; Niazi, A.; Johari-daha, F.; Alirezapour, B.; Ramezanpour, S. Development of a 68Ga-Peptide Tracer for PET GnRH1-Imaging. Ann. Nucl. Med. 2016, 30, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Berger, S.; Poethko, T.; Schwaiger, M.; Wester, H.-J. Development of Novel 68Ga- and 18F-Labeled GnRH-I Analogues with High GnRHR-Targeting Efficiency. Bioconjug. Chem. 2008, 19, 1256–1268. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Han, Y.; Fu, L.; Ren, Y.; Zhang, Y.; Li, Y.; Sun, P.; Wang, M.; Wu, H.; et al. Synthesis and Evaluation of 18F-Labeled Peptide for Gonadotropin-Releasing Hormone Receptor Imaging. Contrast Media Mol. Imaging 2019, 2019, 5635269. [Google Scholar] [CrossRef]
- Huang, S.; Wu, H.; Li, B.; Fu, L.; Sun, P.; Wang, M.; Hu, K. Automated Radiosynthesis and Preclinical Evaluation of Al[18F]F-NOTA-P-GnRH for PET Imaging of GnRH Receptor-Positive Tumors. Nucl. Med. Biol. 2020, 82–83, 64–71. [Google Scholar] [CrossRef]
- De, K.; Tanbir, S.K.E.; Sinha, S.; Mukhopadhyay, S. Lipid-Based Nanocarrier by Targeting with LHRH Peptide: A Promising Approach for Prostate Cancer Radio-Imaging and Therapy. Mol. Pharm. 2024, 21, 4128–4146. [Google Scholar] [CrossRef]
- Gao, F.; Cai, P.; Yang, W.; Xue, J.; Gao, L.; Liu, R.; Wang, Y.; Zhao, Y.; He, X.; Zhao, L.; et al. Ultrasmall [64Cu]Cu Nanoclusters for Targeting Orthotopic Lung Tumors Using Accurate Positron Emission Tomography Imaging. ACS Nano 2015, 9, 4976–4986. [Google Scholar] [CrossRef]
- Han, W.; Yang, W.; Gao, F.; Cai, P.; Wang, J.; Wang, S.; Xue, J.; Gao, X.; Liu, Y. Iodine-124 Labeled Gold Nanoclusters for Positron Emission Tomography Imaging in Lung Cancer Model. J. Nanosci. Nanotechnol. 2020, 20, 1375–1382. [Google Scholar] [CrossRef]
- Zoghi, M.; Nosrati, S.A.; Rogni, F.; Rajabifar, S. Preparation of a Radiolabeled GnRH-I Analogue Derivative with 111In as a New Anti-Proliferative Agent. J. Label. Compd. Radiopharm. 2018, 61, 903–911. [Google Scholar] [CrossRef]
- Zoghi, M.; Attar Nosrati, S.; Rogni, F.; Shirvani, G.; Johari Daha, F. Preclinical Evaluation of New GnRH-I Receptor Radionuclide Therapy with 177Lu-Peptide Tracer. J. Label. Compd. Radiopharm. 2019, 62, 310–320. [Google Scholar] [CrossRef]
- Chang, C.-H.; Hsu, W.-C.; Wang, C.-Y.; Jan, M.-L.; Tsai, T.-H.; Lee, T.-W.; Lynn, S.-G.; Yeh, C.-H.; Chang, T.-J. Longitudinal MicroSPECT/CT Imaging and Pharmacokinetics of Synthetic Luteinizing Hormone-Releasing Hormone (LHRH) Vaccine in Rats. Anticancer Res. 2007, 27, 3251–3257. [Google Scholar]
- Fan, Q.R.; Hendrickson, W.A. Structure of Human Follicle-Stimulating Hormone in Complex with Its Receptor. Nature 2005, 433, 269–277. [Google Scholar] [CrossRef]
- Santa Coloma, T.A.; Dattatreyamurty, B.; Reichert, L.E. A Synthetic Peptide Corresponding to Human FSH Beta-Subunit 33-53 Binds to FSH Receptor, Stimulates Basal Estradiol Biosynthesis, and Is a Partial Antagonist of FSH. Biochemistry 1990, 29, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Santa-Coloma, T.A.; Crabb, J.W.; Reichert, L.E. Serine Analogues of hFSH-Beta-(33–53) and hFSH-Beta-(81–95) Inhibit hFSH Binding to Receptor. Biochem. Biophys. Res. Commun. 1992, 184, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dias, J.A.; He, X. Structural Biology of Glycoprotein Hormones and Their Receptors: Insights to Signaling. Mol. Cell. Endocrinol. 2014, 382, 424–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, P.; Pan, D.; Zhang, P.; Bai, Z.; Xu, Y.; Wang, L.; Yan, J.; Yan, Y.; Liu, X.; et al. An Investigation on a Novel Anti-Tumor Fusion Peptide of FSH33-53-IIKK. J. Cancer 2016, 7, 1010–1019. [Google Scholar] [CrossRef][Green Version]
- Varani, M.; Bentivoglio, V.; Lauri, C.; Ranieri, D.; Signore, A. Methods for Radiolabelling Nanoparticles: SPECT Use (Part 1). Biomolecules 2022, 12, 1522. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Varani, M.; Lauri, C.; Ranieri, D.; Signore, A. Methods for Radiolabelling Nanoparticles: PET Use (Part 2). Biomolecules 2022, 12, 1517. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Nayak, P.; Varani, M.; Lauri, C.; Signore, A. Methods for Radiolabeling Nanoparticles (Part 3): Therapeutic Use. Biomolecules 2023, 13, 1241. [Google Scholar] [CrossRef]
- Ozgenc, E.; Karpuz, M.; Arzuk, E.; Gonzalez-Alvarez, M.; Sanz, M.B.; Gundogdu, E.; Gonzalez-Alvarez, I. Radiolabeled Trastuzumab Solid Lipid Nanoparticles for Breast Cancer Cell: In Vitro and in Vivo Studies. ACS Omega 2022, 7, 30015–30027. [Google Scholar] [CrossRef]
- Giorgio, A.; Del Gatto, A.; Pennacchio, S.; Saviano, M.; Zaccaro, L. Peptoids: Smart and Emerging Candidates for the Diagnosis of Cancer, Neurological and Autoimmune Disorders. Int. J. Mol. Sci. 2023, 24, 16333. [Google Scholar] [CrossRef]
- Bentivoglio, V.; D’Ippolito, E.; Nayak, P.; Giorgio, A.; Lauri, C. Bispecific Radioligands (BRLs): Two Is Better Than One. J. Clin. Med. 2025, 14, 5628. [Google Scholar] [CrossRef]


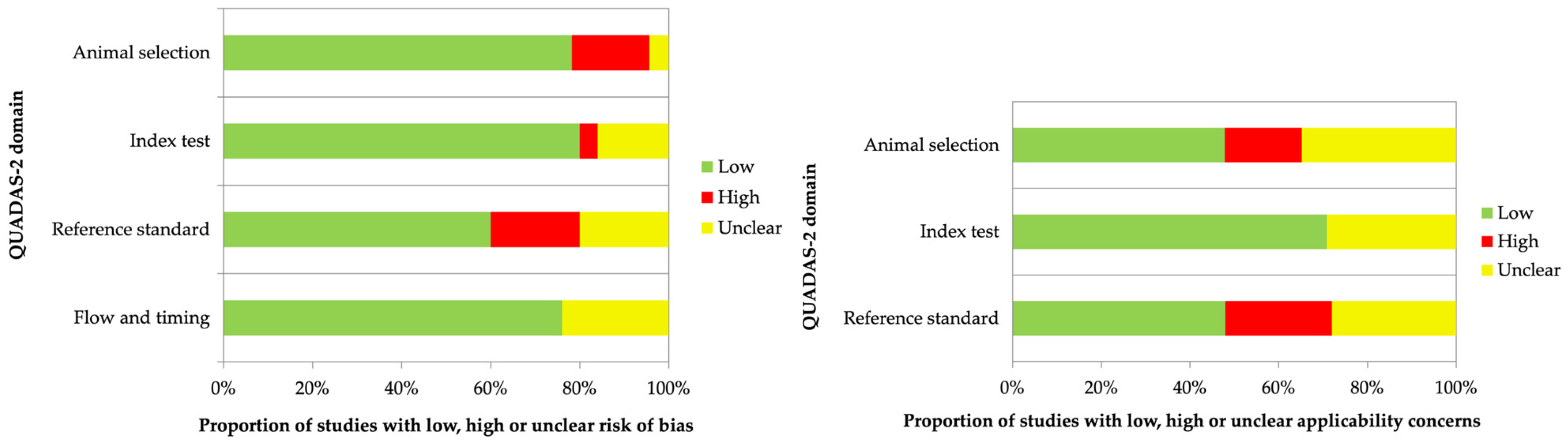
| First Name and Ref. | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Animal Selection | Index Test | Reference Standard | Flow and Timing | Animal Selection | Index Test | Reference Standard | |
| Radiolabeled biomolecules targeting FSH-R | |||||||
| Hong [28] |  |  |  |  |  |  |  |
| Yang [29] |  |  |  |  |  |  |  |
| Xu [30] |  |  |  |  |  |  |  |
| Zhu [31] |  |  |  |  |  |  |  |
| Pan [32] |  |  |  |  |  |  |  |
| Pan [33] |  |  |  |  |  |  |  |
| Radiolabeled biomolecules targeting LHRH-R | |||||||
| SPECT applications | |||||||
| Zoghi [34] |  |  |  |  |  |  |  |
| Guo [35] |  |  |  |  |  |  |  |
| Guo [36] |  |  |  |  |  |  |  |
| Xu [37] |  |  |  |  |  |  |  |
| Farahani [38] |  |  |  |  |  |  |  |
| Masteri Farahani [39] |  |  |  |  |  |  |  |
| Hao [40] | - ** |  |  |  | - ** |  |  |
| Calderon [41] | - ** |  |  |  | - ** |  |  |
| PET applications | |||||||
| Zoghi [42] |  |  |  |  |  |  |  |
| Zoghi [43] |  |  |  |  |  |  |  |
| Schottelius [44] |  |  |  |  |  |  |  |
| Huang [45] |  |  |  |  |  |  |  |
| Huang [46] |  |  |  |  |  |  |  |
| Nanotechnologies | |||||||
| De [47] |  |  |  |  |  |  |  |
| Gao [48] |  |  |  |  |  |  |  |
| Han [49] |  |  |  |  |  |  |  |
| Therapeutic applications | |||||||
| Zoghi [50] |  |  |  |  |  |  |  |
| Zoghi [51] |  |  |  |  |  |  |  |
| Other applications | |||||||
| Chang [52] |  |  |  |  |  |  |  |
 is “low risk of bias”,
is “low risk of bias”,  is “high risk of bias”,
is “high risk of bias”,  is “unclear risk of bias”; ** missing data because studies are not uniform or missing.
is “unclear risk of bias”; ** missing data because studies are not uniform or missing.| Radiopharmaceutical | mAb | Delivery System | Radionuclide | Chelator | Cancer Model | Ref. |
|---|---|---|---|---|---|---|
| 64Cu-NOTA-FSH-R-mAb | FSH-R mAb | No drug delivery system | 64Cu | p-SCN-Bn-NOTA | CAOV-3, SKOV-3, MDA-MB-231, PC-3 | [28] |
| 64Cu-NOTA-GO-FSH-R-mAb | FSH-R mAb | Nano-graphene oxide (GO) platform | 64Cu | p-SCN-Bn-NOTA | MDA-MB-231, cbgLuc-MDA-MB-231, SKOV-3 | [29] |
| Radiopharmaceutical | Peptide | Radionuclide | Chelator | RCP % | Cancer Model | Ref. |
|---|---|---|---|---|---|---|
| 18F-Al-NOTA-MAL-FSH1 | FSH1 (FSHβ33–53 *) | 18F | Al-NOTA-MAL | >98% | PC-3 | [30] |
| 18F-Al-NOTA-MAL-FSH2 | FSH2 (GGGRDN-FSHβ33–53 *) | 18F | Al-NOTA-MAL | >95% | PC-3 | [31] |
| [68Ga]Ga-NOTA-MAL-FSH1 | FSH1 (FSHβ33–53 *) | 68Ga | NOTA-MAL | >95% | PC-3 | [32] |
| [68Ga]Ga-NOTA-MAL-FSH4 | FSH4 (FSHβ33–53 *-NDRGGG) | 68Ga | NOTA-MAL | >95% | PC-3 | [33] |
| Radiopharmaceutical | Peptide | Radionuclide | Chelator | RCP% | Cancer Model | Ref. | |
|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | ||||||
| [111In]-DOTA-TRP | TRP * | 111In | DOTA-NCS | >95% | - ** | 4T1 tumor-bearing female mice | [34] |
| 111In-DOTA-Ahx-(D-Lys6-GnRH1) | D-Lys6-GnRH1 | 111In | DOTA | >95% | MDA-MB-231 | MDA-MB-231 cancer-xenografted nude mice | [35] |
| 111In-DOTA-Ahx-(D-Lys6-GnRH2) | D-Lys6-GnRH2 | ||||||
| 111In-DOTA-Ahx-(L-Lys6-GnRH3) | L-Lys6-GnRH3 | ||||||
| 111In-DOTA-Ahx-(D-Lys6-GnRH1) | D-Lys6-GnRH1 | 111In | DOTA | >95% | - ** | DU145 cancer-xenografted nude mice | [36] |
| 111In-DOTA-Aoc-D-Phe-(D-Lys6-GnRH) | D-Lys6-GnRH | 111In | DOTA | >98% | - ** | DU145 cancer-xenografted nude mice | [37] |
| 111In-DOTA-βAla-D-Phe-(D-Lys6-GnRH) | |||||||
| 111In-DOTA-Aun-D-Phe-(D-Lys6-GnRH) | |||||||
| 99mTc-HYNIC-GABA-D-Lys6-GnRH | D-Lys6-GnRH | 99mTc | HYNIC | ~97% | LN-CaP, DU-145, PC-3 | LN-CaP xenografted mice | [38] |
| 99mTc-HYNIC-Ahx-DLys6-GnRH | D-Lys6-GnRH | 99mTc | HYNIC | ~97% | LN-CaP, DU-145 | LN-CaP xenografted mice | [39] |
| 99mTc-LHRH | GnRH | 99mTc | Direct labeling | 93.9–96.4% | - ** | - ** | [40] |
| 99mTc-Acdien-LHRH | D-Lys6-GnRH | 99mTc | Acdien | >99% | MDA-MB-231 | - ** | [41] |
| Radiopharmaceutical | Peptide | Radionuclide | Chelator | RCP% | Cancer Model | Ref. | |
|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | ||||||
| [68Ga]-DOTA-Hyd-TRP | Triptorelin | 68Ga | pSCN-Bn-DOTA | >99% | - * | 4T1 tumor-bearing female mice | [42] |
| [68Ga]-DOTA-TRP | Triptorelin | 68Ga | pSCN-Bn-DOTA | 91–95% | - * | 4T1 tumor-bearing female mice | [43] |
| D-Lys6-[68Ga]DOTA-GnRH-I | D-Lys6-GnRH-I | 68Ga | DOTA | >99% | EFO-27, SKOV-3, LNCaP, DU-145, MDA-MB-231, SKBR-3 | OVCAR-3 tumor-bearing female mice | [44] |
| D-Lys6-Ahx([18F]FBOA)-GnRH-I | D-Lys6-GnRH-I | 18F | 4-[18F] fluorobenzaldehyde | >99% | |||
| D-Lys6-β-Ala([18F]FBOA)-GnRH-I | |||||||
| [18F]FP-D-Lys6-GnRH | D-Lys6-GnRH | 18F | 4-nitrophenyl-2-[18F]fluoropropionate | >95% | PC-3 | PC-3 and SKBR-3 xenografts | [45] |
| Al [18F]F-NOTA-PEG3-D-Lys6-GnRH | D-Lys6-GnRH | 18F | NOTA + PEG3 linker | ≥98% | - * | MDA-MB-231; rat hypophysis (ex vivo) | [46] |
| NPs | Peptide | Radionuclide | Chelator/Linker | Physicochemical Characteristics | RCP% | Cancer Model | Ref. | |
|---|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||||
| SLNPs | LHRH | DX + 99mTc | HYNIC, PEG spacer to DPPE | Size: 245 ± 54 nm (F-DX-SLN); PDI 0.23 ± 0.07; Zeta +33.6 ± 3.4 mV | ~92% | PC3 and SKBR3 | PC3 xenograft nude mice | [47] |
| Nanoclusters | LHRH | 64Cu | Chelator-free [64Cu]Cu; LHRH–BSA via EDC/NHS amide | Size: 3.8 ± 0.5 nm (CuNC@BSA-LHRH); Zeta -15.8 mV | 97.8% | A549 (LHRH-R+) and MRC-5 (LHRH-R−) | A549 subcutaneous xenografted tumors | [48] |
| AuNCs | LHRH | 124I | Chelator-free radioiodination of Tyr residues using chloramine-T | Size: 6 ± 0.5 nm (LHRH-HSA AuNCs) | 98% | - * | A549 xenografted and A549 orthotopic lung cancer model | [49] |
| Radiopharmaceutical | Peptide | Radionuclide | RCP% | Chelator | Cancer Model | Ref. |
|---|---|---|---|---|---|---|
| [111In]-DOTA-TRP-HYD | D-Trp6-GnRH-I | 111In | >95% | p-SCN-Bn-DOTA | 4T1 tumor-bearing female mice | [50] |
| [177Lu]-DOTA-TRPHYD | D-Trp6-GnRH-I | 177Lu | >98% | p-SCN-Bn-DOTA | 4T1 tumor-bearing female mice | [51] |
| Radiopharmaceutical | Peptide | Radionuclide | RCP% | Ref. |
|---|---|---|---|---|
| 131I-labeled LHRH immunogens | LHRH | 131I | >95% | [52] |
| Radiopharmaceutical | Biomolecule | Cancer | Ref. |
|---|---|---|---|
| Radiolabeled biomolecule targeting FSH-R | |||
| 64Cu-NOTA-FSH-R-mAb | FSH-R mAb | Ovarian, breast and prostate | [28] |
| 64Cu-NOTA-GO-FSH-R-mAb | FSH-R mAb | Ovarian and breast | [29] |
| 18F-Al-NOTA-MAL-FSH1 | FSH1 (FSHβ33–53) | Prostate | [30] |
| 18F-Al-NOTA-MAL-FSH2 | FSH2 (GGGRDN-FSHβ33–53) | Prostate | [31] |
| [68Ga]Ga-NOTA-MAL-FSH1 | FSH1 (FSHβ33–53) | Prostate | [32] |
| [68Ga]Ga-NOTA-MAL-FSH4 | FSH4 (FSHβ33–53-NDRGGG) | Prostate | [33] |
| Radiolabeled biomolecule targeting LHRH-R | |||
| SPECT applications | |||
| [111In]-DOTA-TRP | TRP | Breast | [34] |
| 111In-DOTA-Ahx-(D-Lys6-GnRH1) 111In-DOTA-Ahx-(D-Lys6-GnRH2) 111In-DOTA-Ahx-(L-Lys6-GnRH3) | D-Lys6-GnRH1 D-Lys6-GnRH2 D-Lys6-GnRH3 | Breast | [35] |
| 111In-DOTA-Ahx-(D-Lys6-GnRH1) | D-Lys6-GnRH | Prostate | [36] |
| 111In-DOTA-Aoc-D-Phe-(D-Lys6-GnRH) 111In-DOTA-βAla-D-Phe-(D-Lys6-GnRH) 111In-DOTA-Aun-D-Phe-(D-Lys6-GnRH) | D-Lys6-GnRH | Prostate | [37] |
| 99mTc-HYNIC-GABA-D-Lys6-GnRH | D-Lys6-GnRH | Prostate | [38] |
| 99mTc-HYNIC-Ahx-DLys6-GnRH | D-Lys6-GnRH | Prostate | [39] |
| 99mTc-LHRH | GnRH | - | [40] |
| 99mTc-Acdien-LHRH | D-Lys6-GnRH | Breast | [41] |
| PET applications | |||
| [68Ga]-DOTA-Hyd-TRP | Triptorelin | Breast | [42] |
| [68Ga]-DOTA-TRP | Triptorelin | Breast | [43] |
| D-Lys6-[68Ga]DOTA-GnRH-I D-Lys6-Ahx([18F]FBOA)-GnRH-I D-Lys6-β-Ala([18F]FBOA)-GnRH-I | D-Lys6-GnRH-I | Ovarian | [44] |
| [18F]FP-D-Lys6-GnRH | D-Lys6-GnRH | Prostate and breast | [45] |
| Al [18F]F-NOTA-PEG3-D-Lys6-GnRH | D-Lys6-GnRH | Breast | [46] |
| Nanotechnologies | |||
| 99mTc-DX-SLN | LHRH | Prostate and breast | [47] |
| [64Cu]CuNC@BSA-LHRH | LHRH | Lung | [48] |
| 124I-LHRH-HSA AuNCs | LHRH | Lung | [49] |
| Therapeutic applications | |||
| [111In]-DOTA-TRP-HYD | D-Trp6-GnRH-I | Breast | [50] |
| [177Lu]-DOTA-TRPHYD | D-Trp6-GnRH-I | Breast | [51] |
| Other applications | |||
| 131I-labeled LHRH immunogens | LHRH | - | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgio, A.; Varani, M.; Lauri, C.; Bentivoglio, V.; Nayak, P. Radiolabeled LHRH and FSH Analogues as Cancer Theranostic Agents: A Systematic Review. J. Clin. Med. 2025, 14, 7811. https://doi.org/10.3390/jcm14217811
Giorgio A, Varani M, Lauri C, Bentivoglio V, Nayak P. Radiolabeled LHRH and FSH Analogues as Cancer Theranostic Agents: A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7811. https://doi.org/10.3390/jcm14217811
Chicago/Turabian StyleGiorgio, Anna, Michela Varani, Chiara Lauri, Valeria Bentivoglio, and Pallavi Nayak. 2025. "Radiolabeled LHRH and FSH Analogues as Cancer Theranostic Agents: A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7811. https://doi.org/10.3390/jcm14217811
APA StyleGiorgio, A., Varani, M., Lauri, C., Bentivoglio, V., & Nayak, P. (2025). Radiolabeled LHRH and FSH Analogues as Cancer Theranostic Agents: A Systematic Review. Journal of Clinical Medicine, 14(21), 7811. https://doi.org/10.3390/jcm14217811






