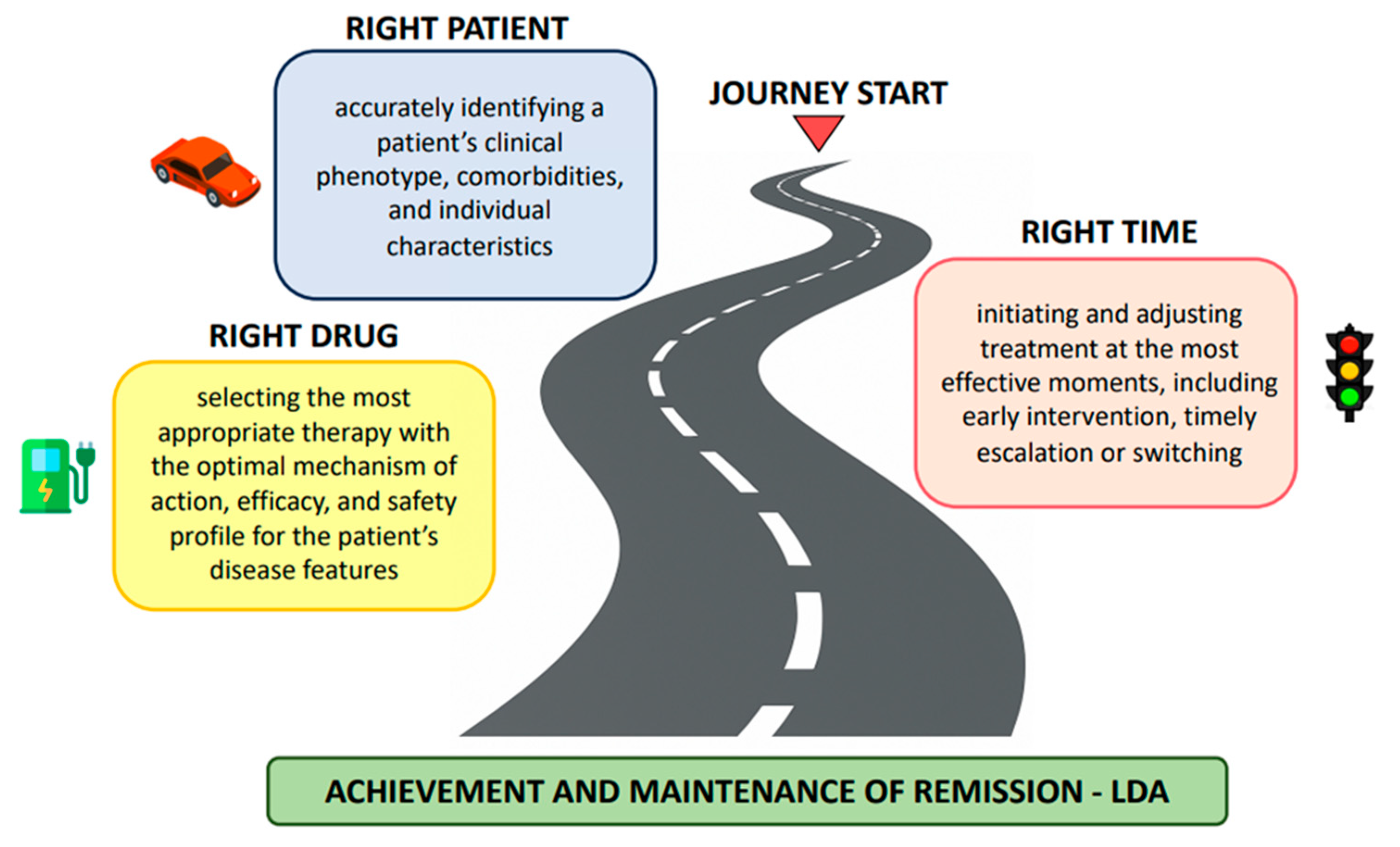
Navigating the Journey in Psoriatic Arthritis: Matching the Right Patient, the Right Drug, and the Right Time
Abstract
1. Introduction
2. The Patient as the Vehicle
3. The Drug as the Fuel
4. Time as the Road Conditions and Signals
5. Conclusions
- Enhance early detection and intervention—Define preclinical stages and strategies to reduce diagnostic delays.
- Link phenotypes and endotypes to therapy response—Identify molecular and clinical signatures predictive of treatment outcomes.
- Develop decision-support tools—Integrate clinical, imaging, biomarker, and patient-reported data to guide individualized therapy.
- Leverage real-world evidence and registries—Monitor long-term outcomes and optimize T2T strategies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bDMARD | Biologic disease-modifying antirheumatic drug |
| csDMARD | Conventional synthetic disease-modifying antirheumatic drug |
| DAPSA | Disease Activity in PSoriatic Arthritis score |
| EULAR | European Alliance of Associations for Rheumatology |
| GRAPPA | Group for Research and Assessment of Psoriasis and Psoriatic Arthritis |
| IL | Interleukin |
| JAK | Janus kinase |
| LDA | Low disease activity |
| MDA | Minimal disease activity |
| NSAID | Non-steroidal anti-inflammatory drug |
| PDE4 | Phosphodiesterase-4 |
| PsA | Psoriatic arthritis |
| PsO | Psoriasis |
| T2T | Treat-to-target |
| TNF | Tumor necrosis factor |
References
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970, Erratum in N. Engl. J. Med. 2017, 376, 2097. [Google Scholar] [CrossRef]
- Coates, L.C.; Helliwell, P.S. Psoriatic arthritis: State of the art review. Clin. Med. 2017, 17, 65–70. [Google Scholar] [CrossRef]
- Caso, F.; Fatica, M.; Ferraioli, M.; Megna, M.; Potestio, L.; Ruggiero, A.; Tommasino, N.; Maione, F.; Scarpa, R.; Chimenti, M.S.; et al. The role of bDMARDs in the prevention and treatment of inflammatory-related comorbidities in Psoriatic Arthritis. Expert Opin. Biol. Ther. 2024, 24, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Bohannan, B.; Mburu, S.; Coates, L.C.; Ogdie, A.; Alarcon, I.; Kasparek, T.; Frade, S.; Barrio, S.F.; Augustin, M. Patient Perspectives on Psoriatic Disease Burden: Results from the Global Psoriasis and Beyond Survey. Dermatology 2023, 239, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Hwang, M.; Veeranki, P.; Portelli, A.; Sison, S.; Shafrin, J.; Pedro, S.; Hass, S.; Hur, P.; Kim, N.; et al. Health care utilization and costs associated with functional status in patients with psoriatic arthritis. J. Manag. Care Spec. Pharm. 2022, 28, 997–1007. [Google Scholar]
- Nash, P.; Clegg, D.O. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann. Rheum. Dis. 2005, 64 Suppl 2, ii74–ii77. [Google Scholar] [CrossRef]
- Mease, P. A short history of biological therapy for psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S104–S108. [Google Scholar]
- Sunzini, F.; D’Antonio, A.; Fatica, M.; Triggianese, P.; Conigliaro, P.; Greco, E.; Bergamini, A.; Chimenti, M.S. What’s new and what’s next for biological and targeted synthetic treatments in psoriatic arthritis? Expert Opin. Biol. Ther. 2022, 22, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Helliwell, P.S. Treat to target in psoriatic arthritis-evidence, target, research agenda. Curr. Rheumatol. Rep. 2015, 17, 517. [Google Scholar] [CrossRef]
- Schoels, M.M.; Aletaha, D.; Alasti, F.; Smolen, J.S. Disease Activity in Psoriatic Arthritis (DAPSA): Defining Remission and Treatment Success Using the OMERACT Filter. Ann. Rheum. Dis. 2016, 75, 811–818. [Google Scholar] [CrossRef]
- Helliwell, P.S.; Wanxman, R. Modification of the Psoriatic Arthritis Disease Activity Score (PASDAS). Ann. Rheum. Dis. 2018, 77, 467–468. [Google Scholar] [CrossRef]
- Coates, L.C.; Fransen, J.; Helliwell, P.S. Defining Minimal Disease Activity in Psoriatic Arthritis: A Proposed Objective Target for Treatment. Ann. Rheum. Dis. 2010, 69, 48–53. [Google Scholar] [CrossRef]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Callis Duffin, K.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; FitzGerald, O.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef]
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.O.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef]
- Scriffignano, S.; Perrotta, F.M.; Conigliaro, P.; Ferraioli, M.; Triggianese, P.; Chimenti, M.S.; Lubrano, E. Identification of the Minimal Disease Activity Domains Achieved Based on Different Treatments in Psoriatic Arthritis. Rheumatol. Ther. 2023, 10, 1785–1794. [Google Scholar] [CrossRef]
- Moll, J.M.; Wright, V. Psoriatic arthritis. Semin. Arthritis. Rheum. 1973, 3, 55–78. [Google Scholar] [CrossRef] [PubMed]
- López-Medina, C.; Chevret, S.; Molto, A.; Sieper, J.; Duruöz, T.; Kiltz, U.; Elzorkany, B.; Hajjaj-Hassouni, N.; Burgos-Vargas, R.; Maldonado-Cocco, J.; et al. Identification of clinical phenotypes of peripheral involvement in patients with spondyloarthritis, including psoriatic arthritis: A cluster analysis in the worldwide ASAS-PerSpA study. RMD Open 2021, 7, e001728. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, G.M.; Delrosso, C.A.; Nerviani, A.; Boutet, M.A. Molecular portrait of chronic joint diseases: Defining endotypes toward personalized medicine. Joint Bone Spine 2024, 91, 105692. [Google Scholar] [CrossRef]
- Najm, A.; Goodyear, C.S.; McInnes, I.B.; Siebert, S. Phenotypic heterogeneity in psoriatic arthritis: Towards tissue pathology-based therapy. Nat. Rev. Rheumatol. 2023, 19, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, I.; Nakayamada, S.; Ueno, M.; Miyazaki, Y.; Ohkubo, N.; Inoue, Y.; Kubo, S.; Tanaka, Y. Precision medicine based on the phenotypic differences in peripheral T helper cells in patients with psoriatic arthritis: One year follow-up outcomes. Front. Med. 2022, 9, 934937. [Google Scholar] [CrossRef]
- Pitzalis, C.; Choy, E.H.S.; Buch, M.H. Transforming clinical trials in rheumatology: Towards patient-centric precision medicine. Nat. Rev. Rheumatol. 2020, 16, 590–599. [Google Scholar] [CrossRef]
- Lubrano, E.; Scriffignano, S.; Perrotta, F.M. Multimorbidity and comorbidity in psoriatic arthritis—A perspective. Expert Rev. Clin. Immunol. 2020, 16, 963–972. [Google Scholar] [CrossRef]
- Cagnotto, G.; Bruschettini, M.; Stróżyk, A.; Scirè, C.A.; Compagno, M. Tumor necrosis factor (TNF) inhibitors for psoriatic arthritis. Cochrane Database Syst. Rev. 2025, 13, CD013614. [Google Scholar] [CrossRef]
- Savage, L.J.; Wittmann, M.; McGonagle, D.; Helliwell, P.S. Ustekinumab in the Treatment of Psoriasis and Psoriatic Arthritis. Rheumatol. Ther. 2015, 2, 1–16. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Siebert, S. The role of IL-23 and the use of IL-23 inhibitors in psoriatic arthritis. Musculoskeletal. Care 2022, 20 (Suppl. 1), S12–S21. [Google Scholar] [CrossRef]
- Simopoulou, T.; Tsiogkas, S.G.; Zafiriou, E.; Bogdanos, D.P. Secukinumab, ixekizumab, bimekizumab and brodalumab for psoriasis and psoriatic arthritis. Drugs Today 2023, 59, 135–167. [Google Scholar] [CrossRef] [PubMed]
- Keeling, S.; Maksymowych, W.P. JAK inhibitors, psoriatic arthritis, and axial spondyloarthritis: A critical review of clinical trials. Expert Rev. Clin. Immunol. 2021, 17, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Apremilast in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S98–S100. [Google Scholar] [PubMed]
- Mease, P.J.; Chandran, V.; Armstrong, A.W.; Blanco, R.; Ogdie, A.; Siegel, E.; Gottlieb, A.B.; Zeng, X.; Thaçi, D.; Kishimoto, M.; et al. OP0095 Efficacy and safety of deucravacitinib up to week 52 from POETYK PsA-2: A multicenter, randomized, double-blind, placebo-controlled, phase 3 study in patients with psoriatic arthritis. Ann. Rheum. Dis. 2025, 84 (Suppl. 1), 84–85. [Google Scholar] [CrossRef]
- McInnes, I.B.; Eder, L.; Ritchlin, C.T.; Ogdie, A.; Kavanaugh, A.; Coates, L.C.; Schett, G.; Kivitz, A.; Brennan, N.; Godwood, A.; et al. OP0096 Impact of sonelokimab, a novel IL-17A- and IL-17F-inhibiting Nanobody, on multidomain clinical outcomes in active psoriatic arthritis: Results from the randomized, double-blind, placebo-controlled Phase 2 ARGO trial. Ann. Rheum. Dis. 2025, 84 (Suppl. 1), 85–86. [Google Scholar] [CrossRef]
- Taylor, P.C.; Mease, P.J.; de Vlam, K.; Mpofu, S.; Wetzel, D.; Stevens, A.M.; Wiens, B.; Koskinen, L.O.; Ohlman, S.; Feldwisch, J.; et al. Efficacy and safety of izokibep in patients with active psoriatic arthritis: A randomised, double-blind, placebo-controlled, phase 2 study. Ann. Rheum. Dis. 2025, 84, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Reddy, S.; Ross, S.; Lisse, J.R.; Reis, P.; Griffing, K.; Sapin, C.; Vadhariya, A.; Furst, D.E. Evaluating the efficacy of biologics with and without methotrexate in the treatment of psoriatic arthritis: A network meta-analysis. RMD Open 2024, 10, e003423. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, A.; Smolen, J.S.; Ferreira, R.J.O.; Bertheussen, H.; Baraliakos, X.; Aletaha, D.; McGonagle, D.G.; van der Heijde, D.; McInnes, I.B.; Esbensen, B.A.; et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: A systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 2024, 83, 760–774. [Google Scholar] [CrossRef]
- Skaarup, L.; Ingrid, E.; Sepriano, A.; Nikiphorou, E.; Østgård, R.; Lauper, K.; Grosse-Michaelis, I.; Kloppenburg, M.; Glintborg, B.; Liew, D.F.L.; et al. A Systematic Overview of Contraindications and Special Warnings for Biologic and Targeted Synthetic Disease Modifying Antirheumatic Drugs: Establishing a Framework to Create a “Safety Checklist”. Drug Saf. 2024, 47, 1075–1093. [Google Scholar] [CrossRef]
- Magee, C.; Jethwa, H.; FitzGerald, O.M.; Jadon, D.R. Biomarkers predictive of treatment response in psoriasis and psoriatic arthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211014010. [Google Scholar] [CrossRef]
- Karmacharya, P.; Wright, K.; Achenbach, S.J.; Bekele, D.; Crowson, C.S.; Ogdie, A.; Duarte-García, A.; Ernste, F.C.; Tollefson, M.M.; Davis, J.M. Diagnostic Delay in Psoriatic Arthritis: A Population-based Study. J. Rheumatol. 2021, 48, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Gallagher, P.; FitzGerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1045–1050. [Google Scholar] [CrossRef]
- Snoeck Henkemans, S.V.J.; de Jong, P.H.P.; Luime, J.J.; Kok, M.R.; Tchetverikov, I.; Korswagen, L.A.; van der Kooij, S.M.; van Oosterhout, M.; Baudoin, P.; Bijsterbosch, J.; et al. Window of opportunity in psoriatic arthritis: The earlier the better? RMD Open 2024, 10, e004062. [Google Scholar] [CrossRef]
- Coates, L.C.; Moverley, A.R.; McParland, L.; Brown, S.; Navarro-Coy, N.; O’Dwyer, J.L.; Meads, D.M.; Emery, P.; Conaghan, P.G.; Helliwell, P.S. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): A UK multicentre, open-label, randomised controlled trial. Lancet 2015, 386, 2489–2498. [Google Scholar] [CrossRef]
- Gossec, L.; Coates, L.C.; Gladman, D.D.; Aelion, J.A.; Vasandani, J.; Pinter, A.; Merola, J.F.; Kavanaugh, A.; Reddy, J.; Wang, R.; et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: Primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann. Rheum. Dis. 2024, 83, 1480–1488. [Google Scholar] [CrossRef]
- Perez-Chada, L.M.; Haberman, R.H.; Chandran, V.; Rosen, C.F.; Ritchlin, C.; Eder, L.; Mease, P.; Reddy, S.; Ogdie, A.; Merola, J.F.; et al. Consensus terminology for preclinical phases of psoriatic arthritis for use in research studies: Results from a Delphi consensus study. Nat. Rev. Rheumatol. 2021, 17, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Zabotti, A.; De Marco, G.; Gossec, L.; Baraliakos, X.; Aletaha, D.; Iagnocco, A.; Gisondi, P.; Balint, P.V.; Bertheussen, H.; Boehncke, W.H.; et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann. Rheum. Dis. 2023, 82, 1162–1170. [Google Scholar] [CrossRef]
- Zabotti, A.; Fagni, F.; Gossec, L.; Giovannini, I.; Sticherling, M.; Tullio, A.; Baraliakos, X.; De Marco, G.; De Vita, S.; Errichetti, E.; et al. Risk of developing psoriatic arthritis in psoriasis cohorts with arthralgia: Exploring the subclinical psoriatic arthritis stage. RMD Open 2024, 10, e004314. [Google Scholar] [CrossRef]
- Gisondi, P.; Bellinato, F.; Maurelli, M.; Geat, D.; Zabotti, A.; McGonagle, D.; Girolomoni, G. Reducing the Risk of Developing Psoriatic Arthritis in Patients with Psoriasis. Psoriasis 2022, 12, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Scher, J.U.; Ogdie, A.; Ritchlin, C.; Merola, J.F. Prevention of Psoriatic Arthritis: The Need for Prospective Studies. Dermatol. Clin. 2024, 42, 429–438. [Google Scholar] [CrossRef] [PubMed]
- López-Medina, C.; McGonagle, D.; Gossec, L. Subclinical psoriatic arthritis and disease interception-where are we in 2024? Rheumatology 2025, 64, 56–64. [Google Scholar] [CrossRef]
- Ciccia, F.; Gandolfo, S.; Caporali, R.; Scher, J.U. Understanding the spectrum from preclinical psoriatic arthritis to early diagnosis of the disease. Lancet Rheumatol. 2025, 7, e208–e211. [Google Scholar] [CrossRef]
- Acosta Felquer, M.L.; LoGiudice, L.; Galimberti, M.L.; Rosa, J.; Mazzuoccolo, L.; Soriano, E.R. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 74–79. [Google Scholar] [CrossRef]
- Aronovich, A.; Novikov, I.; Pavlovsky, L. Do Biologic Treatments for Psoriasis Lower the Risk of Psoriatic Arthritis? A Systematic Review. Am. J. Clin. Dermatol. 2023, 24, 865–873. [Google Scholar] [CrossRef]
- Errichetti, E.; Zabotti, A. Biologics in Prevention of Psoriasis to Psoriatic Arthritis Transition: The Need of Prospective Analyses and Stratification According to Time-Related Risk Factors. Dermatol. Ther. 2024, 14, 1–3. [Google Scholar] [CrossRef]
- de Camargo, M.C.; Barros, B.C.A.; Fulone, I.; Silva, M.T.; Silveira, M.S.D.N.; de Camargo, I.A.; Barberato-Filho, S.; Del Fiol, F.S.; Lopes, L.C. Adverse Events in Patients With Rheumatoid Arthritis and Psoriatic Arthritis Receiving Long-Term Biological Agents in a Real-Life Setting. Front. Pharmacol. 2019, 10, 965. [Google Scholar] [CrossRef]
- Lumetti, F.; Ariani, A.; Marchesoni, A.; Becciolini, A.; Giuggioli, D.; Sandri, G. Cycling versus swapping strategies with TNF-α inhibitors and IL-17 inhibitors in psoriatic arthritis in clinical practice. Sci. Rep. 2024, 14, 24922. [Google Scholar] [CrossRef]
- Ariani, A.; Santilli, D.; Mozzani, F.; Lumetti, F.; Lucchini, G.; Di Donato, E.; Giordano, S.; Riva, M.; Becciolini, A. Cycling or swap biologics and small molecules in psoriatic arthritis: Observations from a real-life single center cohort. Medicine 2021, 100, e25300. [Google Scholar] [CrossRef] [PubMed]
- van Es, I.; Vriezekolk, J.E.; den Broeder, N.; de Beijer, L.; den Broeder, A.A.; van Herwaarden, N.; Mahler, E.; Leijten, E.F.A. Cycle versus swap strategy after TNFi discontinuation in psoriatic arthritis and axial spondyloarthritis: A quasi-experimental study. RMD Open 2025, 11, e005566. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, F.; Ferreira, M.; Soares, C.; Parente, H.; Ochoa Matos, C.; Costa, R.; Oliveira, D.; Abreu, C.; Teixeira, R.; Azevedo, S.; et al. Cycling versus swapping strategies in psoriatic arthritis: Results from the rheumatic diseases Portuguese register. ARP Rheumatol. 2023, 2, 200. [Google Scholar]
- Lubrano, E.; Scriffignano, S.; Perrotta, F.M. Psoriatic Arthritis, Psoriatic Disease, or Psoriatic Syndrome? J. Rheumatol. 2019, 46, 1428–1430. [Google Scholar] [CrossRef]
| Index | Components/Formula | Cut-Offs for Disease Activity States | Pros/Cons | Monitoring |
|---|---|---|---|---|
| DAPSA (Disease Activity index for PSoriatic Arthritis) | DAPSA = TJC(68) + SJC(66) + PtGA (0–10 VAS) + Pt Pain (0–10 VAS) + CRP (mg/dL or mg/L) | Remission: ≤4 Low: >4–14 Moderate: >14–28 High: >28 | Pros: Quick, feasible, easy to use, sensitive to change Cons: do not include skin/nail, enthesitis, axial disease and dactylitis. Cut-offs based on physician evaluation | 3–6 months |
| cDAPSA (Clinical DAPSA) | Same as DAPSA but excludes CRP: cDAPSA = TJC(68) + SJC(66) + PtGA + Pt Pain | Remission: ≤4 Low: >4–13 Moderate: >13–27 High: >27 | Pros: Quick, feasible, easy to use, sensitive to change Cons: do not include skin/nail, enthesitis, axial disease and dactylitis. Cut-offs based on physician evaluation. Absence of laboratory parameters | 3–6 months |
| PASDAS (Psoriatic Arthritis Disease Activity Score) | Composite index combining multiple domains: Formula: PASDAS = (0.18 × √Physician Global VAS) + (0.159 × √PtGA VAS) + (0.253 × √SF-36 PCS) − (0.101 × √SJC66) − (0.048 × √TJC68) + (0.23 × ln(Swollen Entheses + 1)) + (0.37 × ln(CRP + 1)) + 0.102 Range ≈ 0–10. | Remission: ≤1.9 Low: >1.9–3.2 Moderate: >3.2–5.4 High: >5.4 | Pros: accounts for nearly all disease for most people. Developed on real life treatment decision. Disease state based on Physician and patient opinion. Cons: Bit slower. Need SF-36. Need online calculator | 3–6 months |
| MDA (Minimal Disease Activity) | Binary outcome (Yes/No). MDA = achieved if ≥5 of 7 criteria met: TJC ≤ 1; SJC ≤ 1; PASI ≤ 1 or BSA ≤ 3%; Patient Pain on VAS ≤ 15 mm; PtGA VAS ≤ 20 mm; HAQ-DI ≤ 0.5; Tender entheseal points ≤ 1 | MDA achieved: ≥5/7 criteria met Non-MDA: <5/7 | Pros: Quick and feasible. Easy to calculate. Correlates with patient opinion. Account for nearly all disease domains Cons: Only a measure of disease state. Could allow active joint disease. Absence of laboratory parameter | 3–6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubrano, E.; Fatica, M.; Italiano, N.; Perrotta, F.M. Navigating the Journey in Psoriatic Arthritis: Matching the Right Patient, the Right Drug, and the Right Time. J. Clin. Med. 2025, 14, 7713. https://doi.org/10.3390/jcm14217713
Lubrano E, Fatica M, Italiano N, Perrotta FM. Navigating the Journey in Psoriatic Arthritis: Matching the Right Patient, the Right Drug, and the Right Time. Journal of Clinical Medicine. 2025; 14(21):7713. https://doi.org/10.3390/jcm14217713
Chicago/Turabian StyleLubrano, Ennio, Mauro Fatica, Noemi Italiano, and Fabio Massimo Perrotta. 2025. "Navigating the Journey in Psoriatic Arthritis: Matching the Right Patient, the Right Drug, and the Right Time" Journal of Clinical Medicine 14, no. 21: 7713. https://doi.org/10.3390/jcm14217713
APA StyleLubrano, E., Fatica, M., Italiano, N., & Perrotta, F. M. (2025). Navigating the Journey in Psoriatic Arthritis: Matching the Right Patient, the Right Drug, and the Right Time. Journal of Clinical Medicine, 14(21), 7713. https://doi.org/10.3390/jcm14217713







