Emerging Thrombolysis Technologies in Vascular Thrombosis
Abstract
1. Introduction
2. Methods
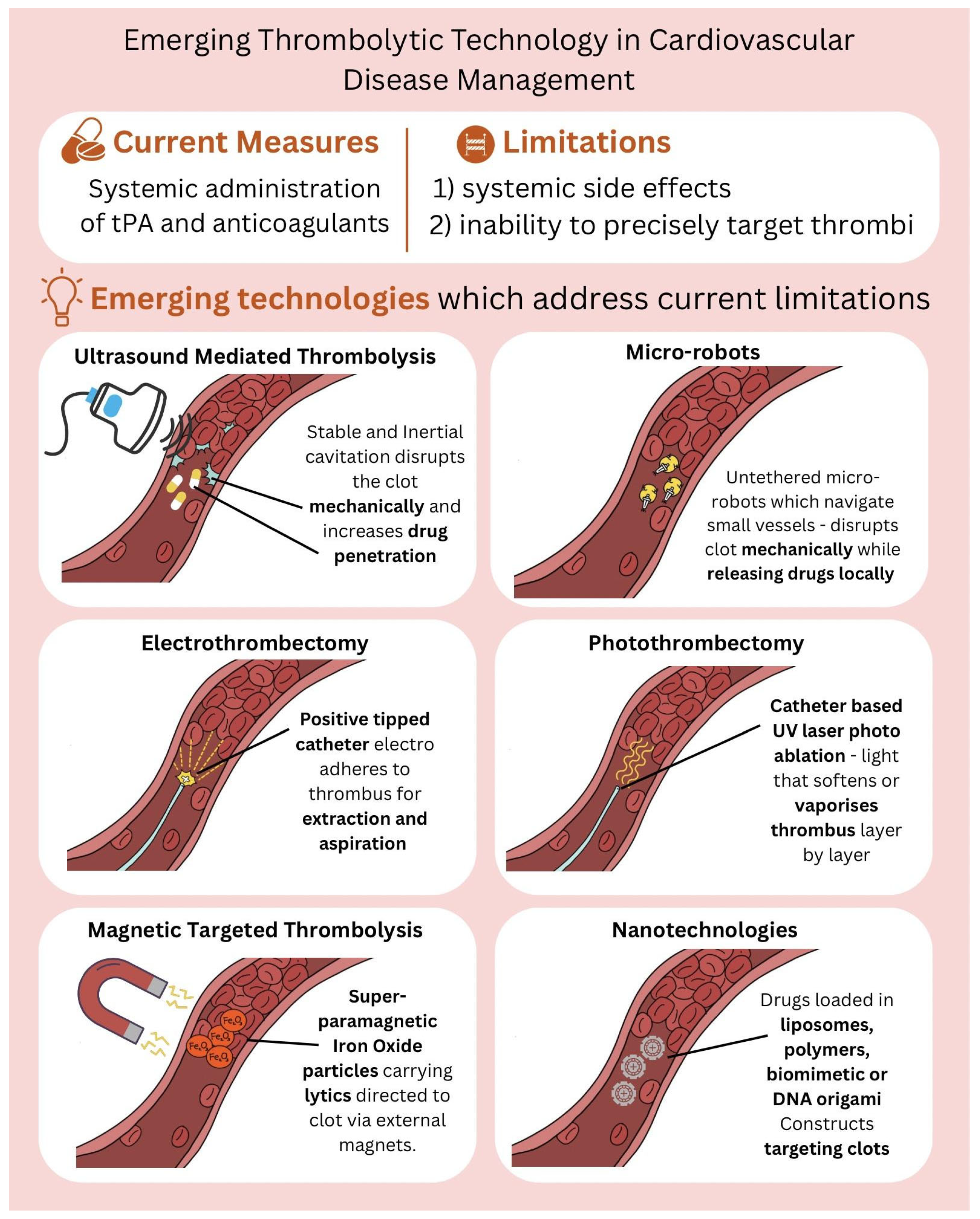
3. Thrombolysis Technologies
3.1. Ultrasound-Mediated Thrombolysis (Sonothrombolysis): Mechanism, Technologies, and Clinical Applications
| No. | Study/Reference | Model/Setting | Intervention & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Wildberger et al., Cardiovasc Intervent Radiol, 2001 [14] | In vitro haemodialysis access model | Thrombus ablation in occluded haemodialysis access shunts utilizing ultrasound | Demonstrated effective clot fragmentation and removal without damage to vascular graft materials. | Provided early evidence supporting ultrasound as a safe, non-invasive tool for thrombus removal in vascular access. |
| 2 | Datta S. et al., Ultrasound Med Biol, 2006 [15] | In-vitro fibrin clot model | Diagnostic US (120 kHz–1 MHz) + tPA; cavitation monitoring | Cavitation dose correlated strongly with lysis rate enhancement | Defined quantitative cavitation–lysis relationship foundational for later energy-dosing strategies |
| 3 | Maxwell et al., Ultrasound Med Biol, 2009 [27] | In vivo canine clot model | Histotripsy using focused ultrasound pulses to induce controlled cavitation for mechanical clot fractionation. | Achieved complete thrombus fractionation without thermal injury or embolization; no vessel damage observed. | Established histotripsy as a non-thermal, non-pharmacologic thrombolytic modality. |
| 4 | Chuang YH et al., Ultrason Imaging, 2010 [16] | In-vitro fibrin clot dissolution | Controlled inertial cavitation via 1 MHz pulsed US | Enhanced fibrinolysis up to 3× baseline; optimal duty cycle identified | Clarified inertial cavitation threshold for enzymatic thrombolysis optimization |
| 5 | Hua et al., J Thromb Thrombolysis, 2014 [24] | Rabbit femoral artery thrombosis model | tPA-loaded targeted microbubbles activated with diagnostic ultrasound | Enhanced thrombus lysis and recanalization with reduced tPA dose compared to systemic therapy. | Demonstrated synergistic benefit of tPA-microbubbles with ultrasound for targeted thrombolysis. |
| 6 | Zhang et al., IEEE Trans Ultrason Ferroelectr Freq Control, 2015 [28] | In vitro thrombus model | Microtripsy technique using short, high-pressure ultrasound pulses | Enabled localized thrombus disintegration with minimized collateral damage and high reproducibility. | Improved control and safety profile over conventional histotripsy for clinical translation. |
| 7 | Petit B et al., Ultrasound Med Biol, 2015 [17] | In-vitro human clot model | Quantified stable vs. inertial cavitation using contrast microbubbles | Both cavitation types synergistically improved clot lysis; stable cavitation dominant | Established mechanistic framework distinguishing cavitation regimes |
| 8 | Porter TR et al., Invest Radiol, 2017 [19] | Porcine carotid thromboembolism | Diagnostic US-induced microbubble cavitation (no tPA) | Achieved >70% recanalization; no major hemorrhage | Proof of mechanical-only sonothrombolysis feasibility |
| 9 | Suo D et al., Ultrason Sonochem, 2018 [18] | Computational model | Multi-frequency cavitation modeling | Lower inertial threshold achieved with dual frequencies | Supports development of multi-frequency sonothrombolysis devices |
| 10 | Hu B et al., Int J Cardiol, 2018 [20] | Rat coronary microcirculation model | Acoustic phase-change dodecafluoropentane nanoparticles (PCNDs) + US | Significantly improved microvascular flow; no endothelial damage | Demonstrated translational feasibility of nanoparticle-assisted UMT |
| 11 | Guo S et al., Ultrason Sonochem, 2019 [26] | Rabbit femoral | Phase-change nanodroplet-assisted UMT | Reduced clot debris size; faster lysis kinetics | Advanced hybrid acoustic–nanocarrier approach |
| 12 | Kim et al., Ultrasound Med Biol, 2020 [25] | In vitro aged bovine clot models | Comparison of phase-change nanodroplets versus microbubbles under low-intensity ultrasound. | Phase-change nanodroplets achieved superior lysis efficiency in aged clots | Supports development of next-generation nanodroplet agents for resistant thrombi. |
| 13 | Xie Y et al., Ultrason Sonochem, 2022 [22] | Computational & in-vitro focused US field | Cavitation bubble–endothelium interaction | Predicted thresholds for vascular injury | Quantified safety envelope for high-intensity UMT |
| 14 | Wang et al., Front Bioeng Biotechnol, 2022 [11] | In vitro flow model and in vivo rabbit inferior vena cava thrombosis model | Endovascular low-frequency ultrasound combined with bifunctional microbubbles | Significantly improved clot dissolution and recanalization rates without endothelial damage. | Demonstrated potential of combined ultrasound-microbubble system for deep vein thrombosis (DVT) therapy. |
| 15 | Chen J et al., Front Bioeng Biotechnol, 2023 [12] | Rabbit inferior vena cava thrombosis model | Targeted microbubbles combined with low-power focused ultrasound | Achieved near-complete thrombus resolution | Validated targeted, low-intensity ultrasound-guided microbubbles as a safe and effective DVT treatment modality. |
| No. | Study/Reference | Clinical Condition/Setting | Modality & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Alexandrov AV et al., N Engl J Med, 2004 [9] | 126 Acute Ischemic Stroke (AIS) patients (MCA occlusion) | Transcranial Doppler (TCD) Ultrasonography (2-MHz, continuous) + IV t-PA | TCD significantly augmented t-PA-induced arterial recanalization (49% vs. 30% for placebo; p = 0.03) | First major randomized trial (CLOTBUST) to demonstrate that non-invasive ultrasound can safely boost systemic thrombolysis for stroke. |
| 2 | Slikkerveer J et al., Ultrasound Med Biol, 2012 [30] | 10 Acute ST-Elevation Myocardial Infarction (STEMI) patients/Prehospital | Sonothrombolysis (Pulsatile Ultrasound + Microbubbles + Alteplase) | No significant difference between treatment and control group in safety (minor adverse events 2/5 vs. 2/5, p = NS) and outcome (TIMI III flow 3/5 vs. 1/5 respectively, p = 0.23) was recorded | Pilot study to demonstrate study protocol is feasible and safe in acute cardiac setting |
| 3 | Al-Terki H et al., J Clin Med, 2023 [13] | 20 Intermediate-High-Risk Pulmonary Embolism (PE) patients | Ultrasound-Accelerated Catheter-Directed Thrombolysis (USAT) | USAT improved echocardiographic measures of left ventricular function (e.g., RV/LV ratio decreased) and pulmonary arterial obstruction scores, with a relatively low complication rate. | Supports the safety and efficacy of local, low-dose thrombolysis combined with ultrasound to quickly relieve left heart strain in high-risk PE. |
| 4 | Sterling KM et al., Circ Cardiovasc Interv, 2024 [31] | 489 Intermediate-High & High-Risk PE patients/Prospective Intl. Registry (KNOCOUT PE) | Ultrasound-Facilitated, Catheter-Directed Thrombolysis (US-CDT) | Significant reduction in RV/LV ratio by 0.49 at 24 h; 98.4% survival to discharge; low rate of major bleeding (1.7%). | Provides prospective, real-world evidence confirming the high efficacy and safety of US-CDT in a broad population of patients with severe PE. |
| 5 | Prasad R et al., J Vasc Access, 2024 [32] | Thrombosed Native Arteriovenous Fistula (AVF) (Dialysis Access) | Direct Percutaneous Thrombolyhsis (DPT) with Ultrasound Guidance + Urokinase | High technical success rate for salvaging thrombosed AVFs (84.2% for no-stenosis group, 97.5% for stenosis group followed by angioplasty). | Introduces DPT as a safe, economical, and minimally invasive technique for salvaging vital dialysis access sites |
3.2. Microrobots in Thrombolysis
| No. | Study/Reference | Model/Setting | Intervention & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Yang et al., Sci Adv, 2023 [36] | In vivo rat femoral vein thrombosis | Swarming magnetic nanorobots coated with heparinoid-polymer brushes enabling anticoagulant surface and magnetic propulsion | Complete recanalization within 40 min; no haemorrhage or organ toxicity; effective under physiological flow | Demonstrated biocompatible, non-immunogenic magnetic nanorobot design suitable for translation; validates safety of swarm actuation in mammals |
| 2 | Zhang et al., Nat Commun, 2023 [37] | In vivo mouse tail thrombosis model and rat cerebral ischaemia/reperfusion injury model | Self-fuelled nano-penetrators composed of polyoxometalate–carbon composites that generate propulsion with endogenous H2O2 | Achieved significant recanalization and improved neurological recovery without haemorrhagic complications. | First-in-class non-pharmaceutical, chemically self-driven electro-nanomechanical thrombolytic platform. Represents a paradigm shift from external energy or drug-dependent thrombolysis toward autonomous nanomechanical therapy; positions technology at TRL 4–5 (preclinical efficacy validated) |
| 3 | Wang B et al., Sci Adv, 2024 [34] | In vivo occlusion (rabbit carotid artery + rat femoral vein models) | tPA-anchored magnetic nanorobots (~300 nm Fe3O4 cores) propelled by rotating magnetic fields for localized fibrinolysis | Average recanalization time was 37 min, perfusion rates increased to ~100% after targeted therapy | First demonstration of autonomous magnetic nanorobots performing mechanical + enzymatic clot lysis in vivo; establishes scale-down feasibility for end-arterial applications |
| 4 | Pontius et al., PNAS, 2024 [35] | In vivo zebrafish thrombosis model | Magnetically powered “microwheels” composed of tPA-conjugated 4 µm magnetic particles rotating under external field | Microwheels recanalized occlusive thrombi within 30 min; tPA retention higher than diffusion-only controls | Provides real-time visualization of microrobotic swarm behavior and confirms efficacy of localized mechanical–enzymatic synergy |
3.3. Electrothrombectomy: Harnessing Electrical Energy for Clot Removal
| No. | Study/Reference | Model/Setting | Intervention & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Magneto Thrombectomy Solutions, Endovascular Today, Biomed Israel Conference Press Release, 2022 [38] | Preclinical and feasibility testing (bench and ex vivo clot models) | eTrieve™ Electrothrombectomy System—catheter applying localized positive voltage to attract and adhere to negatively charged thrombi for mechanical extraction. | Demonstrated strong electrostatic capture of thrombi of varying composition, enabling intact clot retrieval with minimal vessel trauma. | Proof-of-concept validation of a novel electroadhesion-based thrombectomy mechanism, supporting transition to human feasibility studies. |
| No. | Study/Reference | Model/Setting | Intervention & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Andersen A., Musialek P., Araszkiewicz A. et al., J Am Coll Cardiol Intv, 2023 [39] | First-in-human, 10 patients with intermediate-risk pulmonary embolism | Mechanical–electric hybrid thrombectomy (eTrieve™ system) combining electro-adhesion and mechanical aspiration | 100% procedural success and clot clearance Significant reduction in RV/LV ratio and pulmonary arterial pressure No major bleeding or device-related adverse events | Demonstrated safety and feasibility of photo-electric/electro-mechanical energy–assisted thrombus extraction; supports ongoing clinical development of hybrid device-assisted thrombolysis |
3.4. Photothrombectomy: Light-Based Approaches to Clot Dissolution
| No. | Study/Reference | Model/Setting | Modality & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Jawad-Ul-Qamar M. et al. Open Heart, 2021 [40] | 50 patients undergoing elective or emergency PCI | Excimer laser coronary angioplasty (ELCA) using 308 nm ultraviolet laser pulses to photo-ablate fibrin and platelet aggregates | - High procedural success with acceptable complication rate - Reduced residual thrombus burden; improved distal flow | Demonstrated clinical feasibility of photochemical–photothermal ablation of thrombus within coronary circulation |
| 2 | Kujiraoka et al., Yoshida K., Fukamizu S. Lasers Med Sci, 2023 [41] | Clinical series, 319 patients with STEMI | ELCA-assisted primary PCI for thrombotic occlusions | - Shorter procedural times and improved TIMI flow when ELCA used in early presentation (<3 h)—Comparable safety vs. standard PCI | Confirms ELCA’s role in acute coronary thrombosis; provides translational link from photothermal ablation to modern endovascular reperfusion strategies |
| 3 | Song J et al., Nat Commun, 2023 [42] | In vitro & in vivo mouse carotid artery thrombus model | Fibrin-specific homopolymer nanoparticles with NIR-II photoacoustic imaging + photo-triggered thermal release | Precise NIR-II-induced clot disintegration; dual imaging and therapy with minimal tissue heating | Demonstrates fully integrated imaging–therapy (PA/photothermal) system for real-time thrombus monitoring |
3.5. Magnetic Targeted Thrombolysis: Iron Based Intravascular Therapy
| No. | Study/Reference | Model/Setting | Modality & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Zhang Y et al., Int J Nanomedicine, 2019 [54] | In vitro simulated circulatory device & in vivo mixed thrombus mouse model | Polydopamine-modified dual-ligand NPs for MRI/PA dual-modality imaging | High fibrin affinity and strong imaging contrast | Early example of theranostic dual-imaging thrombus agents |
| 2 | Zhang Y et al., ACS Appl Mater Interfaces, 2021 [51] | In vitro clots & in vivo rat thrombosis model | MOF-derived carbon nanoplatforms with multimodal (photo + magnetic) capabilities | Enhanced fibrin breakdown with optical tracking; low bleeding risk | Early MOF-based multimodal template for current hybrid designs |
| 3 | Choi W et al., Biomater Res, 2022 [47] | In vivo photothrombotic stroke (mouse) | Magneto-acoustic particles targeted to occlusion site | Restored cerebral perfusion to ~80% baseline; improved neurological score; no haemorrhage | Extends magneto-acoustic synergy to neurovascular (stroke) models |
| 4 | Tang X et al., Small, 2022 [49] | In vivo rat venous thrombosis | Enzyme–magnetite nanoparticle swarms for low-dose pharmacomechanical thrombolysis | Tenfold thrombolytic efficiency achieved when compared to pure rTPA | Demonstrates enzyme-magnetic swarm synergy for dose minimization |
| 5 | Cabrera D et al., J Thromb Haemost, 2022 [48] | In vitro human plasma clots | Magnetic hyperthermia using clot-targeted Fe3O4 NPs | Local heating (≈42 °C) permeabilized fibrin, increasing tPA susceptibility | Establishes adjunctive magnetothermal pre-conditioning concept |
| 6 | Liu KT et al., Adv Healthc Mater, 2023 [46] | Microfluidic & in vivo rodent models | Self-indicating biomimetic nanoassembly with site-specific photothermal activation | Real-time optical signal correlates with local clot lysis; reduced systemic exposure | Adds theranostic self-reporting functionality for precision feedback |
| 7 | Jheng PR et al., Mater Today Bio, 2023 [50] | In vitro + in vivo murine thrombosis model | Cold-plasma–enabled platelet-vesicle iron-oxide nano-propellers | Active rotation and localized heating achieved near-complete clot removal; biocompatible | Introduces bio-hybrid nano-propeller concept leveraging plasma activation |
| 8 | Vazquez-Prada KX et al., Small, 2023 [52] | In vivo mouse thrombosis model | Spiky Ag–Fe3O4 nanoparticles for targeted photothermal therapy + multimodal imaging | Local temperature rise ≈ 45 °C induced thrombus ablation within 5 min; high imaging contrast | Improved photothermal efficiency and MRI/PA visibility for precision targeting |
| 9 | Ruan R et al., Adv Healthc Mater, 2024 [43] | In vitro 3D printed vein vasculature model & in vivo rat thrombosis model | Targeting nanomotor with NIR + ultrasound dual-triggered transformation for staged cascade thrombolysis | Achieved multistage propulsion and complete recanalization; enhanced safety by sequential energy activation | Introduces polystage cascade paradigm combining NIR and US energy cues |
| 10 | Zhu L et al., Adv Healthc Mater, 2024 [45] | In vivo mouse tail vein thrombosis model | Erythrocyte-membrane-camouflaged magnetic nanocapsules with photothermal + magnetothermal dual modes | Rapid thrombus clearance; prolonged circulation; no organ toxicity | Biomimetic stealth carrier combines immune-evasion + dual heating |
| 11 | Jacqmarcq C et al., Nat Commun, 2024 [53] | In vivo stroke mouse model | Polydopamine-coated iron-oxide nanoparticles enabling MRI detection of microthrombi | High-resolution MRI tracking of microthrombi; potential for targeted therapy | Provides diagnostic integration layer for image-guided nano-thrombolysis |
| 12 | Vazquez-Prada KX et al., Biomater Sci, 2025 [44] | In vitro human blood clot and thrombosis mouse model | Branched Ag–Fe3O4 nanoparticles enabling drug-free magnetothermal ablation | Localized hyperthermia disrupted fibrin mesh within minutes without lytic drug | First demonstration of purely physical, magnetothermal thrombolysis—bleeding-sparing concept |
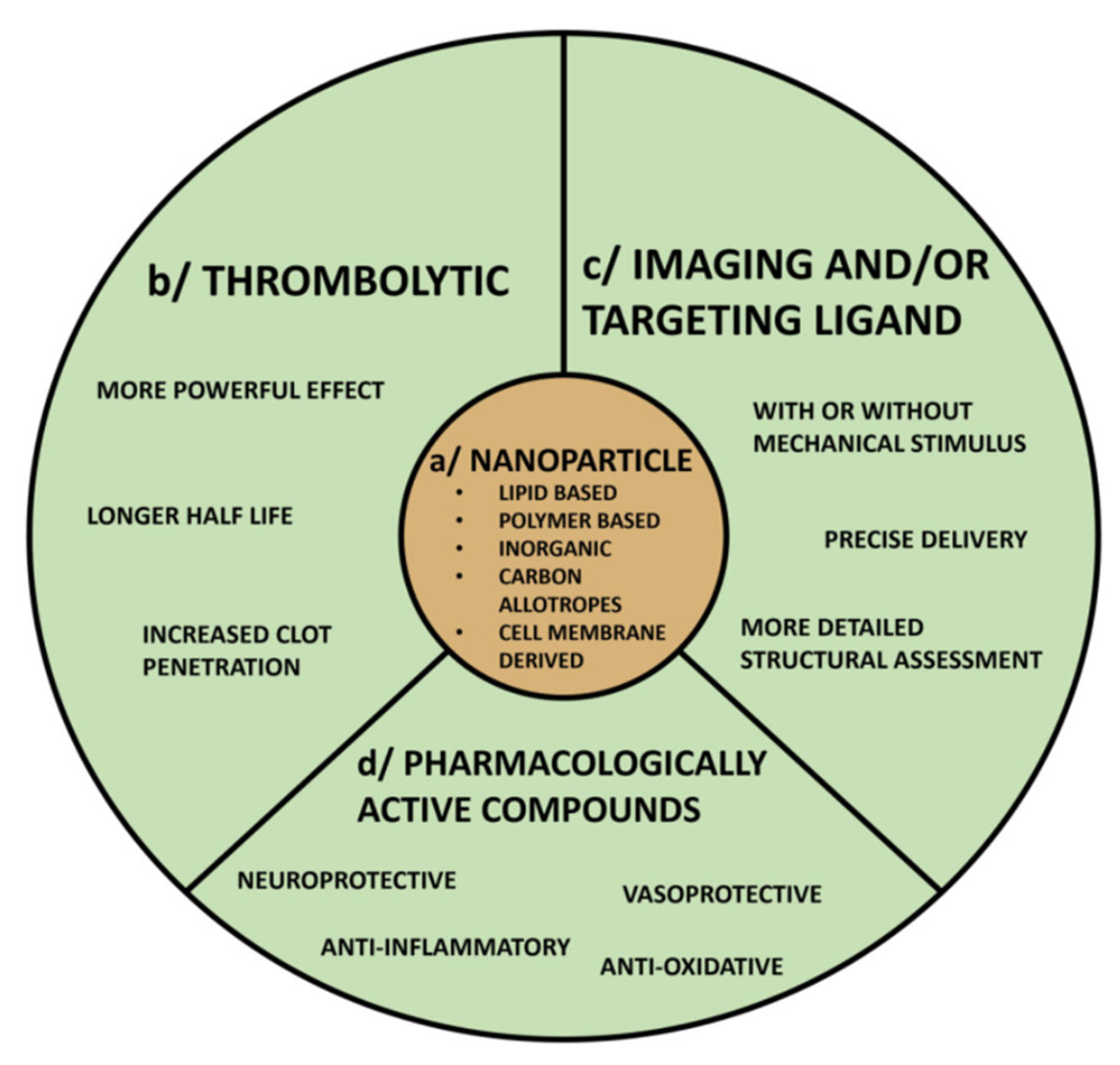
3.6. Nanoparticle Technology
| No. | Study/Reference | Model/Setting | Modality & Mechanism | Key Findings | Translational Relevance |
|---|---|---|---|---|---|
| 1 | Chung TW et al., Biomaterials, 2008 [58] | In vitro blood clot model | Chitosan-coated plasminogen activators in PLGA nanoparticles | Sustained release of tPA; accelerated clot lysis with reduced bleeding risk | One of the earliest controlled-release nanocarrier approaches for thrombolysis |
| 2 | Korin N et al., Science, 2012 [59] | Microfluidic and in vivo mice model | Shear-activated nanotherapeutics that unfold under pathologic shear | Selective tPA release in occluded vessels → site-specific clot lysis without systemic bleeding | Pioneering “smart release” platform for occlusion-responsive thrombolysis |
| 3 | Colasuonno M et al., ACS Nano, 2018 [60] | Microfluidic chip and in vivo murine thrombosis model | Erythrocyte-inspired discoidal polymeric nanoconstructs carrying tPA | 2× faster clot lysis vs. free tPA; prolonged circulation | Validated biomimetic nanoplatform with enhanced hemodynamic stability |
| 4 | Blum NT et al., ACS Appl Mater Interfaces, 2019 [61] | In vitro clot and HIFU setup | Phospholipid-coated hydrophobic mesoporous silica NPs to enhance HIFU thrombectomy | Improved lysis with low debris generation | Demonstrates safe energy–nanoparticle coupling |
| 5 | Refaat A et al., J Control Release, 2021 [62] | In vitro halo-clot model | NIR-responsive liposomes for protein delivery | Triggered on-demand release by light; no off-target effect | Prototype for controlled, non-systemic activation |
| 6 | Hu L et al., Int J Nanomedicine, 2022 [63] | In vitro and rat model | Hybrid nanoplatform combining mechanical ultrasound blasting and drug delivery | Synergistic mechanical + pharmacologic lysis with reduced dose | Bridges mechanical and nano-drug approaches |
| 7 | Yu W et al., Acta Biomater, 2022 [64] | Mouse ischemic stroke model | Biomimetic nanovesicles mimicking platelet membrane for thrombus targeting + ischemia-reperfusion protection | Enhanced clot lysis and post-ischemic tissue repair | Dual-action (blood–brain barrier and reperfusion protection) platform |
| 8 | Chen YT et al., ACS Appl Mater Interfaces 2023 [65] | In vitro + in vivo rodent thrombosis model | Biomimetic platelet nanomotors for site-specific thrombolysis | Autonomous motion toward thrombus; reduced reperfusion injury | Proof of concept for biohybrid motile nanoplatforms |
| 9 | Wang Z et al., J Nanobiotechnol, 2024 [66] | In vivo rat arterial thrombosis | Dual-mode nanoprobe integrating ultrasound + NIR activation | Synergistic lysis with enhanced targeting and monitoring | Validates multi-energy dual-mode activation strategy |
| 10 | Yin L et al., Nat Mater, 2024 [67] | In vivo mouse arterial and venous thrombosis models | Intelligent DNA nanodevice for precision thrombolysis | The device actively binds and penetrates the thrombus; achieves complete clot removal in vivo with low bleeding risk due to enzyme-free action. | Major advance in active, non-enzymatic thrombolysis using programmable DNA nanotechnology |
3.7. Types of Nanomaterials for Thrombolysis and Mechanism of Action
- 1.
- Lipid-Based Nanocarriers:
- 2.
- Biodegradable Polymeric Nanoparticles:
- 3.
- Magnetic Nanoparticles:
- 4.
- Inorganic and Photothermal Nanoparticles:
- 5.
- Biomimetic Nanocarriers:
- 6.
- DNA Origami Nanodevices:
4. Discussion: Advancing Thrombolysis Through Precision Platforms
| Technology | Core Mechanism | Evidence/Efficacy Signal | Technology Readiness Level * | Key Benefits | Potential Clinical Risks | Translational Barriers | Representative Platforms |
|---|---|---|---|---|---|---|---|
| Ultrasound-Mediated Thrombolysis (UMT) | Acoustic cavitation (stable + inertial) enhances drug penetration & mechanical clot disruption; catheter or external US. | ↑ Recanalization in stroke (CLOTBUST); RV strain reduction & clot debulking in PE/DVT (EKOS); lower lytic dose requirements. | TRL 9 | Targeted thrombolysis with reduced systemic tPA; adaptable (neuro, venous, coronary); can shorten ICU time. | Vessel injury if high energy; embolic debris; haemorrhage if parameters mis-set; skull attenuation limits transcranial use. | Parameter standardization; access cost; operator training; patient-specific acoustic planning (bone, body habitus). | EKOS™; CLOTBUST protocols; histotripsy/microtripsy variants (investigational). |
| Electrothrombectomy | Positively charged catheter tip electro-adheres to negatively charged thrombus for intact extraction ± aspiration. | Small PE feasibility series: 100% technical success; ~36% RV/LV reduction at 48 h; broad clot phenotype capture. | TRL 3–4 | Intact clot removal; minimal fragmentation; drug-sparing (reduced bleeding); rapid hemodynamic response. | Large-bore access (20F) bleeding; vascular trauma at access; incomplete capture in branching anatomy; electrical malfunction (rare). | Miniaturization for smaller vessels; randomized outcome trials; comparative cost-effectiveness; electrical safety standards. | eTrieve™ electro-adhesion catheter |
| Microrobots | Untethered magnetic/acoustic micro- or nanorobots that navigate small, tortuous vessels; mechanical boring + local drug release. | Rodent/zebrafish models: near-complete flow restoration within ~40 min; effective tPA delivery at low dose. | TRL 6 | Access to distal microvasculature; ultra-localized therapy; potential multiplex (drug, sensors). | Off-target lodging; immune/toxic material response; uncontrolled migration; retrieval failure; microembolization. | Real-time tracking & control in humans; biocompatible, biodegradable materials; scalable manufacture; novel regulatory pathway. | Magnetically driven “microwheels”; heparin-mimetic swarming nanorobots; ultrasound-driven nanomotors (experimental). |
| Photothrombectomy | Light energy (UV laser photoablation; NIR photothermal/photochemical activation of clot-bound agents) to vaporize or soften thrombus. | ELCA improves myocardial blush/reduces no-reflow in late STEMI; peripheral thrombus debulking reports; NIR nanoparticles dissolve clots in animals without systemic lytics. | TRL 4 (NIR nanoparticles); TRL 9 (ELCA) | Precise on-demand lysis; low systemic drug use; adjunct to PCI; theranostic potential with photoactive probes. | Thermal or photochemical vessel injury; perforation if mis-fired; need for line-of-sight/fiber delivery; off-target activation of photosensitizers. | Efficient deep-tissue light delivery; selective targeting; device cost/training; regulatory clearance for new photo-agents. | ELCA (excimer laser, coronary); NIR-responsive NO or photothermal nanoparticles (preclinical). |
| Magnetic Targeted Thrombolysis | Superparamagnetic iron-oxide (SPIO) particles carrying lytics steered & concentrated at clot via external magnets; can add mechanical oscillation. | Animal data: effective lysis with ≤20% standard tPA dose; ~80% dose reduction shown in models; MRI-trackable constructs. | TRL 4 | Major lytic dose reduction (lower bleeding); image-guided targeting; combinable with mechanical or thermal triggers. | Particle aggregation/microvascular obstruction; complement activation; iron overload or RES sequestration; magnet mis-targeting. | Human-scale magnetic steering hardware; GMP nanoparticle production; long-term biodistribution safety; combo product regulation. | Fibrin-targeted IO-rtPA conjugates; erythrocyte-camouflaged magnetic nanocapsules; magnetoacoustic particles. |
| Nanotechnology (Targeted/Stimuli-Responsive Nanocarriers) | Drug (tPA/urokinase) loaded in liposomes, polymers, biomimetic or DNA origami constructs; clot-targeting ligands; triggered release (shear, US, pH, NIR, enzymes). | Liposomal tPA: ~22× half-life extension; ~50% faster lysis; CREKA targeting ~3× clot uptake; polymer NPs preserve 80% activity/↑ lysis; DNA origami reduces hemorrhage ~70% in stroke models. | TRL 4–5 | Lower systemic exposure/bleeding; prolonged circulation; multi-cargo (cytoprotectants, imaging); customizable to clot biology (arterial vs. venous). | Material toxicity; immune recognition; off-target deposition (RES, lung); variable clot penetration; manufacturing heterogeneity. | GMP scale-up; regulatory path as drug–device combo; stratified clinical trial design; cost of complex biologics; personalized targeting biomarkers. | CREKA-tPA liposomes; platelet-membrane nanovesicles; shear-activated nano-aggregates; thrombin-responsive DNA nanodevice. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- Kim, S.J. Global Awareness of Myocardial Infarction Symptoms in General Population. Korean Circ. J. 2021, 51, 997–1000. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Quencer, K.B.; Oklu, R. Hemodialysis access thrombosis. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 3), S299–S308. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Omran, S.S.; Gialdini, G.; Lerario, M.P.; Yaghi, S.; Elkind, M.S.; Navi, B.B. Safety Outcomes After Thrombolysis for Acute Ischemic Stroke in Patients with Recent Stroke. Stroke 2017, 48, 2282–2284. [Google Scholar] [CrossRef]
- Goel, L.; Jiang, X. Advances in Sonothrombolysis Techniques Using Piezoelectric Transducers. Sensors 2020, 20, 1288. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, A.V.; Molina, C.A.; Grotta, J.C.; Garami, Z.; Ford, S.R.; Alvarez-Sabin, J.; Montaner, J.; Saqqur, M.; Demchuk, A.M.; Moyé, L.A.; et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N. Engl. J. Med. 2004, 351, 2170–2178. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, N.; Wang, Z.; Deng, Q.; Zhou, Q.; Hu, B. Ultrasound-mediated cardiovascular thrombolysis: From Sonothrombolysis to Sonoperfusion. Postgrad. Med. J. 2024, 101, 275–282. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Y.; Huang, H.; Zhang, Y.; Li, Y.; Zou, C.; Huang, G.; Chen, Y.; Li, Y.; Li, J.; et al. Enhanced thrombolysis by endovascular low-frequency ultrasound with bifunctional microbubbles in venous thrombosis: In vitro and in vivo study. Front. Bioeng. Biotechnol. 2022, 10, 965769. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Li, Y.; Xu, L.; Zhao, C.; Chen, Q.; Lu, Y. Targeted microbubbles combined with low-power focused ultrasound promote the thrombolysis of acute deep vein thrombosis. Front. Bioeng. Biotechnol. 2023, 11, 1163405. [Google Scholar] [CrossRef]
- Al-Terki, H.; Mügge, A.; Gotzmann, M.; Tiyerili, V.; Klein, F.; Franz, M.; Möbius-Winkler, S.; Elhakim, A. The Safety and Efficacy of Ultrasound-Accelerated Catheter-Directed Thrombolysis in Patients with Intermediate-High-Risk Pulmonary Embolism: Bo-NE-Experience. J. Clin. Med. 2023, 12, 3459. [Google Scholar] [CrossRef]
- Wildberger, J.E.; Schmitz-Rode, T.; Haage, P.; Pfeffer, J.; Ruebben, A.; Günther, R.W. Ultrasound thrombolysis in hemodialysis access: In vitro investigation. Cardiovasc. Interv. Radiol. 2001, 24, 53–56. [Google Scholar] [CrossRef]
- Datta, S.; Coussios, C.-C.; McAdory, L.E.; Tan, J.; Porter, T.; De Courten-Myers, G.; Holland, C.K. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med. Biol. 2006, 32, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.H.; Cheng, P.W.; Chen, S.C.; Ruan, J.L.; Li, P.C. Effects of ultrasound-induced inertial cavitation on enzymatic thrombolysis. Ultrason. Imaging 2010, 32, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Petit, B.; Bohren, Y.; Gaud, E.; Bussat, P.; Arditi, M.; Yan, F.; Tranquart, F.; Allémann, E. Sonothrombolysis: The contribution of stable and inertial cavitation to clot lysis. Ultrasound Med. Biol. 2015, 41, 1402–1410. [Google Scholar] [CrossRef]
- Suo, D.; Govind, B.; Zhang, S.; Jing, Y. Numerical investigation of the inertial cavitation threshold under multi-frequency ultrasound. Ultrason. Sonochem. 2018, 41, 419–426. [Google Scholar] [CrossRef]
- Porter, T.R.M.; Xie, F.; Lof, J.; Powers, J.; Vignon, F.; Shi, W.; White, M. The thrombolytic effect of diagnostic ultrasound-induced microbubble cavitation in acute carotid thromboembolism. Investig. Radiol 2017, 52, 477–481. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, N.; Zhou, Q.; Cao, S.; Gao, S.; Zhang, B.; Chen, J.; Guo, R. Stable cavitation using acoustic phase-change dodecafluoropentane nanoparticles for coronary micro-circulation thrombolysis. Int. J. Cardiol. 2018, 272, 1–6. [Google Scholar] [CrossRef]
- Weiss, H.L.; Selvaraj, P.; Okita, K.; Matsumoto, Y.; Voie, A.; Hoelscher, T.; Szeri, A.J. Mechanical clot damage from cavitation during sonothrombolysis. J. Acoust. Soc. Am. 2013, 133, 3159–3175. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, J.; Lei, W.; Qian, S. Prediction of vascular injury by cavitation microbubbles in a focused ultrasound field. Ultrason. Sonochem. 2022, 88, 106103. [Google Scholar] [CrossRef]
- Xie, F.; Lof, J.; Matsunaga, T.; Zutshi, R.; Porter, T.R. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation 2009, 119, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhou, L.; Liu, P.; He, Y.; Tan, K.; Chen, Q.; Gao, Y.; Gao, Y. In vivo thrombolysis with targeted microbubbles loading tissue plasminogen activator in a rabbit femoral artery thrombus model. J. Thromb. Thrombolysis 2014, 38, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; DeRuiter, R.M.; Goel, L.; Xu, Z.; Jiang, X.; Dayton, P.A. A Comparison of Sonothrombolysis in Aged Clots between Low-Boiling-Point Phase-Change Nanodroplets and Microbubbles of the Same Composition. Ultrasound Med. Biol. 2020, 46, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, X.; Wang, X.; Zhou, D.; Du, X.; Han, M.; Zong, Y.; Wan, M. Reduced clot debris size in sonothrombolysis assisted with phase-change nanodroplets. Ultrason. Sonochem. 2019, 54, 183–191. [Google Scholar] [CrossRef]
- Maxwell, A.D.; Cain, C.A.; Duryea, A.P.; Yuan, L.; Gurm, H.S.; Xu, Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy—Histotripsy. Ultrasound Med. Biol. 2009, 35, 1982–1994. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, L.; Vlaisavljevich, E.; Owens, G.E.; Gurm, H.S.; Cain, C.A.; Xu, Z. Noninvasive thrombolysis using microtripsy: A parameter study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 2092–2105. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Alexandrov, A.V. Ultrasound Enhanced Thrombolysis: Applications in Acute Cerebral Ischemia. J. Clin. Neurol. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Slikkerveer, J.; Kleijn, S.A.; Appelman, Y.; Porter, T.R.; Veen, G.; van Rossum, A.C.; Kamp, O. Ultrasound enhanced prehospital thrombolysis using microbubbles infusion in patients with acute ST elevation myocardial infarction: Pilot of the Sonolysis study. Ultrasound Med. Biol. 2012, 38, 247–252. [Google Scholar] [CrossRef]
- Sterling, K.M.; Goldhaber, S.Z.; Sharp, A.S.; Kucher, N.; Jones, N.; Maholic, R.; Meneveau, N.; Zlotnick, D.; Sayfo, S.; Konstantinides, S.V.; et al. Prospective Multicenter International Registry of Ultrasound-Facilitated Catheter-Directed Thrombolysis in Intermediate-High and High-Risk Pulmonary Embolism (KNOCOUT PE). Circ. Cardiovasc. Interv. 2024, 17, e013448. [Google Scholar] [CrossRef]
- Prasad, R.; Vignesh, S.; Yadav, R.R.; Sharma, S.; Hasani, P.; Yadav, T.; Israrahmed, A.; Lal, H. Direct Percutaneous Thrombolysis (DPT): A novel method of salvaging Thrombosed Native Arteriovenous Fistula. J. Vasc. Access 2024, 25, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, N.; Jiang, Z.; Deng, Q.; Zhou, Q.; Hu, B. Beyond silence: Evolving ultrasound strategies in the battle against cardiovascular thrombotic challenges. J. Thromb. Thrombolysis 2024, 57, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Q.; Chan, K.F.; Ning, Z.; Wang, Q.; Ji, F.; Yang, H.; Jiang, S.; Zhang, Z.; Ip, B.Y.M.; et al. tPA-anchored nanorobots for in vivo arterial recanalization at submillimeter-scale segments. Sci. Adv. 2024, 10, eadk8970. [Google Scholar] [CrossRef] [PubMed]
- Pontius, M.H.H.; Ku, C.J.; Osmond, M.J.; Disharoon, D.; Liu, Y.; Warnock, M.; Lawrence, D.A.; Marr, D.W.M.; Neeves, K.B.; Shavit, J.A. Magnetically powered microwheel thrombolysis of occlusive thrombi in zebrafish. Proc. Natl. Acad. Sci. USA 2024, 121, e2315083121. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Mou, F.; Cao, C.; Yu, L.; Li, Z.; Guan, J. Swarming magnetic nanorobots bio-interfaced by heparinoid-polymer brushes for in vivo safe synergistic thrombolysis. Sci. Adv. 2023, 9, eadk7251. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Sun, S.; Zhang, S.; Wang, Y.; Zhang, X.; Sun, J.; He, Z.; Zhang, S.; Luo, C. Molecularly self-fueled nano-penetrator for nonpharmaceutical treatment of thrombosis and ischemic stroke. Nat. Commun. 2023, 14, 255. [Google Scholar] [CrossRef]
- Magneto Thrombectomy Solutions. First-in-human results of the eTrieve electrothrombectomy catheter in acute PE (Press Release, Biomed Israel Conference). Endovasc. Today 2022, 10, 2022. [Google Scholar]
- Andersen, A.; Musialek, P.; Araszkiewicz, A.; Schultz, J.; Nielsen-Kudsk, J.E.; Tekieli, L.; Zajdel, W.; Sławek-Szmyt, S.; Taff, Y.; Weinberg, I. First-in-Human. Trial of Mechanical-Electric Thrombectomy in Acute Pulmonary Embolism. J. Am. Coll. Cardiol. Interv. 2023, 16, 623–625. [Google Scholar] [CrossRef]
- Jawad-Ul-Qamar, M.; Sharma, H.; Vetrugno, V.; Sandhu, K.; Ludman, P.F.; Doshi, S.N.; Townend, J.N.; Osheiba, M.; Zaphiriou, A.; Khan, S.Q. Contemporary use of excimer laser in percutaneous coronary intervention with indications, procedural characteristics, complications and outcomes in a university teaching hospital. Open Heart 2021, 8, e001522. [Google Scholar] [CrossRef]
- Kujiraoka, H.; Tsuchiyama, T.; Inagaki, D.; Yoshida, K.; Fukamizu, S. Comparison of the efficacy of excimer laser coronary angioplasty for ST-segment elevation myocardial infarction with onset-to-balloon time. Lasers Med. Sci. 2023, 38, 126. [Google Scholar] [CrossRef]
- Song, J.; Kang, X.; Wang, L.; Ding, D.; Kong, D.; Li, W.; Qi, J. Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles. Nat. Commun. 2023, 14, 6881. [Google Scholar] [CrossRef]
- Ruan, R.; Chen, S.; Su, J.; Liu, N.; Feng, H.; Xiao, P.; Zhang, X.; Pan, G.; Hou, L.; Zhang, J. Targeting Nanomotor with Near-Infrared/Ultrasound Triggered-Transformation for Polystage-Propelled Cascade Thrombolysis and Multimodal Imaging Diagnosis. Adv. Healthc. Mater. 2024, 13, e2302591. [Google Scholar] [CrossRef]
- Vazquez-Prada, K.X.; Moonshi, S.S.; Wu, Y.; Peter, K.; Wang, X.; Xu, Z.P.; Ta, H.T. Branched silver-iron oxide nanoparticles enabling highly effective targeted and localised drug-free thrombolysis. Biomater. Sci. 2025, 13, 1683–1696. [Google Scholar] [CrossRef]
- Zhu, L.; Lian, W.; Yu, N.; Meng, J.; Zeng, H.; Wang, Y.; Wang, X.; Wen, M.; Chen, Z. Erythrocyte-Membrane-Camouflaged Magnetic Nanocapsules With Photothermal/Magnetothermal Effects for Thrombolysis. Adv. Healthc. Mater. 2024, 13, e2400127. [Google Scholar] [CrossRef]
- Liu, K.T.; Quiñones, E.D.; Liu, M.H.; Lin, C.W.; Chen, Y.T.; Chiang, C.C.; Wu, K.C.; Fan, Y.J.; Chuang, E.Y.; Yu, J. A Biomimicking and Multiarm Self-Indicating Nanoassembly for Site-Specific Photothermal-Potentiated Thrombolysis Assessed in Microfluidic and In Vivo Models. Adv. Healthc. Mater. 2023, 12, e2300682. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Cho, H.; Kim, G.; Youn, I.; Key, J.; Han, S. Targeted thrombolysis by magnetoacoustic particles in photothrombotic stroke model. Biomater. Res. 2022, 26, 58. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, D.; Eizadi Sharifabad, M.; Ranjbar, J.A.; Telling, N.D.; Harper, A.G.S. Clot-targeted magnetic hyperthermia permeabilizes blood clots to make them more susceptible to thrombolysis. J. Thromb. Haemost. 2022, 20, 2556–2570. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Manamanchaiyaporn, L.; Zhou, Q.; Huang, C.; Li, L.; Li, Z.; Wang, L.; Wang, J.; Ren, L.; Xu, T.; et al. Synergistic Integration and Pharmacomechanical Function of Enzyme-Magnetite Nanoparticle Swarms for Low-Dose Fast Thrombolysis. Small 2022, 18, e2202848. [Google Scholar] [CrossRef]
- Jheng, P.R.; Chiang, C.C.; Kang, J.H.; Fan, Y.J.; Wu, K.C.; Chen, Y.T.; Liang, J.W.; Bolouki, N.; Lee, J.W.; Hsieh, J.H.; et al. Cold atmospheric plasma-enabled platelet vesicle incorporated iron oxide nano-propellers for thrombolysis. Mater. Today Bio 2023, 23, 100876. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, T.; Wang, Q.; Huang, L.; Zhong, Z.; Lin, J.; Hu, K.; Xin, H.; Wang, X. Targeted Thrombolytic Therapy with Metal-Organic-Framework-Derived Carbon Based Platforms with Multimodal Capabilities. ACS Appl. Mater. Interfaces 2021, 13, 24453–24462. [Google Scholar] [CrossRef]
- Vazquez-Prada, K.X.; Moonshi, S.S.; Wu, Y.; Akther, F.; Tse, B.W.C.; Sokolowski, K.A.; Peter, K.; Wang, X.; Xu, G.; Ta, H.T. A Spiky Silver-Iron Oxide Nanoparticle for Highly Efficient Targeted Photothermal Therapy and Multimodal Imaging of Thrombosis. Small 2023, 19, e2205744. [Google Scholar] [CrossRef] [PubMed]
- Jacqmarcq, C.; Picot, A.; Flon, J.; Lebrun, F.; de Lizarrondo, S.M.; Naveau, M.; Bernay, B.; Goux, D.; Rubio, M.; Malzert-Fréon, A.; et al. MRI-based microthrombi detection in stroke with polydopamine iron oxide. Nat. Commun. 2024, 15, 5070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, Y.; Ye, M.; Xu, J.; Liu, J.; Zhou, J.; Wang, S.; Guo, D.; Wang, Z.; Ran, H. Polydopamine-modified dual-ligand nanoparticles as highly effective and targeted magnetic resonance/photoacoustic dual-modality thrombus imaging agents. Int. J. Nanomed. 2019, 14, 7155–7171. [Google Scholar] [CrossRef]
- Bonnard, T.; Gauberti, M.; Martinez de Lizarrondo, S.; Campos, F.; Vivien, D. Recent Advances in Nanomedicine for Ischemic and Hemorrhagic Stroke. Stroke 2019, 50, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Disharoon, D.; Marr, D.W.M.; Neeves, K.B. Engineered microparticles and nanoparticles for fibrinolysis. J. Thromb. Haemost. 2019, 17, 2004–2015. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, Z.; Xu, J.; Liu, J.; Guo, Z.N. Targeted nano-delivery strategies for facilitating thrombolysis treatment in ischemic stroke. Drug Deliv. 2021, 28, 357–371. [Google Scholar] [CrossRef]
- Chung, T.W.; Wang, S.S.; Tsai, W.J. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials 2008, 29, 228–237. [Google Scholar] [CrossRef]
- Korin, N.; Kanapathipillai, M.; Matthews, B.D.; Crescente, M.; Brill, A.; Mammoto, T.; Ghosh, K.; Jurek, S.; Bencherif, S.A.; Bhatta, D.; et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012, 337, 738–742. [Google Scholar] [CrossRef]
- Colasuonno, M.; Palange, A.L.; Aid, R.; Ferreira, M.; Mollica, H.; Palomba, R.; Emdin, M.; Del Sette, M.; Chauvierre, C.; Letourneur, D.; et al. Erythrocyte-Inspired Discoidal Polymeric Nanoconstructs Carrying Tissue Plasminogen Activator for the Enhanced Lysis of Blood Clots. ACS Nano 2018, 12, 12224–12237. [Google Scholar] [CrossRef]
- Blum, N.T.; Gyorkos, C.M.; Narowetz, S.J.; Mueller, E.N.; Goodwin, A.P. Phospholipid-Coated Hydrophobic Mesoporous Silica Nanoparticles Enhance Thrombectomy by High-Intensity Focused Ultrasound with Low Production of Embolism-Inducing Clot Debris. ACS Appl. Mater. Interfaces 2019, 11, 36324–36332. [Google Scholar] [CrossRef]
- Refaat, A.; del Rosal, B.; Palasubramaniam, J.; Pietersz, G.; Wang, X.; Moulton, S.E.; Peter, K. Near-infrared light-responsive liposomes for protein delivery: Towards bleeding-free photothermally-assisted thrombolysis. J. Control. Release 2021, 337, 212–223. [Google Scholar] [CrossRef]
- Hu, L.; Xu, J.; Zhang, W.; Wang, J.; Fang, N.; Luo, Y.; Xu, L.; Liu, J.; Zhang, Y.; Ran, H.; et al. A Synergistic and Efficient Thrombolytic Nanoplatform: A Mechanical Method of Blasting Combined with Thrombolytic Drugs. Int. J. Nanomed. 2022, 17, 5229–5246. [Google Scholar] [CrossRef]
- Yu, W.; Yin, N.; Yang, Y.; Xuan, C.; Liu, X.; Liu, W.; Zhang, Z.; Zhang, K.; Liu, J.; Shi, J. Rescuing ischemic stroke by biomimetic nanovesicles through accelerated thrombolysis and sequential ischemia-reperfusion protection. Acta Biomater. 2022, 140, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Liu, C.H.; Pan, W.Y.; Jheng, P.R.; Hsieh, Y.S.Y.; Burnouf, T.; Fan, Y.J.; Chiang, C.C.; Chen, T.Y.; Chuang, E.Y. Biomimetic Platelet Nanomotors for Site-Specific Thrombolysis and Ischemic Injury Alleviation. ACS Appl. Mater. Interfaces 2023, 15, 32967–32983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, N.; Jiang, Z.; Wang, H.; Guo, Y.; Zhong, F.; Gui, B.; Chen, Y.; Deng, Q.; Zhou, Q.; et al. Dual-mode nanoprobe strategy integrating ultrasound and near-infrared light for targeted and synergistic arterial thrombolysis. J. Nanobiotechnol. 2024, 22, 311. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, S.; Wang, J.; Zhang, Y.; Fan, C.; Chao, J.; Gao, Y.; Wang, L. An intelligent DNA nanodevice for precision thrombolysis. Nat. Mater. 2024, 23, 854–862. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X.; Jin, R.; Li, G. Tissue plasminogen activator-based nanothrombolysis for ischemic stroke. Expert Opin. Drug Deliv. 2018, 15, 173–184. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, X. Magnetic Nanoparticles Mediated Thrombolysis—A Review. IEEE Open J. Nanotechnol. 2023, 4, 109–132. [Google Scholar] [CrossRef]
- Alexandrov, A.V.; Köhrmann, M.; Soinne, L.; Tsivgoulis, G.; Barreto, A.D.; Demchuk, A.M.; Sharma, V.K.; Mikulik, R.; Muir, K.W.; Brandt, G.; et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: A multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol. 2019, 18, 338–347. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.E.; Kok, Y.J.; Tan, C.W.; Hew, Y.Y.; Ong, B.J.A.; Tan, B.Y.-Q.; Teo, W.Z.Y.; Dalan, R.; Chee, Y.L.; Yap, E.S. Emerging Thrombolysis Technologies in Vascular Thrombosis. J. Clin. Med. 2025, 14, 7758. https://doi.org/10.3390/jcm14217758
Fan BE, Kok YJ, Tan CW, Hew YY, Ong BJA, Tan BY-Q, Teo WZY, Dalan R, Chee YL, Yap ES. Emerging Thrombolysis Technologies in Vascular Thrombosis. Journal of Clinical Medicine. 2025; 14(21):7758. https://doi.org/10.3390/jcm14217758
Chicago/Turabian StyleFan, Bingwen Eugene, Yixin Jamie Kok, Chuen Wen Tan, Yu Yue Hew, Brandon Jin An Ong, Benjamin Yong-Qiang Tan, Winnie Z. Y. Teo, Rinkoo Dalan, Yen Lin Chee, and Eng Soo Yap. 2025. "Emerging Thrombolysis Technologies in Vascular Thrombosis" Journal of Clinical Medicine 14, no. 21: 7758. https://doi.org/10.3390/jcm14217758
APA StyleFan, B. E., Kok, Y. J., Tan, C. W., Hew, Y. Y., Ong, B. J. A., Tan, B. Y.-Q., Teo, W. Z. Y., Dalan, R., Chee, Y. L., & Yap, E. S. (2025). Emerging Thrombolysis Technologies in Vascular Thrombosis. Journal of Clinical Medicine, 14(21), 7758. https://doi.org/10.3390/jcm14217758






