Bidirectional Interplay Between Traumatic Brain Injury and Cardiovascular Dysfunction in Athletes
Abstract
1. Introduction
2. Methods
3. Cardiovascular Benefits and Risks of Sports Participation
4. The Neurocardiac Consequences of Sports-Related Brain Injury
4.1. Experimental and Clinical Evidence
4.2. Sport-Related Concussion and Cardiovascular Dysfunction
4.3. Acute Autonomic and Hemodynamic Changes
4.4. Chronic Sequelae
4.5. Putative Mechanisms
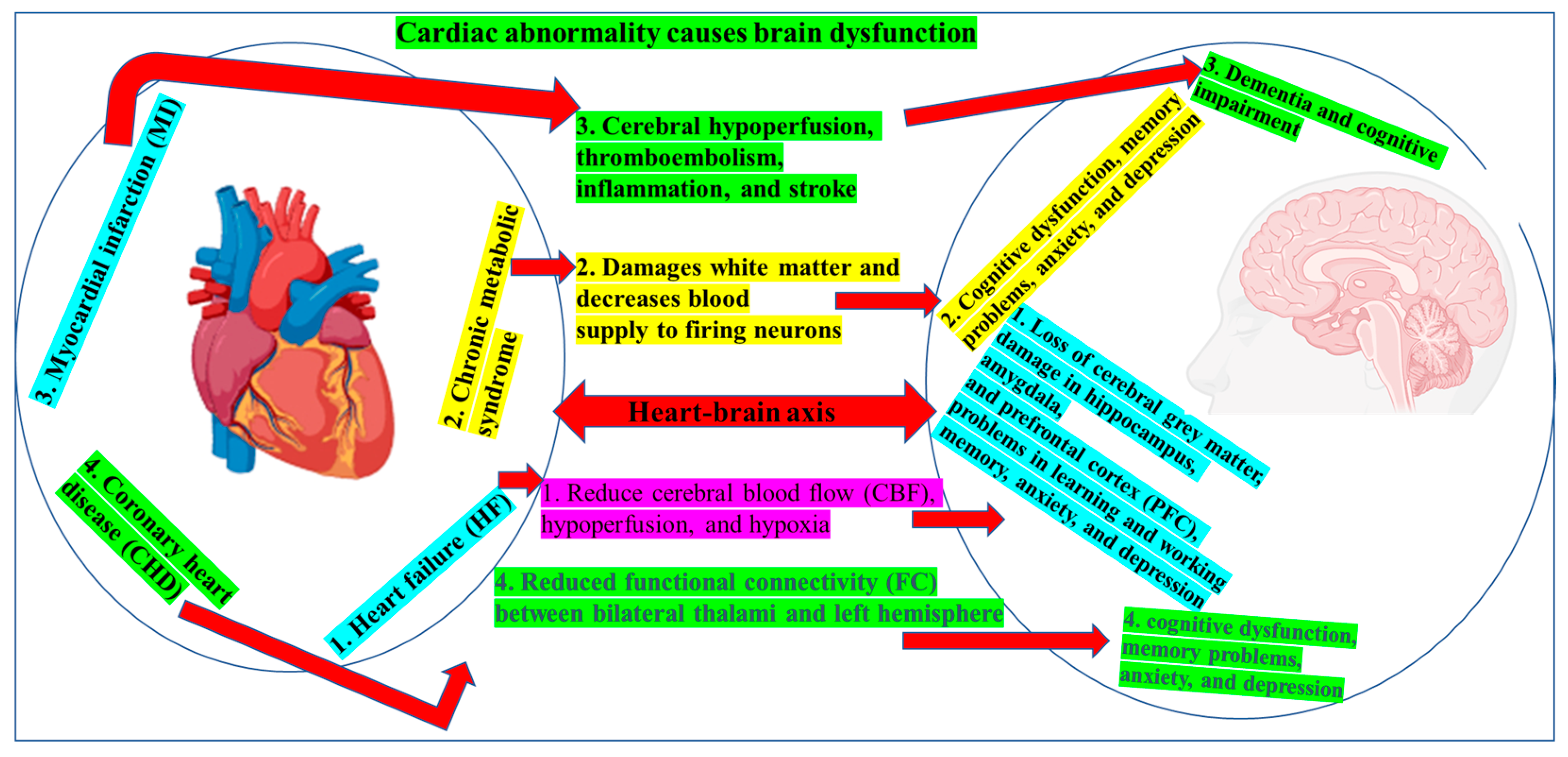
5. Cardiac Contribution to Brain Dysfunction
5.1. HF
5.2. MI
5.3. Arrhythmias
5.4. Metabolic Syndrome and Coronary Disease
5.5. Congenital and Premature Heart Disease
6. Conclusions
- Screening and surveillance must be bilateral. Pre-participation and post-injury evaluations should integrate basic neuro-cardiac metrics—heart rate variability, dynamic cerebral autoregulation, high-sensitivity troponin, and plasma inflammatory markers—to detect subclinical dysfunction in either organ early.
- Rehabilitation should be network-based. Return-to-play protocols that combine graded aerobic exercise, autonomic retraining, and cognitive remediation hold promise for restoring both cardiac conditioning and cerebral plasticity after SRC or cardiac events.
- Research priorities are clear. Prospective, sport-specific cohorts with unified neuro-cardiac phenotyping are needed to define dose–response curves for concussion burden and extreme training loads. Mechanistic trials should test whether targeting inflammasome signaling, catecholamine surges, or endothelial dysfunction mitigates long-term morbidity.
Author Contributions
Funding
Conflicts of Interest
Figures and Graphics
References
- Eather, N.; Wade, L.; Pankowiak, A.; Eime, R. The impact of sports participation on mental health and social outcomes in adults: A systematic review and the ‘Mental Health through Sport’ conceptual model. Syst. Rev. 2023, 12, 102. [Google Scholar] [CrossRef]
- Ishihara, T.; Nakajima, T.; Yamatsu, K.; Okita, K.; Sagawa, M.; Morita, N. Relationship of participation in specific sports to academic performance in adolescents: A 2-year longitudinal study. Scand. J. Med. Sci. Sports 2020, 30, 1471–1482. [Google Scholar] [CrossRef]
- Malm, C.; Jakobsson, J.; Isaksson, A. Physical Activity and Sports-Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports 2019, 7, 127. [Google Scholar] [CrossRef]
- Latino, F.; Tafuri, F. Physical Activity and Cognitive Functioning. Medicina 2024, 60, 216. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Leal-Nazaré, C.G.; Santos-Sacramento, L.; Lopes-Araújo, A.; Freire Royes, L.F.; Crespo-Lopez, M.E. Exercise Reshapes the Brain: Molecular, Cellular, and Structural Changes Associated with Cognitive Improvements. Mol. Neurobiol. 2023, 60, 6950–6974. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.; Gostian-Ropotin, L.A.; Beltrán-Velasco, A.I.; Belando-Pedreño, N.; Simón, J.A.; López-Mora, C.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Sporting Mind: The Interplay of Physical Activity and Psychological Health. Sports 2024, 12, 37. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Ruilope, L.M.; Santos-Lozano, A.; Wilhelm, M.; Kränkel, N.; Fiuza-Luces, C.; Lucia, A. Exercise benefits in cardiovascular diseases: From mechanisms to clinical implementation. Eur. Heart J. 2023, 44, 1874–1889. [Google Scholar] [CrossRef]
- Vettor, R.; Valerio, A.; Ragni, M.; Trevellin, E.; Granzotto, M.; Olivieri, M.; Tedesco, L.; Ruocco, C.; Fossati, A.; Fabris, R.; et al. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: Role in adaptation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E519–E528. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.M.; Gerszten, R.E. Exercise, exerkines, and cardiometabolic health: From individual players to a team sport. J. Clin. Investig. 2023, 133, e168121. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Medicine. Sports Injury Statistics. 2024. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/sports-injuries/sports-injury-statistics (accessed on 15 July 2024).
- Han, J.; Lalario, A.; Merro, E.; Sinagra, G.; Sharma, S.; Papadakis, M.; Finocchiaro, G. Sudden Cardiac Death in Athletes: Facts and Fallacies. J. Cardiovasc. Dev. Dis. 2023, 10, 68. [Google Scholar] [CrossRef]
- Petek, B.J.; Churchill, T.W.; Moulson, N.; Kliethermes, S.A.; Baggish, A.L.; Drezner, J.A.; Patel, M.R.; Ackerman, M.J.; Kucera, K.L.; Siebert, D.M.; et al. Sudden Cardiac Death in National Collegiate Athletic Association Athletes: A 20-Year Study. Circulation 2024, 149, 80–90. [Google Scholar] [CrossRef]
- Bohm, P.; Meyer, T.; Narayanan, K.; Schindler, M.; Weizman, O.; Beganton, F.; Schmied, C.; Bougouin, W.; Barra, S.; Dumas, F.; et al. Sports-related sudden cardiac arrest in young adults. EP Eur. 2022, 25, 627–633. [Google Scholar] [CrossRef]
- Weizman, O.; Empana, J.P.; Blom, M.; Tan, H.L.; Jonsson, M.; Narayanan, K.; Ringh, M.; Marijon, E.; Jouven, X. Incidence of Cardiac Arrest During Sports Among Women in the European Union. J. Am. Coll. Cardiol. 2023, 81, 1021–1031. [Google Scholar] [CrossRef]
- Deo, R.; Albert, C.M. Epidemiology and Genetics of Sudden Cardiac Death. Circulation 2012, 125, 620–637. [Google Scholar] [CrossRef] [PubMed]
- The University of Kansas Health System. The Scary Side of Sports. 2023. Available online: https://www.kansashealthsystem.com/news-room/blog/0001/01/cardiac-death-young-athletes (accessed on 20 March 2025).
- Hallock, H.; Mantwill, M.; Vajkoczy, P.; Wolfarth, B.; Reinsberger, C.; Lampit, A.; Finke, C. Sport-Related Concussion: A Cognitive Perspective. Neurol. Clin. Pract. 2023, 13, e200123. [Google Scholar] [CrossRef]
- Esterov, D.; Greenwald, B.D. Autonomic Dysfunction after Mild Traumatic Brain Injury. Brain Sci. 2017, 7, 100. [Google Scholar] [CrossRef]
- Keane, R.W.; Hadad, R.; Scott, X.O.; Cabrera Ranaldi, E.D.L.R.M.; Pérez-Bárcena, J.; de Rivero Vaccari, J.P. Neural-Cardiac Inflammasome Axis after Traumatic Brain Injury. Pharmaceuticals 2023, 16, 1382. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilpa, N.; Krishnamoorthy, V.; Lele, A.V.; Qiu, Q.; Vavilala, M.S. Characterizing the relationship between systemic inflammatory response syndrome and early cardiac dysfunction in traumatic brain injury. J. Neurosci. Res. 2018, 96, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Wee, I.C.; Arulsamy, A.; Corrigan, F.; Collins-Praino, L. Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk. Molecules 2024, 29, 1470. [Google Scholar] [CrossRef]
- Stewart, I.J.; Amuan, M.E.; Wang, C.P.; Kennedy, E.; Kenney, K.; Werner, J.K.; Carlson, K.F.; Tate, D.F.; Pogoda, T.K.; Dismuke-Greer, C.E.; et al. Association Between Traumatic Brain Injury and Subsequent Cardiovascular Disease Among Post-9/11–Era Veterans. JAMA Neurol. 2022, 79, 1122–1129. [Google Scholar] [CrossRef]
- Izzy, S.; Grashow, R.; Radmanesh, F.; Chen, P.; Taylor, H.; Formisano, R.; Wilson, F.; Wasfy, M.; Baggish, A.; Zafonte, R. Long-term risk of cardiovascular disease after traumatic brain injury: Screening and prevention. Lancet Neurol. 2023, 22, 959–970. [Google Scholar] [CrossRef]
- Sobolewska-Nowak, J.; Wachowska, K.; Nowak, A.; Orzechowska, A.; Szulc, A.; Płaza, O.; Gałecki, P. Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines 2023, 11, 1903. [Google Scholar] [CrossRef]
- Shen, M.J. The cardiac autonomic nervous system: An introduction. Herzschrittmacherther. Elektrophysiol. 2021, 32, 295–301. [Google Scholar] [CrossRef]
- Manolis, A.A.; Manolis, T.A.; Apostolopoulos, E.J.; Apostolaki, N.E.; Melita, H.; Manolis, A.S. The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends Cardiovasc. Med. 2021, 31, 290–302. [Google Scholar] [CrossRef]
- Fu, Q. Autonomic dysfunction and cardiovascular risk in post-traumatic stress disorder. Auton. Neurosci. 2022, 237, 102923. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ruiz, A.; Racosta, J.M.; Kimpinski, K.; Hilz, M.J.; Sposato, L.A. Cardiovascular autonomic dysfunction after stroke. Neurol. Sci. 2021, 42, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lassarén, P.; Lindblad, C.; Frostell, A.; Carpenter, K.L.H.; Guilfoyle, M.R.; Hutchinson, P.J.A.; Helmy, A.; Thelin, E.P. Systemic inflammation alters the neuroinflammatory response: A prospective clinical trial in traumatic brain injury. J. Neuroinflamm. 2021, 18, 221. [Google Scholar] [CrossRef]
- McDonald, S.J.; Sharkey, J.M.; Sun, M.; Kaukas, L.M.; Shultz, S.R.; Turner, R.J.; Leonard, A.V.; Brady, R.D.; Corrigan, F. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 770–781. [Google Scholar] [CrossRef]
- Sabet, N.; Soltani, Z.; Khaksari, M. Multipotential and systemic effects of traumatic brain injury. J. Neuroimmunol. 2021, 357, 577619. [Google Scholar] [CrossRef]
- Rachfalska, N.; Putowski, Z.; Krzych, Ł.J. Distant Organ Damage in Acute Brain Injury. Brain Sci. 2020, 10, 1019. [Google Scholar] [CrossRef]
- Wallner, M.; Duran, J.M.; Mohsin, S.; Troupes, C.D.; Vanhoutte, D.; Borghetti, G.; Vagnozzi, R.J.; Gross, P.; Yu, D.; Trappanese, D.M.; et al. Acute Catecholamine Exposure Causes Reversible Myocyte Injury Without Cardiac Regeneration. Circ. Res. 2016, 119, 865–879. [Google Scholar] [CrossRef]
- Sethi, P.; Peiris, C.D. A Review of Catecholamine Associated Cardiomyopathies and Channelopathies. Cureus 2020, 12, e6957. [Google Scholar] [CrossRef]
- Sinderby, C.; Beck, J. Proportional Assist Ventilation and Neurally Adjusted Ventilatory Assist—Better Approaches to Patient Ventilator Synchrony? Clin. Chest Med. 2008, 29, 329–342. [Google Scholar] [CrossRef]
- Ellingson, C.J.; Shafiq, M.A.; Ellingson, C.A.; Neary, J.P.; Dehghani, P.; Singh, J. Assessment of cardiovascular functioning following sport-related concussion: A physiological perspective. Auton. Neurosci. 2024, 252, 103160. [Google Scholar] [CrossRef]
- Mrozek, S.; Gobin, J.; Constantin, J.M.; Fourcade, O.; Geeraerts, T. Crosstalk between brain, lung and heart in critical care. Anaesth. Crit. Care Pain. Med. 2020, 39, 519–530. [Google Scholar] [CrossRef]
- Biso, S.; Wongrakpanich, S.; Agrawal, A.; Yadlapati, S.; Kishlyansky, M.; Figueredo, V. A Review of Neurogenic Stunned Myocardium. Cardiovasc. Psychiatry Neurol. 2017, 2017, 5842182. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Bonatti, G.; Pelosi, P.; Citerio, G. Extracranial complications after traumatic brain injury: Targeting the brain and the body. Curr. Opin. Crit. Care 2020, 26, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.; Oh, J.K.; Mulvagh, S.L.; Wijdicks, E.F. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2006, 5, 243–249. [Google Scholar] [CrossRef]
- Gopinath, R.; Ayya, S.S. Neurogenic stress cardiomyopathy: What do we need to know. Ann. Card. Anaesth. 2018, 21, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef]
- Dantzer, R.; Konsman, J.P.; Bluthé, R.M.; Kelley, K.W. Neural and humoral pathways of communication from the immune system to the brain: Parallel or convergent? Auton. Neurosci. 2000, 85, 60–65. [Google Scholar] [CrossRef]
- Kerro, A.; Woods, T.; Chang, J.J. Neurogenic stunned myocardium in subarachnoid hemorrhage. J. Crit. Care 2017, 38, 27–34. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Robba, C.; Menon, D.K.; Citerio, G.; Sandroni, C.; Sekhon, M.S. Clinical targeting of the cerebral oxygen cascade to improve brain oxygenation in patients with hypoxic–ischaemic brain injury after cardiac arrest. Intensive Care Med. 2023, 49, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Neumar, R.; Hsu, C.H.; Hirsch, K.G.; Aneman, A.; Becker, L.B.; Couper, K.; Callaway, C.W.; Hoedemaekers, C.W.E.; Lim, S.L.; et al. Improving Outcomes After Post–Cardiac Arrest Brain Injury: A Scientific Statement from the International Liaison Committee on Resuscitation. Circulation 2024, 150, e158–e180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Du, J.; Lachance, B.B.; Chen, S.; Polster, B.M.; Jia, X. Neuroprotection of NSC Therapy is Superior to Glibenclamide in Cardiac Arrest-Induced Brain Injury via Neuroinflammation Regulation. Transl. Stroke Res. 2023, 14, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, M.; Jia, X.; Zhang, W.; Shi, Y.; Bai, S.; Rampes, S.; Vizcaychipi, M.P.; Wu, C.; Wang, K.; et al. Inflammation Disrupts the Brain Network of Executive Function after Cardiac Surgery. Ann. Surg. 2023, 277, e689. [Google Scholar] [CrossRef]
- Zenger, B.; Rizzi, S.; Steinberg, B.A.; Ranjan, R.; Bunch, T.J. This is Your Brain, and This is Your Brain on Atrial Fibrillation: The Roles of Cardiac Malperfusion Events and Vascular Dysfunction in Cognitive Impairment. Arrhythm. Electrophysiol. Rev. 2023, 12, e01. [Google Scholar] [CrossRef]
- Boere, K.; Lloyd, K.; Binsted, G.; Krigolson, O.E. Exercising is good for the brain but exercising outside is potentially better. Sci. Rep. 2023, 13, 1140. [Google Scholar] [CrossRef]
- Jerome, G.J.; Boyer, W.R.; Bustamante, E.E.; Kariuki, J.; Lopez-Jimenez, F.; Paluch, A.E.; Swift, D.L.; Webber-Ritchey, K.J.; Barone Gibbs, B.; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Increasing Equity of Physical Activity Promotion for Optimal Cardiovascular Health in Adults: A Scientific Statement from the American Heart Association. Circulation 2023, 147, 1951–1962. [Google Scholar] [CrossRef]
- Przewłócka, K.; Korewo-Labelle, D.; Berezka, P.; Karnia, M.J.; Kaczor, J.J. Current Aspects of Selected Factors to Modulate Brain Health and Sports Performance in Athletes. Nutrients 2024, 16, 1842. [Google Scholar] [CrossRef]
- Nobari, H.; Azarian, S.; Saedmocheshi, S.; Valdés-Badilla, P.; García Calvo, T. Narrative review: The role of circadian rhythm on sports performance, hormonal regulation, immune system function, and injury prevention in athletes. Heliyon 2023, 9, e19636. [Google Scholar] [CrossRef]
- Borst, S.E. Interventions for sarcopenia and muscle weakness in older people. Age Ageing 2004, 33, 548–555. [Google Scholar] [CrossRef]
- Watts, E.L.; Matthews, C.E.; Freeman, J.R.; Gorzelitz, J.S.; Hong, H.G.; Liao, L.M.; McClain, K.M.; Saint-Maurice, P.F.; Shiroma, E.J.; Moore, S.C. Association of Leisure Time Physical Activity Types and Risks of All-Cause, Cardiovascular, and Cancer Mortality Among Older Adults. JAMA Netw. Open 2022, 5, e2228510. [Google Scholar] [CrossRef]
- Paluch, A.E.; Bajpai, S.; Bassett, D.R.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Jefferis, B.J.; Kraus, W.E.; Lee, I.M.; et al. Daily steps and all-cause mortality: A meta-analysis of 15 international cohorts. Lancet Public Health 2022, 7, e219–e228. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Mattioli, A.V.; Sciomer, S.; Moscucci, F.; Renda, G.; Gallina, S. The Impact of Physical Activity and Inactivity on Cardiovascular Risk across Women’s Lifespan: An Updated Review. J. Clin. Med. 2023, 12, 4347. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [PubMed]
- World Health Organization. Physical Activity. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 10 February 2025).
- Murray, K.O.; Mahoney, S.A.; Venkatasubramanian, R.; Seals, D.R.; Clayton, Z.S. Aging, aerobic exercise, and cardiovascular health: Barriers, alternative strategies and future directions. Exp. Gerontol. 2023, 173, 112105. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Spanos, M.; Li, G.; Lu, R.; Bei, Y.; Xiao, J. Exercise training maintains cardiovascular health: Signaling pathways involved and potential therapeutics. Signal Transduct. Target. Ther. 2022, 7, 306. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, B.; Zhao, C.; Wang, Y.; Zhou, Y.; Lin, J.; Zhao, R. Irisin Regulates Cardiac Responses to Exercise in Health and Diseases: A Narrative Review. J. Cardiovasc. Transl. Res. 2023, 16, 430–442. [Google Scholar] [CrossRef]
- Paluch, A.E.; Boyer, W.R.; Franklin, B.A.; Laddu, D.; Lobelo, F.; Lee, D.C.; McDermott, M.M.; Swift, D.L.; Webel, A.R.; Lane, A. Resistance Exercise Training in Individuals with and Without Cardiovascular Disease: 2023 Update: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e217–e231. [Google Scholar] [CrossRef]
- Rueegg, C.S.; Zürcher, S.J.; Schindera, C.; Jung, R.; Deng, W.H.; Bänteli, I.; Schaeff, J.; Hebestreit, H.; von der Weid, N.X.; Kriemler, S. Effect of a 1-year physical activity intervention on cardiovascular health in long-term childhood cancer survivors—A randomised controlled trial (SURfit). Br. J. Cancer 2023, 129, 1284–1297. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Feng, X.; Meng, D.; Zhao, X.; Wang, J.; Yu, P.; Xu, G.E.; Hu, M.; Wang, T.; et al. Exercise-induced circular RNA circUtrn is required for cardiac physiological hypertrophy and prevents myocardial ischaemia–reperfusion injury. Cardiovasc. Res. 2023, 119, 2638–2652. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Geng, Q.; Jin, S.; Teng, X.; Xiao, L.; Wu, Y.; Tian, D. Exercise protects vascular function by countering senescent cells in older adults. Front. Physiol. 2023, 14, 1138162. [Google Scholar] [CrossRef]
- Tamariz-Ellemann, A.; Wickham, K.A.; Nørregaard, L.B.; Gliemann, L.; Hellsten, Y. The time is now: Regular exercise maintains vascular health in ageing women. J. Physiol. 2023, 601, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.P.; Evenson, K.R.; Herring, A.H.; Hales, D.; Stevens, J. Cardiometabolic Correlates of Physical Activity and Sedentary Patterns in U.S. Youth. Med. Sci. Sports Exerc. 2017, 49, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Oja, P.; Kelly, P.; Murtagh, E.M.; Murphy, M.H.; Foster, C.; Titze, S. Effects of frequency, intensity, duration and volume of walking interventions on CVD risk factors: A systematic review and meta-regression analysis of randomised controlled trials among inactive healthy adults. Br. J. Sports Med. 2018, 52, 769–775. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Colucci, W.S. Mitochondrial oxidative stress in heart failure: “oxygen wastage” revisited. Circ. Res. 2000, 86, 119–120. [Google Scholar] [CrossRef]
- Powers, S.K.; Quindry, J.C.; Kavazis, A.N. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic. Biol. Med. 2008, 44, 193–201. [Google Scholar] [CrossRef]
- Yamashita, N.; Hoshida, S.; Otsu, K.; Asahi, M.; Kuzuya, T.; Hori, M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J. Exp. Med. 1999, 189, 1699–1706. [Google Scholar] [CrossRef]
- Gao, R.; Wang, L.; Bei, Y.; Wu, X.; Wang, J.; Zhou, Q.; Tao, L.; Das, S.; Li, X.; Xiao, J. Long Noncoding RNA Cardiac Physiological Hypertrophy-Associated Regulator Induces Cardiac Physiological Hypertrophy and Promotes Functional Recovery After Myocardial Ischemia-Reperfusion Injury. Circulation 2021, 144, 303–317. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Guan, L.; Elkin, K.; Xiao, J. Targets identified from exercised heart: Killing multiple birds with one stone. npj Regen. Med. 2021, 6, 23. [Google Scholar] [CrossRef]
- Balligand, J.L.; Feron, O.; Dessy, C. eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009, 89, 481–534. [Google Scholar] [CrossRef]
- Farah, C.; Nascimento, A.; Bolea, G.; Meyer, G.; Gayrard, S.; Lacampagne, A.; Cazorla, O.; Reboul, C. Key role of endothelium in the eNOS-dependent cardioprotection with exercise training. J. Mol. Cell Cardiol. 2017, 102, 26–30. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; Aon, M.A.; Marbán, E.; Winslow, R.L.; O’Rourke, B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 2003, 84, 2734–2755. [Google Scholar] [CrossRef]
- Burelle, Y.; Wambolt, R.B.; Grist, M.; Parsons, H.L.; Chow, J.C.; Antler, C.; Bonen, A.; Keller, A.; Dunaway, G.A.; Popov, K.M.; et al. Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1055–H1063. [Google Scholar] [CrossRef]
- Han, X.; Li, T.; Li, Y.; Yang, J.; Chen, S.; Zhu, X.; Wang, B.; Cheng, W.; Wang, L.; Lu, Z.; et al. Exercise and Circulating Microparticles in Healthy Subjects. J. Cardiovasc. Transl. Res. 2021, 14, 841–856. [Google Scholar] [CrossRef]
- Bei, Y.; Xu, T.; Lv, D.; Yu, P.; Xu, J.; Che, L.; Das, A.; Tigges, J.; Toxavidis, V.; Ghiran, I.; et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic. Res. Cardiol. 2017, 112, 38. [Google Scholar] [CrossRef] [PubMed]
- Vujic, A.; Lerchenmüller, C.; Wu, T.D.; Guillermier, C.; Rabolli, C.P.; Gonzalez, E.; Senyo, S.E.; Liu, X.; Guerquin-Kern, J.L.; Steinhauser, M.L.; et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat. Commun. 2018, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Hanson, J.; Han, J.; Dolezal, B.; Bradfield, J.S.; Boyle, N.G.; Hsu, J.J. Sudden cardiac arrest in athletes and strategies to optimize preparedness. Clin. Cardiol. 2023, 46, 1059–1071. [Google Scholar] [CrossRef]
- Sattler, A.G.; Rozzi, S. Identifying Sudden Cardiac Arrest Risk in Adolescent Male Athletes. Int. J. Exerc. Sci. 2024, 17, 874–886. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Westaby, J.; Sheppard, M.N.; Papadakis, M.; Sharma, S. Sudden Cardiac Death in Young Athletes. J. Am. Coll. Cardiol. 2024, 83, 350–370. [Google Scholar] [CrossRef]
- Sarto, P.; Zorzi, A.; Merlo, L.; Vessella, T.; Pegoraro, C.; Giorgiano, F.; Graziano, F.; Basso, C.; Drezner, J.A.; Corrado, D. Value of screening for the risk of sudden cardiac death in young competitive athletes. Eur. Heart J. 2023, 44, 1084–1092. [Google Scholar] [CrossRef]
- Arthur, M.N.; DeLong, R.N.; Kucera, K.; Goettsch, B.P.; Schattenkerk, J.; Bekker, S.; Drezner, J.A. Socioeconomic deprivation and racialised disparities in competitive athletes with sudden cardiac arrest from the USA. Br. J. Sports Med. 2024, 58, 494. [Google Scholar] [CrossRef]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Mannakkara, N.N.; Finocchiaro, G. Exercise and the Heart: Benefits, Risks and Adverse Effects of Exercise Training. Rev. Cardiovasc. Med. 2023, 24, 94. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Rizzoli, G.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 2003, 42, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Connelly, K.A.; Mooney, D.J.; MacIsaac, A.I.; Prior, D.L. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart 2008, 94, 860–866. [Google Scholar] [CrossRef]
- Sedaghat-Hamedani, F.; Kayvanpour, E.; Frankenstein, L.; Mereles, D.; Amr, A.; Buss, S.; Keller, A.; Giannitsis, E.; Jensen, K.; Katus, H.A.; et al. Biomarker changes after strenuous exercise can mimic pulmonary embolism and cardiac injury--a metaanalysis of 45 studies. Clin. Chem. 2015, 61, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Schnohr, P.; O’Keefe, J.H.; Lavie, C.J.; Holtermann, A.; Lange, P.; Jensen, G.B.; Marott, J.L. U-Shaped Association Between Duration of Sports Activities and Mortality: Copenhagen City Heart Study. Mayo Clin. Proc. 2021, 96, 3012–3020. [Google Scholar] [CrossRef]
- Agarwal, N.; Thakkar, R.; Than, K. Sports-Related Head Injury; American Association of Neurological Surgeons: Rolling Meadows, IL, USA, 2024. [Google Scholar]
- Leyba, K.; Paiyabhroma, N.; Salvas, J.P.; Damen, F.W.; Janvier, A.; Zub, E.; Bernis, C.; Rouland, R.; Dubois, C.J.; Badaut, J.; et al. Neurovascular hypoxia after mild traumatic brain injury in juvenile mice correlates with heart–brain dysfunctions in adulthood. Acta Physiol. 2023, 238, e13933. [Google Scholar] [CrossRef]
- Coppalini, G.; Salvagno, M.; Peluso, L.; Bogossian, E.G.; Quispe Cornejo, A.; Labbé, V.; Annoni, F.; Taccone, F.S. Cardiac Injury After Traumatic Brain Injury: Clinical Consequences and Management. Neurocrit Care 2024, 40, 477–485. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Huber-Lang, M.; Sarma, J.V.; Ward, P.A. Catecholamines—Crafty Weapons in the Inflammatory Arsenal of Immune/Inflammatory Cells or Opening Pandora’s Box? Mol. Med. 2008, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- El-Menyar, A.; Goyal, A.; Latifi, R.; Al-Thani, H.; Frishman, W. Brain-Heart Interactions in Traumatic Brain Injury. Cardiol. Rev. 2017, 25, 279–288. [Google Scholar] [CrossRef]
- Cáceres, E.; Olivella, J.C.; Di Napoli, M.; Raihane, A.S.; Divani, A.A. Immune Response in Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2024, 24, 593–609. [Google Scholar] [CrossRef]
- Rizoli, S.B.; Jaja, B.N.; Di Battista, A.P.; Rhind, S.G.; Neto, A.C.; da Costa, L.; Inaba, K.; da Luz, L.T.; Nascimento, B.; Perez, A.; et al. Catecholamines as outcome markers in isolated traumatic brain injury: The COMA-TBI study. Crit. Care 2017, 21, 37. [Google Scholar] [CrossRef]
- Kerr, N.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Neural-respiratory inflammasome axis in traumatic brain injury. Exp. Neurol. 2020, 323, 113080. [Google Scholar] [CrossRef]
- Izzy, S.; Chen, P.M.; Tahir, Z.; Grashow, R.; Radmanesh, F.; Cote, D.J.; Yahya, T.; Dhand, A.; Taylor, H.; Shih, S.L.; et al. Association of Traumatic Brain Injury with the Risk of Developing Chronic Cardiovascular, Endocrine, Neurological, and Psychiatric Disorders. JAMA Netw. Open 2022, 5, e229478. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.K.; Sridhar, S.; Char, S.; Pandya, K.; Mehta, K. Prevalence of comorbidities post mild traumatic brain injuries: A traumatic brain injury model systems study. Front. Hum. Neurosci. 2023, 17, 1158483. [Google Scholar] [CrossRef] [PubMed]
- Sorek, G.; Gagnon, I.; Schneider, K.; Chevignard, M.; Stern, N.; Fadida, Y.; Kalderon, L.; Shaklai, S.; Katz-Leurer, M. Changes in the cardiac autonomic control system during rehabilitation in children after severe traumatic brain injury. Ann. Phys. Rehabil. Med. 2023, 66, 101652. [Google Scholar] [CrossRef]
- Arakaki, X.; Arechavala, R.J.; Choy, E.H.; Bautista, J.; Bliss, B.; Molloy, C.; Wu, D.A.; Shimojo, S.; Jiang, Y.; Kleinman, M.T.; et al. The connection between heart rate variability (HRV), neurological health, and cognition: A literature review. Front. Neurosci. 2023, 17, 1055445. [Google Scholar] [CrossRef]
- Mairi Ziakaa, A.E. The Heart Is at Risk: Understanding Stroke-Heart-Brain Interactions with Focus on Neurogenic Stress Cardiomyopathy—A Review. J. Stroke 2023, 25, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; De Rosa, S.; Godoy, D.A. Crosstalk Between the Nervous System and Systemic Organs in Acute Brain Injury. Neurocrit. Care 2024, 40, 337–348. [Google Scholar] [CrossRef]
- Hamel, R.N.; Smoliga, J.M. Physical Activity Intolerance and Cardiorespiratory Dysfunction in Patients with Moderate-to-Severe Traumatic Brain Injury. Sports Med. 2019, 49, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Duhaime, A.C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, G.A.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport–Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Dvořák, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus statement on concussion in sport—The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Daneshvar, D.H.; Nowinski, C.J.; McKee, A.C.; Cantu, R.C. The epidemiology of sport-related concussion. Clin. Sports Med. 2011, 30, 1–17. [Google Scholar] [CrossRef]
- Mihalik, J.P.; Teel, E.F.; Ford, C.B.; Amalfe, S.A.; Barczak-Scarboro, N.E.; Lynall, R.C.; Riegler, K.E.; Wasserman, E.B.; Putukian, M. The Effect of Sex, Sport, and Preexisting Histories on Baseline Concussion Test Performance in College Lacrosse and Soccer Athletes. Clin. J. Sport Med. 2022, 32, e461–e468. [Google Scholar] [CrossRef]
- Kwon, J.; Jang, J. Factors Influencing Injury Severity and Frequency among Korean Sports Participants in Their 20s and 30s. Healthcare 2024, 12, 664. [Google Scholar] [CrossRef]
- Ingram, V.; Fielding, M.; Dunne, L.A.M.; Piantella, S.; Weakley, J.; Johnston, R.D.; McGuckian, T.B. The Incidence of Sports-Related Concussion in Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. Open 2025, 11, 36. [Google Scholar] [CrossRef]
- Younger, D.S. Mild traumatic brain injury and sports-related concussion. In Handbook of Clinical Neurology; Younger, D.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 24; pp. 475–494. [Google Scholar]
- Sas, A.R.; Popovich, M.J.; Gillenkirk, A.; Greer, C.; Grant, J.; Almeida, A.; Ichesco, I.K.; Lorincz, M.T.; Eckner, J.T. Orthostatic Vital Signs After Sport-Related Concussion: A Cohort Study. Am. J. Sports Med. 2024, 52, 2902–2910. [Google Scholar] [CrossRef]
- Singh, J.; Ellingson, C.J.; Ellingson, C.A.; Scott, P.; Neary, J.P. Cardiac cycle timing and contractility following acute sport-related concussion. Res. Sports Med. 2024, 32, 260–267. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Burma, J.S.; Smirl, J.D. Sensor-Assisted Analysis of Autonomic and Cerebrovascular Dysregulation following Concussion in an Individual with a History of Ten Concussions: A Case Study. Sensors 2024, 24, 4404. [Google Scholar] [CrossRef] [PubMed]
- Rim, A.J.; Liu, C.; Jackson, M.; Miller, J.T.; Chukwumerije, N.; El Chami, R.; Ibrahim, R.; Kauser, T.; Miller, A.; Simpson, E.; et al. Concussions Are Associated with Increases in Blood Pressure and Cardiovascular Risk in American-Style Football Athletes. JACC Adv. 2025, 4, 101717. [Google Scholar] [CrossRef]
- Terry, G.C.; Kyle, J.M.; Ellis, J.M., Jr.; Cantwell, J.; Courson, R.; Medlin, R. Sudden Cardiac Arrest in Athletic Medicine. J. Athl. Train 2001, 36, 205–209. [Google Scholar] [PubMed]
- Erez, E.; Mazwi, M.L.; Marquez, A.M.; Moga, M.A.; Eytan, D. Hemodynamic Patterns Before Inhospital Cardiac Arrest in Critically Ill Children: An Exploratory Study. Crit. Care Explor. 2021, 3, e0443. [Google Scholar] [CrossRef] [PubMed]
- Dostal, J.; Hybska, T.; Saganelidze, K.; Pudil, R.; Stasek, J. Autonomic dysfunction as a possible cause of sudden cardiac death in swimming sports. Front. Cardiovasc. Med. 2024, 11, 1443214. [Google Scholar] [CrossRef]
- Grashow, R.; Tan, C.O.; Izzy, S.; Taylor, H.A., Jr.; Weisskopf, M.G.; Wasfy, M.M.; Whittington, A.J.; Speizer, F.; Zafonte, R.; Baggish, A.L. Association Between Concussion Burden During Professional American-Style Football and Postcareer Hypertension. Circulation 2023, 147, 1112–1114. [Google Scholar] [CrossRef]
- Tan, C.O.; Grashow, R.; Thorpe, R., Jr.; Miller, K.K.; Nathan, D.M.; Izzy, S.; Radmanesh, F.; Kim, J.H.; Weisskopf, M.G.; Taylor, H.A., Jr.; et al. Concussion burden and later-life cardiovascular risk factors in former professional American-style football players. Ann. Clin. Transl. Neurol. 2024, 11, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. Repeated concussions can alter heart activity and impact the ‘heart-brain’ axis. Conversat. Can. Ed. 2023. [Google Scholar] [CrossRef]
- Pelo, R.; Suttman, E.; Fino, P.C.; McFarland, M.M.; Dibble, L.E.; Cortez, M.M. Autonomic dysfunction and exercise intolerance in concussion: A scoping review. Clin. Auton. Res. 2023, 33, 149–163. [Google Scholar] [CrossRef]
- Doucet, M.; Brisebois, H.; McKerral, M. Heart Rate Variability in Concussed College Athletes: Follow-Up Study and Biological Sex Differences. Brain Sci. 2023, 13, 1669. [Google Scholar] [CrossRef]
- Simons, M.U.; McCrea, M.A.; Broglio, S.; McAllister, T.W.; Nelson, L.D.; Benjamin, H.; Brooks, A.; Buckley, T.; Cameron, K.; Clugston, J.; et al. Latent Profiles of Acute Symptoms, Cognitive Performance, and Balance in Sport-Related Concussions. Am. J. Sports Med. 2024, 52, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.J.; Schloss, M.G.; Gray, J. Refractory Autonomic Instability in Mild Traumatic Brain Injury: A Case Report. Cureus 2024, 16, e55634. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.D.; Li, Y.L.; Ding, Y. Arterial Chemoreceptors and Sympathetic Nerve Activity. Hypertension 2007, 50, 6–13. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Soda, T.; Pasqua, T.; De Sarro, G.; Moccia, F. Cognitive Impairment and Synaptic Dysfunction in Cardiovascular Disorders: The New Frontiers of the Heart-Brain Axis. Biomedicines 2024, 12, 2387. [Google Scholar] [CrossRef]
- Hu, J.-R.; Abdullah, A.; Nanna, M.G.; Soufer, R. The Brain–Heart Axis: Neuroinflammatory Interactions in Cardiovascular Disease. Curr. Cardiol. Rep. 2023, 25, 1745–1758. [Google Scholar] [CrossRef]
- Dridi, H.; Liu, Y.; Reiken, S.; Liu, X.; Argyrousi, E.K.; Yuan, Q.; Miotto, M.C.; Sittenfeld, L.; Meddar, A.; Soni, R.K.; et al. Heart failure-induced cognitive dysfunction is mediated by intracellular Ca2+ leak through ryanodine receptor type 2. Nat. Neurosci. 2023, 26, 1365–1378. [Google Scholar] [CrossRef]
- Doehner, W.; Čelutkienė, J.; Yilmaz, M.B.; Coats, A.J.S. Heart failure and the heart–brain axis. QJM Int. J. Med. 2023, 116, 897–902. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, G.; Liang, Y.; He, S.; Lyu, M.; Zhu, Y. Heart-brain interaction in cardiogenic dementia: Pathophysiology and therapeutic potential. Front. Cardiovasc. Med. 2024, 11, 1304864. [Google Scholar] [CrossRef]
- Osteraas, N.D.; Lee, V.H. Neurocardiology. Handb. Clin. Neurol. 2017, 140, 49–65. [Google Scholar]
- Ziegler, K.A.; Engelhardt, S.; Carnevale, D.; McAlpine, C.S.; Guzik, T.J.; Dimmeler, S.; Swirski, F.K. Neural Mechanisms in Cardiovascular Health and Disease. Circ. Res. 2025, 136, 1233–1261. [Google Scholar] [CrossRef]
- Xu, C.; Tao, X.; Ma, X.; Zhao, R.; Cao, Z. Cognitive Dysfunction after Heart Disease: A Manifestation of the Heart-Brain Axis. Oxidative Med. Cell. Longev. 2021, 2021, 4899688. [Google Scholar] [CrossRef]
- Wang, M.; Xu, B.; Hou, X.; Shi, Q.; Zhao, H.; Gui, Q.; Wu, G.; Dong, X.; Xu, Q.; Shen, M.; et al. Altered brain networks and connections in chronic heart failure patients complicated with cognitive impairment. Front. Aging Neurosci. 2023, 15, 1153496. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, R.; Wang, Y.J.; Xie, X. Predicting depression in patients with heart failure based on a stacking model. World J. Clin. Cases 2024, 12, 4661–4672. [Google Scholar] [CrossRef]
- Villringer, A.; Laufs, U. Heart failure, cognition, and brain damage. Eur. Heart J. 2021, 42, 1579–1581. [Google Scholar] [CrossRef]
- Ovsenik, A.; Podbregar, M.; Fabjan, A. Cerebral blood flow impairment and cognitive decline in heart failure. Brain Behav. 2021, 11, e02176. [Google Scholar] [CrossRef]
- David, H.; Ughetto, A.; Gaudard, P.; Plawecki, M.; Paiyabhroma, N.; Zub, E.; Colson, P.; Richard, S.; Marchi, N.; Sicard, P.; et al. Experimental Myocardial Infarction Elicits Time-Dependent Patterns of Vascular Hypoxia in Peripheral Organs and in the Brain. Front. Cardiovasc. Med. 2020, 7, 615507. [Google Scholar] [CrossRef]
- Díaz, H.S.; Toledo, C.; Andrade, D.C.; Marcus, N.J.; Del Rio, R. Neuroinflammation in heart failure: New insights for an old disease. J. Physiol. 2020, 598, 33–59. [Google Scholar] [CrossRef]
- Lu, Z.; Teng, Y.; Wang, L.; Jiang, Y.; Li, T.; Chen, S.; Wang, B.; Li, Y.; Yang, J.; Wu, X.; et al. Abnormalities of hippocampus and frontal lobes in heart failure patients and animal models with cognitive impairment or depression: A systematic review. PLoS ONE 2022, 17, e0278398. [Google Scholar] [CrossRef]
- Althammer, F.; Roy, R.K.; Kirchner, M.K.; Campos-Lira, E.; Whitley, K.E.; Davis, S.; Montanez, J.; Ferreira-Neto, H.C.; Danh, J.; Feresin, R.; et al. Angiotensin II-Mediated Neuroinflammation in the Hippocampus Contributes to Neuronal Deficits and Cognitive Impairment in Heart Failure Rats. Hypertension 2023, 80, 1258–1273. [Google Scholar] [CrossRef]
- Hagena, H.; Manahan-Vaughan, D. Interplay of hippocampal long-term potentiation and long-term depression in enabling memory representations. Philos. Trans. R Soc. B Biol. Sci. 2024, 379, 20230229. [Google Scholar] [CrossRef]
- Mueller, K.; Thiel, F.; Beutner, F.; Teren, A.; Frisch, S.; Ballarini, T.; Möller, H.E.; Ihle, K.; Thiery, J.; Schuler, G.; et al. Brain Damage with Heart Failure. Circ. Res. 2020, 126, 750–764. [Google Scholar] [CrossRef]
- Johansen, M.C.; Ye, W.; Gross, A.; Gottesman, R.F.; Han, D.; Whitney, R.; Briceño, E.M.; Giordani, B.J.; Shore, S.; Elkind, M.S.V.; et al. Association Between Acute Myocardial Infarction and Cognition. JAMA Neurol. 2023, 80, 723–731. [Google Scholar] [CrossRef]
- Thong, E.H.E.; Quek, E.J.W.; Loo, J.H.; Yun, C.Y.; Teo, Y.N.; Teo, Y.H.; Leow, A.S.T.; Li, T.Y.W.; Sharma, V.K.; Tan, B.Y.Q.; et al. Acute Myocardial Infarction and Risk of Cognitive Impairment and Dementia: A Review. Biology 2023, 12, 1154. [Google Scholar] [CrossRef]
- Chang, H.; Chen, E.; Zhu, T.; Liu, J.; Chen, C. Communication Regarding the Myocardial Ischemia/Reperfusion and Cognitive Impairment: A Narrative Literature Review. J. Alzheimer’s Dis. 2024, 97, 1545–1570. [Google Scholar] [CrossRef]
- Daniele, G.; DiLucia, S.; Masci, P.G.; Del Monte, F. Heart and Brain: Complex Relationships for Left Ventricular Dysfunction. Curr. Cardiol. Rep. 2020, 22, 72. [Google Scholar] [CrossRef]
- Francis, J.; Chu, Y.; Johnson, A.K.; Weiss, R.M.; Felder, R.B. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2264–H2271. [Google Scholar] [CrossRef]
- Quan, N. In-depth conversation: Spectrum and kinetics of neuroimmune afferent pathways. Brain Behav. Immun. 2014, 40, 1–8. [Google Scholar] [CrossRef]
- Srinivas, S.; Vignesh Rk, B.; Ayinapudi, V.N.; Govindarajan, A.; Sundaram, S.S.; Priyathersini, N. Neurological Consequences of Cardiac Arrhythmias: Relationship Between Stroke, Cognitive Decline, and Heart Rhythm Disorders. Cureus 2024, 16, e57159. [Google Scholar] [CrossRef]
- D’Ambrosio, P.; Claessen, G.; Kistler, P.M.; Heidbuchel, H.; Kalman, J.M.; La Gerche, A. Ventricular arrhythmias in association with athletic cardiac remodelling. EP Eur. 2024, 26, euae279. [Google Scholar] [CrossRef]
- Lampert, R.; Chung, E.H.; Ackerman, M.J.; Arroyo, A.R.; Darden, D.; Deo, R.; Dolan, J.; Etheridge, S.P.; Gray, B.R.; Harmon, K.G.; et al. 2024 HRS expert consensus statement on arrhythmias in the athlete: Evaluation, treatment, and return to play. Heart Rhythm 2024, 21, e151–e252. [Google Scholar] [CrossRef]
- Heidbuchel, H. The athlete’s heart is a proarrhythmic heart, and what that means for clinical decision making. EP Eur. 2017, 20, 1401–1411. [Google Scholar] [CrossRef]
- Fekete, M.; Liotta, E.M.; Molnar, T.; Fülöp, G.A.; Lehoczki, A. The role of atrial fibrillation in vascular cognitive impairment and dementia: Epidemiology, pathophysiology, and preventive strategies. Geroscience 2024, 47, 287–300. [Google Scholar] [CrossRef]
- Kogelschatz, B.; Zenger, B.; Steinberg, B.A.; Ranjan, R.; Jared Bunch, T. Atrial fibrillation and the risk of early-onset dementia and cognitive decline: An updated review. Trends Cardiovasc. Med. 2024, 34, 236–241. [Google Scholar] [CrossRef]
- Yong, J.; Song, J. CaMKII activity and metabolic imbalance-related neurological diseases: Focus on vascular dysfunction, synaptic plasticity, amyloid beta accumulation, and lipid metabolism. Biomed. Pharmacother. 2024, 175, 116688. [Google Scholar] [CrossRef]
- Song, J. Amygdala activity and amygdala-hippocampus connectivity: Metabolic diseases, dementia, and neuropsychiatric issues. Biomed. Pharmacother. 2023, 162, 114647. [Google Scholar] [CrossRef]
- Kouvari, M.; D’Cunha, N.M.; Travica, N.; Sergi, D.; Zec, M.; Marx, W.; Naumovski, N. Metabolic Syndrome, Cognitive Impairment and the Role of Diet: A Narrative Review. Nutrients 2022, 14, 333. [Google Scholar] [CrossRef]
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart-Brain Axis. J. Am. Heart Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef]
- Zedde, M.; Pascarella, R. The Cerebrovascular Side of Plasticity: Microvascular Architecture across Health and Neurodegenerative and Vascular Diseases. Brain Sci. 2024, 14, 983. [Google Scholar] [CrossRef]
- Gazdzinski, S.; Kornak, J.; Weiner, M.W.; Meyerhoff, D.J. Body mass index and magnetic resonance markers of brain integrity in adults. Ann. Neurol. 2008, 63, 652–657. [Google Scholar] [CrossRef]
- Lusis, A.J.; Attie, A.D.; Reue, K. Metabolic syndrome: From epidemiology to systems biology. Nat. Rev. Genet. 2008, 9, 819–830. [Google Scholar] [CrossRef]
- Vincent, H.K.; Powers, S.K.; Dirks, A.J.; Scarpace, P.J. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 378–388. [Google Scholar] [CrossRef]
- Russell, A.P.; Gastaldi, G.; Bobbioni-Harsch, E.; Arboit, P.; Gobelet, C.; Dériaz, O.; Golay, A.; Witztum, J.L.; Giacobino, J.P. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: A case of good vs. bad lipids? FEBS Lett. 2003, 551, 104–106. [Google Scholar] [CrossRef]
- Wei, H.-L.; Ao, M.Q.; Wang, M.Y.; Zhou, G.P.; Yu, Y.S.; Tao, Q.; Zhang, H. Disrupted resting-state functional connectivity of the thalamus in patients with coronary heart disease. Heliyon 2023, 9, e13423. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Travieso, A.; Matías-Guiu, J.A.; Yus, M.; Espejo-Paeres, C.; Finocchiaro, F.; Fernández, S.; Gomez-Escalonilla, C.I.; Reneses-Prieto, B.; Gómez-Garré, M.D.; et al. Coronary microvascular dysfunction is associated with impaired cognitive function: The Cerebral-Coronary Connection study (C3 study). Eur. Heart J. 2023, 44, 113–125. [Google Scholar] [CrossRef]
- Zheng, L.; Mack, W.J.; Chui, H.C.; Heflin, L.; Mungas, D.; Reed, B.; DeCarli, C.; Weiner, M.W.; Kramer, J.H. Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J. Am. Geriatr. Soc. 2012, 60, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Fourdain, S.; Provost, S.; Tremblay, J.; Vannasing, P.; Doussau, A.; Caron-Desrochers, L.; Gaudet, I.; Roger, K.; Hüsser, A.; Dehaes, M.; et al. Functional brain connectivity after corrective cardiac surgery for critical congenital heart disease: A preliminary near-infrared spectroscopy (NIRS) report. Child Neuropsychol. 2023, 29, 1088–1108. [Google Scholar] [CrossRef]


| Type of TBI | Sport | Participants | Key Findings | Cardiovascular Relevance | Reference |
|---|---|---|---|---|---|
| Head injury | High schools and community sports | Healthy adolescents (11–18 years) | Glial fibrillary acidic protein decreases by 9.5% with each 1-year increase in age, adjusted for previous concussions | Biomarker changes may reflect neuroinflammation, which can influence autonomic function | [95] |
| Acute and sub-acute concussion | Athletics | Pediatric and collegiate | Contact sports exposure may increase brain age | Accelerated brain aging could predispose to autonomic dysfunction and CVD risk | [96] |
| Contact injury | Football | Pre-collegiate | Higher concussion risk for those starting football before age 12 | Early exposure may lead to cumulative autonomic disruption | [97] |
| Sport and non-sport injury | Participant and non-participant | Children (5–12 years) | Similar injury recovery in both groups | Suggests concussion effects are consistent across contexts, including potential CVD risks | [99] |
| Contact and non-contact injury | Rugby | Retired players | Altered serum measurements (exosome size, t-tau, p-tau181, RBP-4) in retired athletes with concussion history | Biomarker changes may indicate chronic neuroinflammation, potentially affecting heart health | [101] |
| Repetitive head injury | High and low contact sports | High school athletes | Degraded neuropsychological results for high-contact athletes | Cognitive decline may correlate with autonomic dysfunction and altered HRV | [102] |
| Sport- and recreation-related brain injury | Different activities | Children (5–17 years) | Contact sports are more injury-prone | Higher concussion rates in contact sports may increase cumulative risk of autonomic imbalance | [103] |
| Sports- and recreation-related concussion | Different activities | Children (5–18 years) | Post-injury complexity and recovery depend on age | Age-related recovery differences may influence long-term autonomic and cardiovascular health | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kibria, F.; Bragina, O.A.; Trofimov, A.O.; Bragin, D. Bidirectional Interplay Between Traumatic Brain Injury and Cardiovascular Dysfunction in Athletes. J. Clin. Med. 2025, 14, 7712. https://doi.org/10.3390/jcm14217712
Kibria F, Bragina OA, Trofimov AO, Bragin D. Bidirectional Interplay Between Traumatic Brain Injury and Cardiovascular Dysfunction in Athletes. Journal of Clinical Medicine. 2025; 14(21):7712. https://doi.org/10.3390/jcm14217712
Chicago/Turabian StyleKibria, Fazle, Olga A. Bragina, Alex O. Trofimov, and Denis Bragin. 2025. "Bidirectional Interplay Between Traumatic Brain Injury and Cardiovascular Dysfunction in Athletes" Journal of Clinical Medicine 14, no. 21: 7712. https://doi.org/10.3390/jcm14217712
APA StyleKibria, F., Bragina, O. A., Trofimov, A. O., & Bragin, D. (2025). Bidirectional Interplay Between Traumatic Brain Injury and Cardiovascular Dysfunction in Athletes. Journal of Clinical Medicine, 14(21), 7712. https://doi.org/10.3390/jcm14217712






