Early Diagnostic Markers in Crisponi Syndrome: Two Cases and Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection Criteria
2.2. Genetic Analysis
3. Case Descriptions
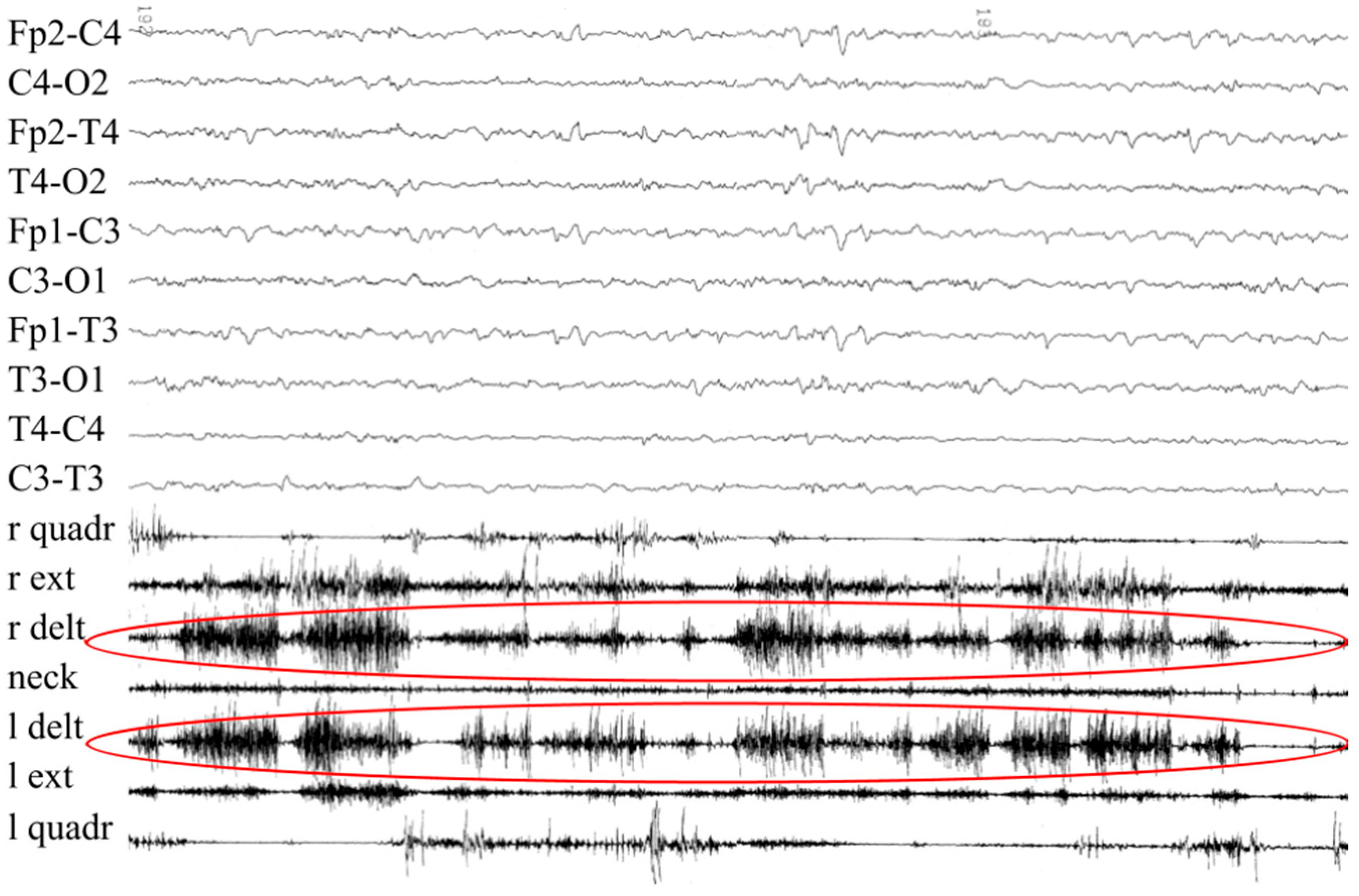
3.1. Patient #1
3.2. Patient #2
4. Discussion
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crisponi, G. Autosomal recessive disorder with muscle contractions resembling neonatal tetanus, characteristic face, camptodactyly, hyperthermia, and sudden death: A new syndrome? Am. J. Med Genet. 1996, 62, 365–371. [Google Scholar] [CrossRef]
- Crisponi, L.; Crisponi, G.; Meloni, A.; Toliat, M.R.; Nürnberg, G.; Usala, G.; Uda, M.; Masala, M.; Höhne, W.; Becker, C.; et al. Crisponi Syndrome Is Caused by Mutations in the CRLF1 Gene and Is Allelic to Cold-Induced Sweating Syndrome Type 1. Am. J. Hum. Genet. 2007, 80, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Dagoneau, N.; Bellais, S.; Blanchet, P.; Sarda, P.; Al-Gazali, L.; Di Rocco, M.; Huber, C.; Djouadi, F.; Le Goff, C.; Munnich, A.; et al. Mutations in Cytokine Receptor-Like Factor 1 (CRLF1) Account for Both Crisponi and Cold-Induced Sweating Syndromes. Am. J. Hum. Genet. 2007, 80, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Nannenberg, E.A.; Bijlmer, R.; Van Geel, B.M.; Hennekam, R.C. Neonatal paroxysmal trismus and camptodactyly: The Crisponi syndrome. Am. J. Med Genet. Part A 2005, 133A, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Herholz, J.; Crisponi, L.; Mallick, B.N.; Rutsch, F. Successful treatment of cold-induced sweating in Crisponi syndrome and its possible mechanism of action. Dev. Med. Child Neurol. 2010, 52, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Fanos, V.; Crisponi, G.; Frau, A.; Ottonello, G. Isolated ‘sign of the horns’: A simple, pathognomonic, prenatal sonographic marker of Crisponi syndrome. J. Obstet. Gynaecol. Res. 2012, 38, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Elson, G.C.A.; Lelièvre, E.; Guillet, C.; Chevalier, S.; Plun-Favreau, H.; Froger, J.; Suard, I.; de Coignac, A.B.; Delneste, Y.; Bonnefoy, J.-Y.; et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat. Neurosci. 2000, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Perret, D.; Guillet, C.; Elson, G.; Froger, J.; Plun-Favreau, H.; Rousseau, F.; Chabbert, M.; Gauchat, J.-F.; Gascan, H. Two Different Contact Sites Are Recruited by Cardiotrophin-like Cytokine (CLC) to Generate the CLC/CLF and CLC/sCNTFRα Composite Cytokines. J. Biol. Chem. 2004, 279, 43961–43970. [Google Scholar] [CrossRef] [PubMed]
- Forger, N.G.; Prevette, D.; Delapeyrière, O.; de Bovis, B.; Wang, S.; Bartlett, P.; Oppenheim, R.W. Cardiotrophin-Like Cytokine/Cytokine-Like Factor 1 is an Essential Trophic Factor for Lumbar and Facial Motoneurons In Vivo. J. Neurosci. 2003, 23, 8854–8858. [Google Scholar] [CrossRef] [PubMed]
- DeChiara, T.M.; Vejsada, R.; Poueymirou, W.T.; Acheson, A.; Suri, C.; Conover, J.C.; Friedman, B.; McClain, J.; Pan, L.; Stahl, N.; et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 1995, 83, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.F.; Knappskog, P.M. Cold-Induced Sweating Syndrome Including Crisponi Syndrome. 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK52917/ (accessed on 25 September 2025).
- Crisponi, L.; Buers, I.; Rutsch, F. CRLF1 and CLCF1 in Development, Health and Disease. Int. J. Mol. Sci. 2022, 23, 992. [Google Scholar] [CrossRef] [PubMed]
- Calà, F.; Sforza, E.; D’alatri, L.; Frassineti, L.; Manfredi, C.; Onesimo, R.; Rigante, D.; Pane, M.; Servidei, S.; Primiano, G.; et al. Do Rare Genetic Conditions Exhibit a Specific Phonotype? A Comprehensive Description of the Vocal Traits Associated with Crisponi/Cold-Induced Sweating Syndrome Type 1. Genes 2025, 16, 881. [Google Scholar] [CrossRef] [PubMed]
- Onesimo, R.; Sforza, E.; Palermo, F.; Giorgio, V.; Leoni, C.; Rigante, D.; Trevisan, V.; Agazzi, C.; Limongelli, D.; Proli, F.; et al. Feeding and Nutritional Key Features of Crisponi/Cold-Induced Sweating Syndrome. Genes 2024, 15, 1109. [Google Scholar] [CrossRef] [PubMed]
- Buers, I.; Persico, I.; Schöning, L.; Nitschke, Y.; Di Rocco, M.; Loi, A.; Sahi, P.K.; Utine, G.E.; Bayraktar-Tanyeri, B.; Zampino, G.; et al. Crisponi/cold-induced sweating syndrome: Differential diagnosis, pathogenesis and treatment concepts. Clin. Genet. 2020, 97, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Herholz, J.; Meloni, A.; Marongiu, M.; Chiappe, F.; Deiana, M.; Herrero, C.R.; Zampino, G.; Hamamy, H.; Zalloum, Y.; Waaler, P.E.; et al. Differential secretion of the mutated protein is a major component affecting phenotypic severity in CRLF1-associated disorders. Eur. J. Hum. Genet. 2011, 19, 525–533. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Patient 1 | Patient 2 | |
|---|---|---|
| DNA variant | Compound heterozygous: C.226T>G C.676-677insA | Homozygous: C.708_709delinsT |
| Exon/intron | Exon 2 Exon 4 | Exon 5 |
| Amino acid change | p.Trp76Gly p.T226NfsX104 | p.Pro238Argfs*6 |
| Clinvar accession number | VCV000005708.3 VCV000005707.4 | VCV000005712.1 |
| Reported before | Yes, both variants | Yes |
| Evidence prompting suspicion of cs/ciss | Orofacial muscle contractions, generalized paroxysmal events, camptodactyly | Prenatal bilateral camptodactyly |
| SWS/SJS2 | NT | CLIFAHDD | CHS/SHFYNG | |
|---|---|---|---|---|
| Inheritance | AR | N/A | AD | AD |
| Gene | LIFR 5p13.1 | N/A | NALCN 13q33.1 | MAGEL2, 15q11.2 |
| Clinical features | BLB, JR, DA, HTE, RD, FSD, DD, PKS | IMT, TS, RF | LD, CD, FA, DF | ID, DPD, NH, CD, DF, FSD, FP, HTE, PSW |
| Difference from CS/CISS | BLB in early childhood | Continuous S without trigger, No DF | Microstomia No HTE | Overlapping phenotype with CS/CISS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perilli, L.; Dzwilewski, K.; Pietruszka, M.; Striano, P.; Capovilla, G.; Mazurkiewicz-Bełdzinska, M. Early Diagnostic Markers in Crisponi Syndrome: Two Cases and Review. J. Clin. Med. 2025, 14, 7757. https://doi.org/10.3390/jcm14217757
Perilli L, Dzwilewski K, Pietruszka M, Striano P, Capovilla G, Mazurkiewicz-Bełdzinska M. Early Diagnostic Markers in Crisponi Syndrome: Two Cases and Review. Journal of Clinical Medicine. 2025; 14(21):7757. https://doi.org/10.3390/jcm14217757
Chicago/Turabian StylePerilli, Lorenzo, Kamil Dzwilewski, Marta Pietruszka, Pasquale Striano, Giuseppe Capovilla, and Maria Mazurkiewicz-Bełdzinska. 2025. "Early Diagnostic Markers in Crisponi Syndrome: Two Cases and Review" Journal of Clinical Medicine 14, no. 21: 7757. https://doi.org/10.3390/jcm14217757
APA StylePerilli, L., Dzwilewski, K., Pietruszka, M., Striano, P., Capovilla, G., & Mazurkiewicz-Bełdzinska, M. (2025). Early Diagnostic Markers in Crisponi Syndrome: Two Cases and Review. Journal of Clinical Medicine, 14(21), 7757. https://doi.org/10.3390/jcm14217757







