Guarding Reflex Inhibition Training Reduces Postoperative Urinary Retention After Urethral Bulking for Stress Urinary Incontinence: A Retrospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
- Over the age of 18.
- Pelvic organ prolapse ≤ II in any compartment.
- Demonstration of SUI on physical examination or urodynamic evaluation.
- Pre-procedure post-void residual (PVR) less than 150 mL.
- Negative pregnancy test at time of procedure.
- No prior surgical or injectable procedure for SUI within 3 months of treatment date.
- Self-reported stress or stress-predominant leakage that is bothersome enough to warrant treatment regardless of frequency or severity of leakage episodes.
- Concomitant surgery for pelvic organ prolapse at time of intervention.
- Active urinary tract infection or asymptomatic bacteriuria at pre-procedure screening.
- Neurogenic lower urinary tract dysfunction (LUTD) due to spinal cord injury, multiple sclerosis etc.
- Uncontrolled bleeding risk or active anti-coagulation, with the exception of prophylactic-dose aspirin (81 mg daily).
- Behavioral therapy and GRIT Protocol:
- Group 1: No GRIT—Standard behavioral therapy (November 2022–March 2023):
- (1)
- Fluid Management: Drinking fluid (32 to 64 oz) evenly throughout the day.
- (2)
- Timed voiding: Voiding every 2 to 3 h (except during sleep) to discourage holding behaviors as well as “just in case” voiding.
- (3)
- Posture training: Assuming a seated and leaning-forward posture for urination and defecation.
- (4)
- Relaxation: Facilitation of voiding and defecation by breathing with the diaphragm instead of bearing down or pushing.
- (5)
- Mindful body awareness: Practicing recognition of involuntary pelvic floor tightening in response to outside stimuli (i.e., stressful situation) or inner stimulus (i.e., heightened emotion).
- (6)
- Limitation: No specific training on reflex inhibition was provided.
- GROUP 2: Behavioral therapy with GRIT (November 2023–March 2024):
- Initial instruction: A 15 min in-office training session at the preoperative visit, including demonstration of the GRIT sequence and provision of reinforcement handouts.
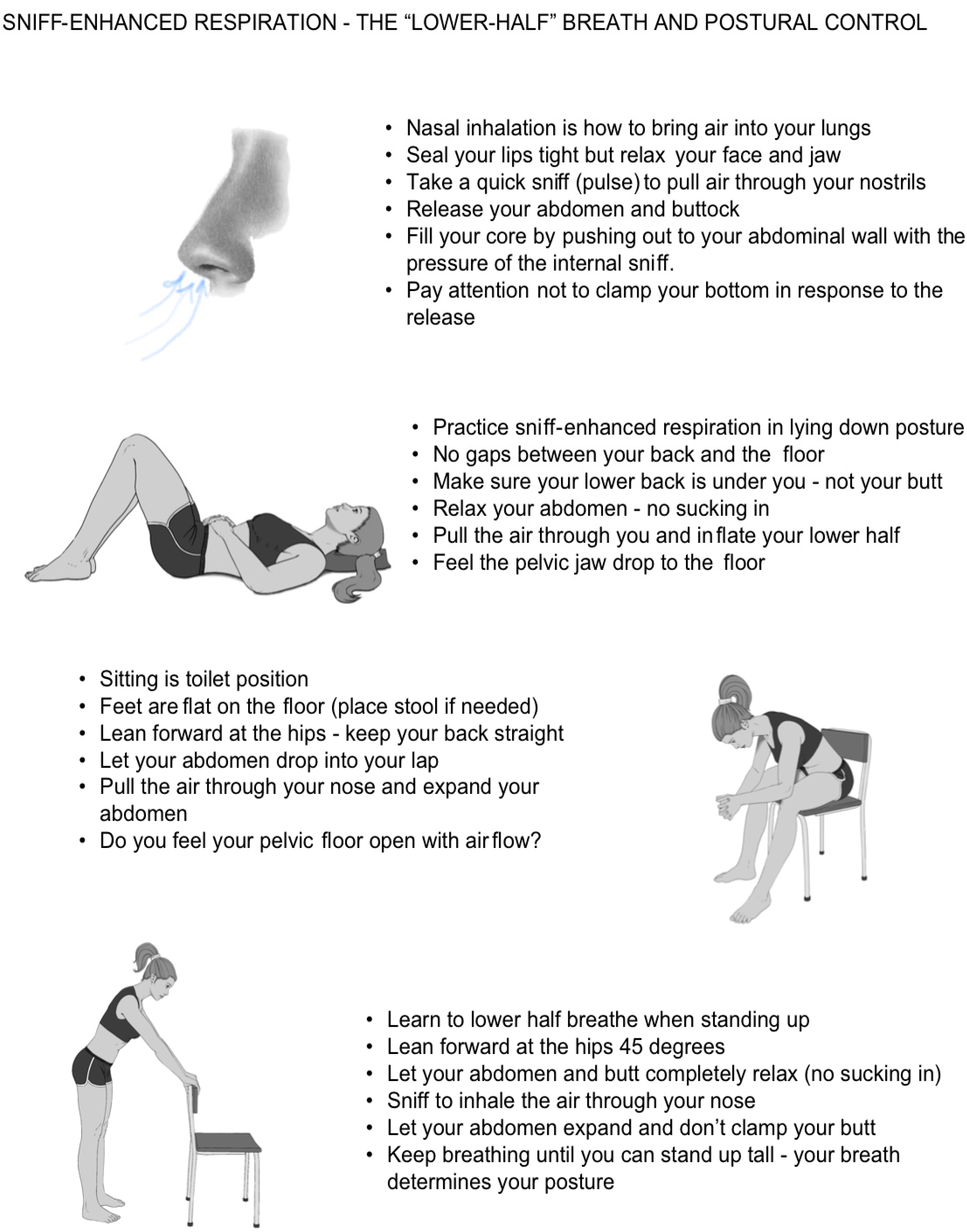
- Breathing initiation: Training with a volitional sniff to activate diaphragmatic descent (sniff-enhanced respiration, SER) and trigger awareness of pelvic release.
- Posture sequencing: SER was practiced across three functional positions—supine, seated/toileting, and upright—each emphasizing a neutral spine and abdominal release.
- GRIT: learning to identify and inhibit daily involuntary guarding reactions using deliberate SER.
- Integration: Repetition of the sniff-posture sequence across positions reinforced cortical awareness, voluntary inhibition of the guarding reflex, and functional voiding mechanics.
- Pre-procedure review: Check for understanding and a brief refresher immediately prior to injection procedure.
2.1. Surgical Procedure
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcome—POUR
- Concomitant surgery: strongly associated with POUR (46.7% vs. 9.4%, p = 0.0008; AOR 5.1, 95% CI: 1.45–17.9, p = 0.011).
- Postmenopausal status: more common among those with POUR (86.7% vs. 51.6%, p = 0.0097), though not significant in adjusted models (AOR 3.53, 95% CI: 0.75–16.7, p = 0.11).
- Diabetes mellitus: higher prevalence in POUR (20% vs. 4.7%, p = 0.021; AOR 9.1, 95% CI: 1.33–61.9, p = 0.025).
4. Discussion
4.1. Postoperative Urinary Retention (POUR)
4.2. POUR as Physiologic Marker Rather than Passive Obstruction
Comparative Context in Behavioral Therapy
4.3. Study Limitations
4.4. Clinical Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| GRIT (N = 39) | No GRIT (N = 106) | Overall (N = 145) | p-Value | |
|---|---|---|---|---|
| Age | 0.0087 | |||
| Mean (SD) | 52.9 (12.8) | 60.0 (14.7) | 58.1 (14.5) | |
| Median [Min, Max] | 51.4 [21.0, 83.2] | 56.8 [37.7, 96.8] | 54.3 [21.0, 96.8] | |
| BMI | 0.25 | |||
| Mean (SD) | 27.3 (6.28) | 28.7 (6.89) | 28.3 (6.74) | |
| Median [Min, Max] | 26.0 [19.0, 43.0] | 27.2 [15.8, 59.9] | 27.0 [15.8, 59.9] | |
| Missing | 0 (0%) | 1 (0.9%) | 1 (0.7%) | |
| Co-surgery | 3 (7.7%) | 16 (15.1%) | 19 (13.1%) | 0.28 |
| Post Menopausal | 14 (35.9%) | 67 (63.2%) | 81 (55.9%) | 0.0033 |
| Smoker | 0.55 | |||
| Never | 31 (79.5%) | 89 (84.0%) | 120 (82.8%) | |
| Former | 8 (20.5%) | 15 (14.2%) | 23 (15.9%) | |
| Current | 0 (0%) | 2 (1.9%) | 2 (1.4%) | |
| Diabetes | 4 (10.3%) | 6 (5.7%) | 10 (6.9%) | 0.46 |
| Recurrent UTI | 4 (10.3%) | 21 (19.8%) | 25 (17.2%) | 0.22 |
| Pelvic Radiation | 0 (0%) | 0 (0%) | 0 (0%) | >0.99 |
| Prior SUI Treatment | 9 (23.1%) | 31 (29.2%) | 40 (27.6%) | 0.44 |
| Mixed Urinary Incontinence | 17 (43.6%) | 64 (60.4%) | 81 (55.9%) | 0.071 |
| Overactive Bladder Treatment | 4 (10.3%) | 17 (16.0%) | 21 (14.5%) | 0.38 |
| No POUR (N = 130) | POUR (N = 15) | Overall (N = 145) | Bivariate, (Unadjusted) p-Value | Multivariate, Adjusted OR, (95% CI), p-Value | |
|---|---|---|---|---|---|
| Treatment Group | 0.012 | AOR 12.0 (0.65–250) p = 0.094 | |||
| GRIT | 39 (30.5%) | 0 (0%) | 39 (27.3%) | ||
| No GRIT | 89 (69.5%) | 15 (100%) | 104 (72.7%) | ||
| Age | 0.055 | ||||
| Mean (SD) | 57.3 (14.5) | 64.9 (14.1) | 58.1 (14.6) | ||
| Median [Min, Max] | 53.6 [21.0, 96.8] | 64.2 [45.1, 91.4] | 54.2 [21.0, 96.8] | ||
| BMI | 0.47 | ||||
| Mean (SD) | 28.4 (6.79) | 27.1 (6.58) | 28.3 (6.76) | ||
| Median [Min, Max] | 27.0 [15.8, 59.9] | 25.0 [19.4, 42.0] | 27.0 [15.8, 59.9] | ||
| Missing | 1 (0.8%) | 0 (0%) | 1 (0.7%) | ||
| Co-surgery | 12 (9.4%) | 7 (46.7%) | 19 (13.3%) | 0.0008 | AOR: 5.1 (1.45–17.9), p = 0.011 |
| Post Menopausal | 66 (51.6%) | 13 (86.7%) | 79 (55.2%) | 0. 0097 | AOR: 3.53 (0.75–16.7), p = 0.11 |
| Smoker | >0.99 | ||||
| Never | 106 (82.8%) | 13 (86.7%) | 119 (83.2%) | ||
| Former | 20 (15.6%) | 2 (13.3%) | 22 (15.4%) | ||
| Current | 2 (1.6%) | 0 (0%) | 2 (1.4%) | ||
| Diabetes | 6 (4.7%) | 3 (20.0%) | 9 (6.3%) | 0.021 | AOR: 9.1 (1.33–61.9), p = 0.025 |
| Recurrent UTI | 20 (15.6%) | 5 (33.3%) | 25 (17.5%) | 0.14 | |
| Pelvic Radiation | 0 (0%) | 0 (0%) | 0 (0%) | >0.99 | |
| Prior SUI Treatment | 33 (25.8%) | 7 (46.7%) | 40 (28.0%) | 0.067 | |
| MUI | 70 (54.7%) | 10 (66.7%) | 80 (55.9%) | 0.38 | |
| OAB treatment | 19 (14.8%) | 2 (13.3%) | 21 (14.7%) | >0.99 |
References
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D.D. Prevalence and trends of symptomatic pelvic floor disorders in U. S. Women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef]
- Reynolds, W.S.; Fowke, J.; Dmochowski, R. The burden of overactive bladder on U. S. Public Health. Curr. Bladder Dysfunct. Rep. 2016, 11, 8–13. [Google Scholar] [CrossRef]
- Smith, M.D.; Coppieters, M.W.; Hodges, P.W. Is balance different in women with and without stress urinary incontinence? Neurourol. Urodyn. 2008, 27, 71–78. [Google Scholar] [CrossRef]
- Kuo, H.C. Urodynamic characteristics and lower urinary tract symptoms of female bladder outlet obstruction. Urology 2005, 66, 1005–1009. [Google Scholar] [CrossRef]
- Karram, M.M.; Bhatia, N.N.; Brubaker, L. Urodynamic characteristics of women with stress urinary incontinence: Correlation with clinical findings. Am. J. Obstet. Gynecol. 1997, 177, 1263–1270. [Google Scholar] [CrossRef]
- Lemack, G.E.; Zimmern, P.E. Female urodynamics: Comparison of non-neurogenic voiding dysfunction patients with and without stress incontinence. Urology 2000, 55, 507–511. [Google Scholar]
- Sapsford, R.R.; Hodges, P.W. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch. Phys. Med. Rehabil. 2001, 82, 1081–1088. [Google Scholar] [CrossRef]
- Thompson, J.A.; O’Sullivan, P.B.; Briffa, N.K.; Neumann, P. Altered muscle activation patterns in symptomatic women during pelvic floor muscle contraction and Valsalva manoeuvre. Neurourol. Urodyn. 2006, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.; Burkhard, F.; Zwahlen, M.; Junginger, B.; Dumoulin, C. Assessment of involuntary pelvic floor muscle contractions in comparison with existing literature and IUGA/ICS terminology reports. Int. Urogynecol. J. 2024, 35, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Torosis, M.; Carey, E.; Christensen, K.; Kaufman, M.R.; Kenton, K.; Kotarinos, R.; Lai, H.H.; Lee, U.; Lowder, J.L.M.; Meister, M.; et al. A treatment algorithm for high-tone pelvic floor dysfunction. Obstet. Gynecol. 2024, 143, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association/International Continence Society joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef]
- O’Connell, K.; Reynolds, W.S.; Kaufman, M.R.; Dmochowski, R.R.; Fowke, J.H. Toileting behaviors, urinary cues, overactive bladder and urinary incontinence in older women. Neurourol. Urodyn. 2019, 38, 210–218. [Google Scholar] [CrossRef]
- Berry, A.; Brady, S.S.; Burgio, K.L.; Cunningham, S.D.; Gahagan, S.; James, A.S.; Low, L.K.; LaCoursiere, D.Y.; Lipman, T.H.; McGwin, G.; et al. Associations between U.S. women’s toileting behaviors and lower urinary tract symptoms: A cross-sectional analysis of RISE for HEALTH study data. J. Womens Health 2025, 34, 653–664. [Google Scholar]
- Newman, D.K.; Borello-France, D.; Sung, V.W. Structured behavioral treatment research protocol for women with mixed urinary incontinence and overactive bladder symptoms. Neurourol. Urodyn. 2018, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; von Gontard, A.; Wright, A.; et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the Standardization Committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016, 35, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.; Austin, P.; Hoebeke, P.; McKenna, P. The management of dysfunctional voiding in children: A report from the Standardisation Committee of the International Children’s Continence Society. J. Urol. 2010, 183, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Epperson, C.N.; Duffy, K.A.; Johnson, R.L.; Sammel, M.D.; Newman, D.K. Enduring impact of childhood adversity on lower urinary tract symptoms in adult women. Neurourol. Urodyn. 2020, 39, 1472–1481. [Google Scholar] [CrossRef]
- Van den Ende, M.; Van de Steen, L.; Everaert, K.; Hervé, F.; Bou Kheir, G. Exploring childhood lower urinary tract symptoms, urinary tract infections and the microbiome: A systematic review. Life 2025, 15, 730. [Google Scholar] [CrossRef]
- Wenske, S.; Van Batavia, J.P.; Combs, A.J.; Glassberg, K.I. Analysis of uroflow patterns in children with dysfunctional voiding. J. Pediatr. Urol. 2014, 10, 250–254. [Google Scholar] [CrossRef]
- Everaert, K.; Van Laecke, E.; De Muynck, M.; Peeters, H.; Hoebeke, P. Urodynamic assessment of voiding dysfunction and dysfunctional voiding in girls and women. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 2000, 11, 254–264. [Google Scholar] [CrossRef]
- Minassian, V.A.; Lovatsis, D.; Pascali, D.; Alarab, M.; Drutz, H.P. Effect of childhood dysfunctional voiding on urinary incontinence in adult women. Obstet. Gynecol. 2006, 107, 1247–1251. [Google Scholar] [CrossRef]
- Selvi, I.; Basar, H.; Baydilli, N.; Kizilay, E.; Demirci, D. Which children are at risk of developing overactive bladder in early adulthood even if lower urinary tract symptoms improve during childhood? Int. J. Urol. 2022, 29, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, M.; Pautasso, S.; Novara, L.; Piat, F.C.; Testa, F.; Arrunategui, V.G.; Sgro, L.G.; Biglia, N. Straining to void at preoperative urodynamic study as a risk factor for prolapse recurrence after surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 283, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Nieuwhof-Leppink, A.; Hussong, J.; Chase, J.; Larsson, J.; Renson, C.; Hoebeke, P.; Yang, S.; von Gontard, A. Definitions, indications and practice of urotherapy in children and adolescents: A standardization document of the International Children’s Continence Society. J. Pediatr. Urol. 2021, 17, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Assis, G.M.; Silva, C.P.C.D.; Martins, G. Urotherapy in the treatment of children and adolescents with bladder and bowel dysfunction: A systematic review. J. Pediatr. (Rio. J.). 2019, 95, 628–641. [Google Scholar] [CrossRef]
- Clearwater, W.L.; Panushka, K.; Najor, A.; Laudano, M.; Fleischmann, N. Reconstruction of urethral sphincter with polyacrylamide hydrogel. Urogynecology 2024, 30, 293–299. [Google Scholar] [CrossRef]
- Kobashi, K.C.; Vasavada, S.; Bloschichak, A.; Hermanson, L.; Kaczmarek, J.; Kim, S.K.; Kirkby, E.; Malik, R. Updates to surgical treatment of female stress urinary incontinence: AUA/SUFU guideline. J. Urol. 2023, 209, 1091–1098. [Google Scholar] [CrossRef]
- Geller, E.J. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int. J. Womens Health 2014, 6, 829–838. [Google Scholar] [CrossRef]
- McDermott, C.D.; Tunitsky-Bitton, E.; Dueñas-Garcia, O.F.; Willis-Gray, M.G.; Cadish, L.A.; Edenfield, A.; Wang, R.; Meriwether, K.; Mueller, E.R. Postoperative urinary retention. Urogynecology 2023, 29, 381–396. [Google Scholar] [CrossRef]
- Barr, S.A.; Thomas, A.; Potter, S.; Melick, C.F.; Gavard, J.A.; McLennan, M.T. Incidence of successful voiding and predictors of early voiding dysfunction after retropubic sling. Int. Urogynecol. J. 2016, 27, 1209–1214. [Google Scholar] [CrossRef]
- Serati, M.; Braga, A.; Salvatore, S.; Torella, M.; Di Dedda, M.C.; Scancarello, C.; Cimmino, C.; De Rosa, A.; Frigerio, M.; Candiani, M.; et al. Up-to-date procedures in female stress urinary incontinence surgery: A concise review on bulking agents. Medicina 2022, 58, 775. [Google Scholar] [CrossRef] [PubMed]
- Kirchin, V.; Page, T.; Keegan, P.E.; Atiemo, K.; Cody, J.D.; McClinton, S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst. Rev. 2017, 7, CD003881. [Google Scholar] [CrossRef]
- Mamut, A.; Carlson, K.V. Periurethral bulking agents for female stress urinary incontinence in Canada. Can. Urol. Assoc. J. 2017, 11 (Suppl. S2), S152–S154. [Google Scholar] [CrossRef]
- McCloskey, K.D.; Kanai, A.; Panicker, J.N.; Hashitani, H.; Fry, C.H. What do we really know about the external urethral sphincter? Continence 2024, 10, 101223. [Google Scholar] [CrossRef]
- Ashton-Miller, J.A.; DeLancey, J.O. Functional anatomy of the female pelvic floor. Ann. N. Y. Acad Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef]
- Hagen, S.; Bugge, C.; Dean, S.G.; Elders, A.; Hay-Smith, J.; Kilonzo, M.; McClurg, D.; Abdel-Fattah, M.; Agur, W.; Andreis, F.; et al. Basic versus biofeedback-mediated intensive pelvic floor muscle training for women with urinary incontinence: The OPAL RCT. Health Technol. Assess. 2020, 24, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, T.; Ioannis, T.; Vasileios, S.; Christina, P.; Michael, S. The effect of pelvic floor muscle training in women with functional bladder outlet obstruction. Arch. Gynecol. Obstet. 2023, 307, 1489–1494. [Google Scholar] [CrossRef]
- Lv, A.; Gai, T.; Zhang, S.; Feng, Q.; Li, Y. Electrical stimulation plus biofeedback improves urination function, pelvic floor function, and distress after reconstructive surgery: A randomized controlled trial. Int. J. Color. Dis. 2023, 38, 226. [Google Scholar] [CrossRef] [PubMed]
- Todhunter-Brown, A.; Hazelton, C.; Campbell, P.; Elders, A.; Hagen, S.; McClurg, D. Conservative interventions for treating urinary incontinence in women: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2022, 9, CD012337. [Google Scholar] [CrossRef]
- Volpe, L.J.; Zugelder, M.; Kotarinos, R.; Kotarinos, E.; Kenton, K.; Geynisman-Tan, J. Objective changes in pelvic floor muscle strength and length in women with high-tone pelvic floor dysfunction after pelvic floor physical therapy (RELAX trial). Urogynecology 2023, 29, 381–396. [Google Scholar] [CrossRef]
- Ludlow, C.L. Laryngeal reflexes: Physiology, technique, and clinical use. J. Clin. Neurophysiol. 2015, 32, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Elsas, S.M.; Gregory, W.L.; White, G.; Navarro, G.; Salinsky, M.C.; Andrews, D.J. Aura interruption: The Andrews/Reiter behavioral intervention may reduce seizures and improve quality of life—A pilot trial. Epilepsy Behav. 2011, 22, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.; Williams, J.; Kessler, T.; Stenzl, A.; Aicher, W.; Andersson, K.-E.; Eberli, D. Treatment of stress urinary incontinence with muscle stem cells and stem cell components: Chances, challenges and future prospects. Int. J. Mol. Sci. 2021, 22, 3981. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Owens, G.E.; Mynatt, C.J.; Brown, E.T.; Badlani, G.H. Regenerative medicine therapies for stress urinary incontinence. J. Urol. 2016, 196, 1619–1626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleischmann, N.; Plagianos, M.; Meiselman, R.; Panushka, K. Guarding Reflex Inhibition Training Reduces Postoperative Urinary Retention After Urethral Bulking for Stress Urinary Incontinence: A Retrospective Single-Center Study. J. Clin. Med. 2025, 14, 7701. https://doi.org/10.3390/jcm14217701
Fleischmann N, Plagianos M, Meiselman R, Panushka K. Guarding Reflex Inhibition Training Reduces Postoperative Urinary Retention After Urethral Bulking for Stress Urinary Incontinence: A Retrospective Single-Center Study. Journal of Clinical Medicine. 2025; 14(21):7701. https://doi.org/10.3390/jcm14217701
Chicago/Turabian StyleFleischmann, Nicole, Marlena Plagianos, Rachel Meiselman, and Katherine Panushka. 2025. "Guarding Reflex Inhibition Training Reduces Postoperative Urinary Retention After Urethral Bulking for Stress Urinary Incontinence: A Retrospective Single-Center Study" Journal of Clinical Medicine 14, no. 21: 7701. https://doi.org/10.3390/jcm14217701
APA StyleFleischmann, N., Plagianos, M., Meiselman, R., & Panushka, K. (2025). Guarding Reflex Inhibition Training Reduces Postoperative Urinary Retention After Urethral Bulking for Stress Urinary Incontinence: A Retrospective Single-Center Study. Journal of Clinical Medicine, 14(21), 7701. https://doi.org/10.3390/jcm14217701







