Neuromuscular Electrical Stimulation During Hemodialysis Enhances Exercise Capacity in Patients with End-Stage Renal Disease: A Pilot Randomized Controlled Trial
Abstract
1. Background
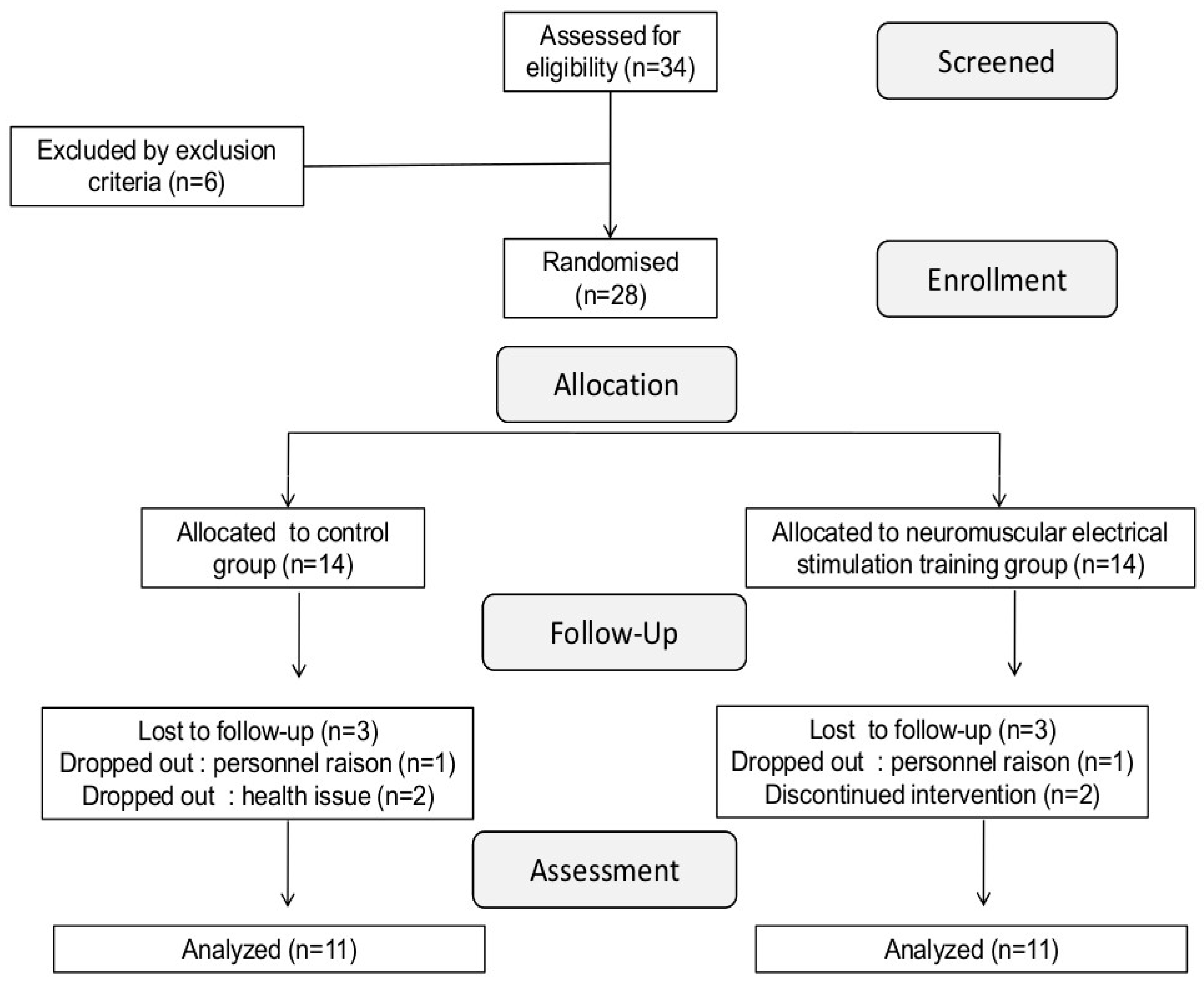
2. Methods
2.1. Participants
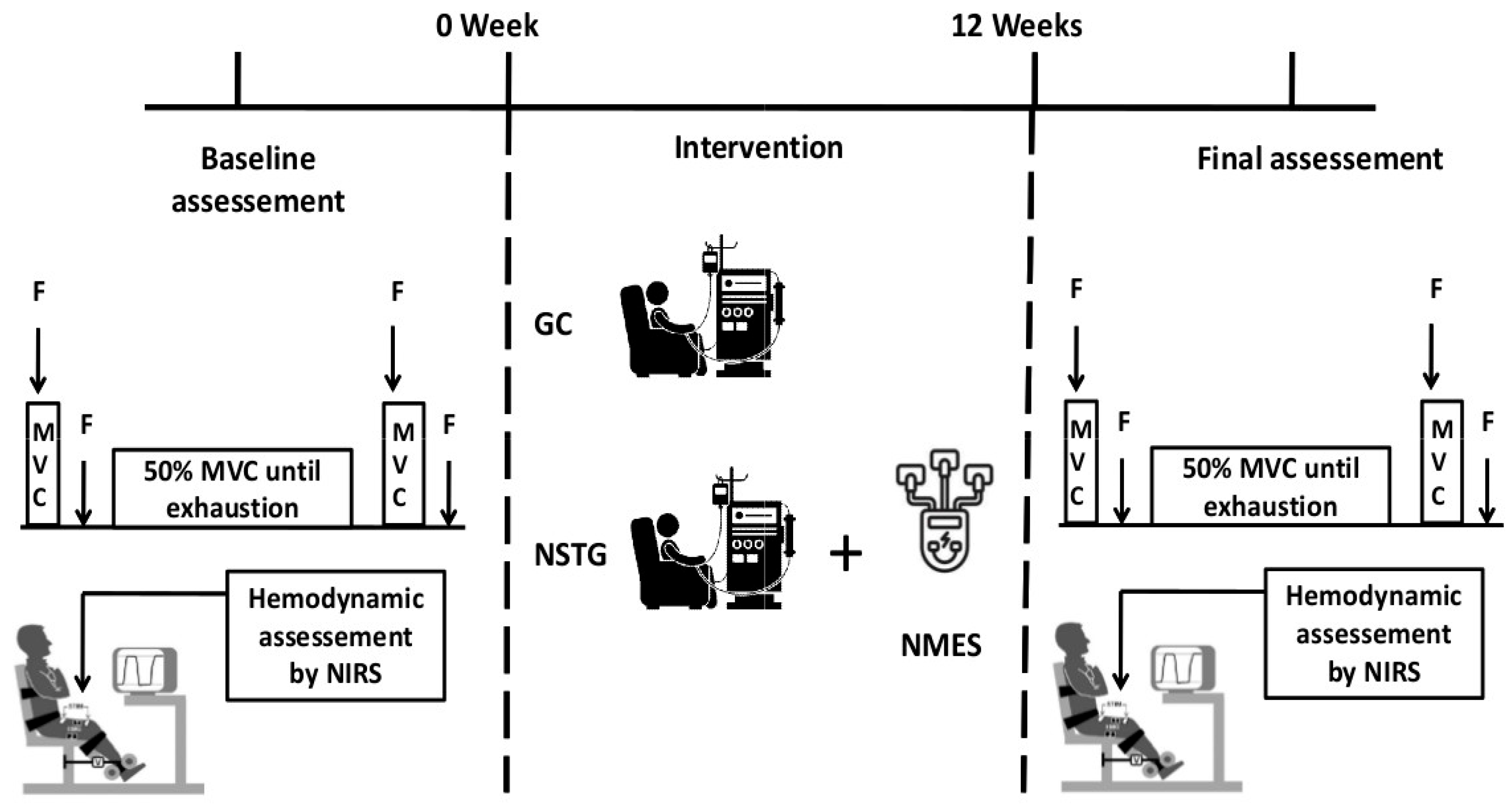
2.2. Study Design
2.3. Intervention Protocol
3. Study Outcomes
3.1. Fatiguing Exercise Protocol
3.2. Data Collection and Analysis
3.2.1. Assessment of Neuromuscular Function
3.2.2. Electromyographic Recordings
3.2.3. Muscular Hemodynamic Measurement
3.3. Statistical Analyses
4. Results
4.1. Exercise Performance
4.2. Central and Peripheral Fatigue
4.3. Electromyography
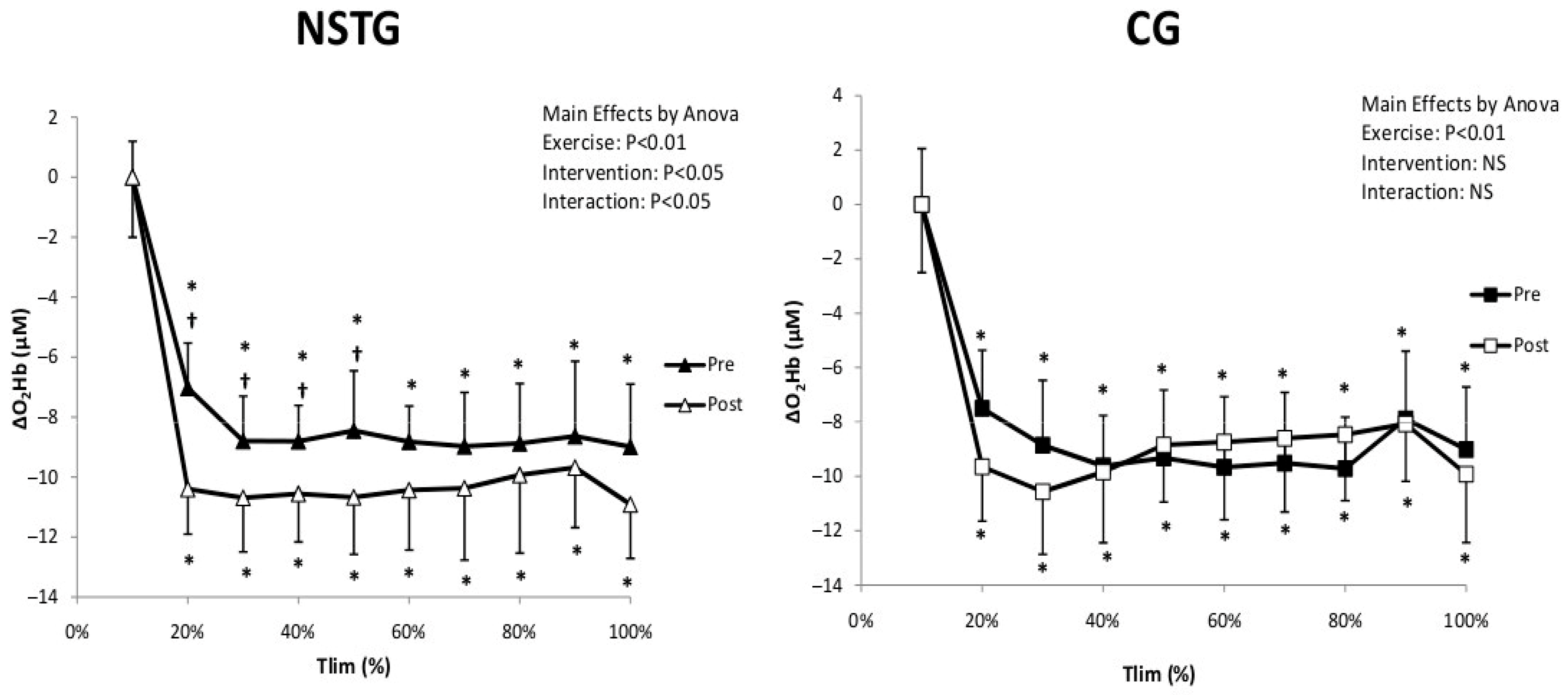
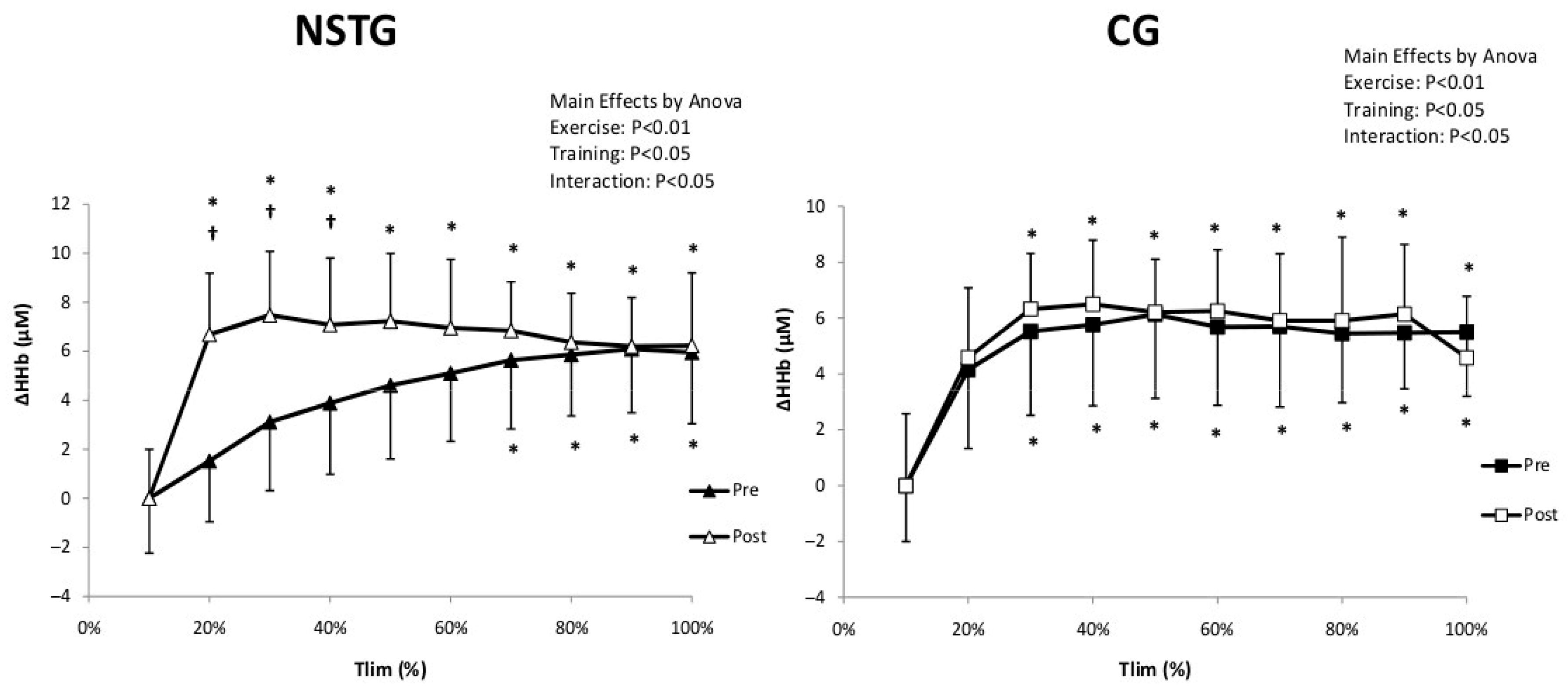
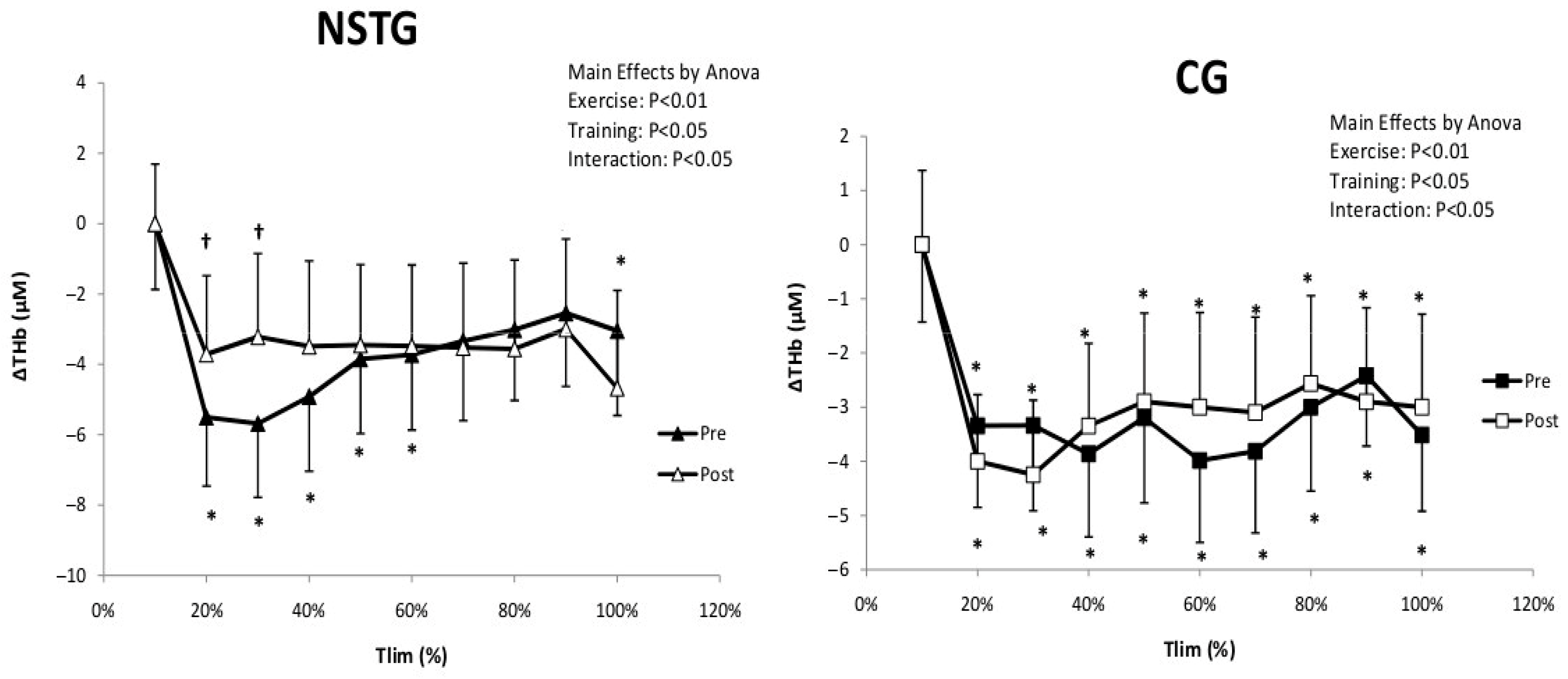
4.4. Vastus Lateralis Hemodynamic and Oxygenation
5. Discussion
5.1. Exercise Capacity
5.2. Neuromuscular Outcomes
5.3. Hemodynamic and Muscle Oxygenation
5.4. Methodological Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatrenet, A.; Beaune, B.; Fois, A.; Pouliquen, C.; Audebrand, J.-M.; Torreggiani, M.; Paris, D.; Durand, S.; Piccoli, G.B. Physiopathology ofNeuromuscular function related to fatigue in chronic Renal disease in the elderly (PIONEER): Study protocol. BMC Nephrol. 2020, 21, 305. [Google Scholar] [CrossRef]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef]
- Adams, G.R.; Vaziri, N.D. Skeletal muscle dysfunction in chronic renal failure: Effects of exercise. Am. J. Physiol. Ren. Physiol. 2006, 290, F753–F761. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Abbasi, A.; Hejazian, S.S.; Hekmatshoar, Y.; Ardalan, M.; Farnood, F.; Vahed, S.Z. Combating chronic kidney disease-associated cachexia: A literature review of recent therapeutic approaches. BMC Nephrol. 2025, 26, 133. [Google Scholar] [CrossRef]
- Greenwood, S.A.; Naish, P.; Clark, R.; O’COnnor, E.; Pursey, V.A.; Macdougall, I.C.; Mercer, T.H.; Koufaki, P. Intra-dialytic exercise training: A pragmatic approach. J. Ren. Care 2014, 40, 219–226. [Google Scholar] [CrossRef]
- Koh, K.P.; Fassett, R.G.; Sharman, J.E.; Coombes, J.S.; Williams, A.D. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: A randomized pilot study. Am. J. Kidney Dis. 2010, 55, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Dobsak, P.; Homolka, P.; Svojanovsky, J.; Reichertova, A.; Soucek, M.; Novakova, M.; Dusek, L.; Vasku, J.; Eicher, J.; Siegelova, J. Intra-dialytic electrostimulation of leg extensors may improve exercise tolerance and quality of life in hemodialyzed patients. Artif. Organs 2012, 36, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Caulfield, B.; Crowe, L.; Clark, A. Prolonged electrical muscle stimulation exercise improves strength and aerobic capacity in healthy sedentary adults. J. Appl. Physiol. 2005, 99, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.J.; E Franssen, F.M.; Gosker, H.R.; Wouters, E.F.M.; A Spruit, M. Metabolic and structural changes in lower-limb skeletal muscle following neuromuscular electrical stimulation: A systematic review. PLoS ONE 2013, 8, e69391. [Google Scholar] [CrossRef]
- Johansen, K.L.; A Kaysen, G.; Young, B.S.; Hung, A.M.; da Silva, M.; Chertow, G.M. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am. J. Clin. Nutr. 2003, 77, 842–846. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Mitch, W.E. Review of muscle wasting associated with chronic kidney disease. Am. J. Clin. Nutr. 2010, 91, 1128S–1132S. [Google Scholar] [CrossRef]
- Hu, L.; Klein, J.D.; Hassounah, F.; Cai, H.; Zhang, C.; Xu, P.; Wang, X.H. Low-frequency electrical stimulation attenuates muscle atrophy in CKD:—A potential treatment strategy. J. Am. Soc. Nephrol. 2015, 26, 626–635. [Google Scholar] [CrossRef]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrica lstimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Cometti, G.; Amiridis, I.G.; Martin, A.; Pousson, M.; Chatard, J.C. The effects of electromyostimulation training and basketball practice on muscle strength and jumping ability. Int. J. Sports Med. 2000, 21, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Bowman, B.R.; Baker, L.L. Effects of wave form parameters on comfort during transcutaneous neuromuscular electrical stimulation. Ann. Biomed. Eng. 1985, 13, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Hainaut, K.; Duchateau, J. Neuromuscular electrical stimulation and voluntary exercise. Sports Med. 1992, 14, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Gueldich, H.; Zarrouk, N.; Chtourou, H.; Zghal, F.; Sahli, S.; Rebai, H. Electrostimulation Training Effects on diurnal Fluctuations of Neuromuscular Performance. Int. J. Sports Med. 2017, 38, 41–47. [Google Scholar] [CrossRef]
- Burke, D. Effects of activity on axonal excitability: Implications for motor control studies. Adv. Exp. Med. Biol. 2002, 508, 33–37. [Google Scholar]
- Merton, P.A. Voluntary strength and fatigue. J. Physiol. 1954, 123, 553–564. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Grassi, B.; Pogliaghi, S.; Rampichini, S.; Quaresima, V.; Ferrari, M.; Marconi, C.; Cerretelli, P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J. Appl. Physiol. 2003, 95, 149–158. [Google Scholar] [CrossRef]
- Machfer, A.; Fekih, N.; Ammar, A.; Hassen, H.B.H.; Daab, W.; Amor, H.I.H.; Bouzid, M.A.; Chtourou, H. Effects of neuromuscular electricalstimulation during hemodialysis on muscle strength, functional capacity and postural balance in patients with end-stage renal disease: A randomized controlled trial. BMC Nephrol. 2025, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Neder, J.; Sword, D.; A Ward, S.; Mackay, E.; Cochrane, L.M.; Clark, C.J. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax 2002, 57, 333–337. [Google Scholar] [CrossRef]
- Banerjee, P.; Caulfield, B.; Crowe, L.; Clark, A.L. Prolonged electrical muscle stimulation exercise improves strength, peak VO2, and exercise capacity in patients with stable chronic heart failure. J. Card. Fail. 2009, 15, 319–326. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Bohmke, N.; Carbone, S.; Garten, R.S.; Rodriguez-Miguelez, P.; Franco, R.L.; Kidd, J.M.; Abbate, A. Exercise intolerance in kidney diseases: Physiological contributors and therapeutic strategies. Am. J. Physiol. Ren. Physiol. 2021, 320, F161–F173. [Google Scholar] [CrossRef]
- Frąk, W.; Dąbek, B.; Balcerczyk-Lis, M.; Motor, J.; Radzioch, E.; Młynarska, E.; Rysz, J.; Franczyk, B. Role of Uremic Toxins, Oxidative Stress, and Renal Fibrosis in Chronic Kidney Disease. Antioxidants 2024, 13, 687. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lopez-Lopez, S.; Romero-Morales, C.; Maffulli, N.; Lippi, G.; Pareja-Galeano, H. Neuromuscular electricalstimulation: A new therapeutic option for chronic diseases based oncontraction-induced myokine secretion. Front. Physiol. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Gondin, J.; Brocca, L.; Bellinzona, E.; D’Antona, G.; Maffiuletti, N.A.; Miotti, D.; Pellegrino, M.A.; Bottinelli, R. Neuromuscular electrical stimulation training induces a typical adaptations of the human skeletal muscle phenotype: A functional and proteomic analysis. J. Appl. Physiol. 2011, 110, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Johansen, K.L.; Doyle, J.; Sakkas, G.K.; Kent-Braun, J.A. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R805–R813. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [PubMed]
- Theodorakopoulou, M.P.; Zafeiridis, A.; Dipla, K.; Faitatzidou, D.; Koutlas, A.; Alexandrou, M.-E.; Doumas, M.; Papagianni, A.; Sarafidis, P. Muscle Oxygenation and Microvascular Reactivity Across Different Stages of CKD: A Near-Infrared Spectroscopy Study. Am. J. Kidney Dis. 2023, 81, 655–664.e1. [Google Scholar] [CrossRef]
- Stefanou, C.; Karatzanos, E.; Mitsiou, G.; Psarra, K.; Angelopoulos, E.; Dimopoulos, S.; Gerovasili, V.; Boviatsis, E.; Routsi, C.; Nanas, S. Neuromuscular electrical stimulation acutely mobilizes endothelial progenitor cells in critically ill patients with sepsis. Ann. Intensive Care 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregg, L.P.; Bossola, M.; Ostrosky-Frid, M.; Hedayati, S.S. Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar]
- Mancinelli, R.; Toniolo, L.; Di Filippo, E.S.; Doria, C.; Marrone, M.; Maroni, C.R.; Verratti, V.; Bondi, D.; Maccatrozzo, L.; Pietrangelo, T.; et al. Neuromuscular electrical stimulation induces skeletal muscle fiber remodeling and specific gene expression profile in healthy elderly. Front. Physiol. 2019, 10, 1459. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Carter, W.; Rivers, J.; Chen, Q.; Lesnefsky, E.J. Skeletal muscle hypertrophy and enhanced mitochondrial bioenergetics following electrical stimulation exercises in spinal cord injury: A randomized clinical trial. Eur. J. Appl. Physiol. 2025, 125, 1075–1089. [Google Scholar]
- Katagiri, M.; Nakabayashi, M.; Matsuda, Y.; Ono, Y.; Ichinose, M. Differential changes in blood flow and oxygen utilization in active muscles between voluntary exercise and electrical muscle stimulation in young adults. J. Appl. Physiol. 2024, 136, 1053–1064. [Google Scholar] [CrossRef] [PubMed]

| NSTG Group | CG Group | p | |
|---|---|---|---|
| Anthropometric data | |||
| Total n | 11 | 11 | NS |
| Age (years) | 37.9 ± 1.8 | 39.2 ± 4.1 | NS |
| Weight (Kg) | 74. 8 ± 2.3 | 75.9 ± 6.3 | NS |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.2 | NS |
| BMI (%) | 25.1 ± 2.2 | 25.3 ± 3.5 | NS |
| Physical activity score | 3.8 ± 2.1 | 4.2 ± 2.4 | NS |
| Comorbidities | |||
| Diabetes mellitus type 2 (%) | 3 (27%) | 2 (18%) | |
| Hypertension (%) | 2 (18%) | 1 (9%) | |
| Clinical parameters | |||
| Time on dialysis (months) | 49.2 ± 6.8 | 54.8 ± 9.2 | NS |
| eGFR (mL/min/1.73 m2) | 9.3 ± 2.2 | 8.9 ± 2.7 | NS |
| Hb (g/dL) | 110.9 ± 8.8 | 9.9 ± 2.7 | NS |
| Blood pressure (systolic) (mmHg) | 151.6 ± 12.3 | 141.6 ± 10.9 | NS |
| Blood pressure (diasystolic) (mmHg) | 91.2 ± 4.5 | 99.5 ± 6.2 | NS |
| Heart rate at rest (bpm) | 79.2 ± 8.8 | 72.9 ± 6.3 | NS |
| Kt/V | 1.99 ± 0.55 | 1.66 ± 0.85 | NS |
| Charlson Comorbidity Index (score) | 3 | 3 | NS |
| CG | NSTG | Effects | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | ||||||
| Rest | Post | Rest | Post | Rest | Post | Rest | Post | ||
| MVC (N) | 379.90 ± 31.51 | 295.50 ± 22.55 * | 398.30 ± 19.02 | 306.50 ± 19.15 * | 421.27 ± 24.90 | 359.73 ± 16.24 * | 550.36 ± 20.33 †# | 388.45 ± 17.05 * | Interaction p < 0.001 Group p < 0.001 Intervention p = 0.02 Exercise p < 0.001 |
| Qtw,pot (N) | 138.11 ± 4.54 | 96.44 ± 6.77 * | 129.89 ± 3.75 | 86.89 ± 9.38 * | 141.00 ± 4.31 | 94.20 ± 2.95 * | 143.60 ± 7.33 # | 81.90 ± 3.89 *† | Interaction p = 0.39 Group p = 0.03 Intervention p = 0.07 Exercise p < 0.001 |
| VA (%) | 91.95 ± 1.98 | 85.85 ± 4.21 * | 89.73 ± 2.63 | 80.60 ± 4.97 * | 90.17 ± 3.66 | 84.71 ± 4.06 * | 96.78 ± 2.50 † | 83.42 ± 6.60 * | Interaction p = 0.22 Group p = 0.18 Intervention p = 0.012 Exercise p < 0.001 |
| VM Max (mV) | 6.52 ± 1.04 | 5.50 ± 0.8 * | 6.23 ± 1.05 | 5.22 ± 0.72 * | 6.75 ± 0.91 | 5.95 ± 0.22 * | 6.48 ± 0.90 | 6.32 ± 0.28 # | Interaction p = 0.22 Group p =0.21 Intervention p = 0.68 Exercise p = 0.04 |
| VM Max (mV) | 5.53 ± 0.97 | 5.64 ± 0.95 | 5.83 ± 0.33 | 5.13 ± 0.64 * | 5.20 ± 0.03 | 5.81 ± 0.55 | 5.47 ± 1.30 | 5.67 ± 0.69 | Interaction p = 0.86 Group p = 0.35 Intervention p = 0.56 Exercise p < 0.001 |
| VM Max (mV) | 6.33 ± 0.97 | 6.44 ± 0.95 | 6.23 ± 1.33 | 6.13 ± 0.64 | 5.86 ± 0.52 # | 6.53 ± 1.39 | 6.14 ± 0.24 | 6.18 ± 1.52 | Interaction p = 0.48 Group p = 0.04 Intervention p = 0.44 Exercise p = 0.99 |
| RMS/Mmax (mV) | 0.11 ± 0.01 | 0.08 ± 0.01 * | 0.12 ± 0.01 | 0.09 ± 0.01 * | 0.10 ± 0.01 | 0.08 ± 0.01 * | 0.11 ± 0.01 * | 0.07 ± 0.01 * | Interaction p =0.35 Group p = 0.33 Intervention p = 0.19 Exercise p = 0.01 |
| Tlim | 107.44 ± 22.03 | - | 99.50 ± 19.6 | - | 103.89± 14.43 | - | 123.33 ± 16.60 †# | - | Interaction p = 0.02 Group p = 0.03 Intervention p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machfer, A.; Ammar, A.; Ceylan, H.İ.; Zghal, F.; Daab, W.; Amor, H.I.H.; Chtourou, H.; Muntean, R.I.; Bouzid, M.A. Neuromuscular Electrical Stimulation During Hemodialysis Enhances Exercise Capacity in Patients with End-Stage Renal Disease: A Pilot Randomized Controlled Trial. J. Clin. Med. 2025, 14, 7702. https://doi.org/10.3390/jcm14217702
Machfer A, Ammar A, Ceylan Hİ, Zghal F, Daab W, Amor HIH, Chtourou H, Muntean RI, Bouzid MA. Neuromuscular Electrical Stimulation During Hemodialysis Enhances Exercise Capacity in Patients with End-Stage Renal Disease: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(21):7702. https://doi.org/10.3390/jcm14217702
Chicago/Turabian StyleMachfer, Amal, Achraf Ammar, Halil İbrahim Ceylan, Firas Zghal, Wael Daab, Hassen Ibn Hadj Amor, Hamdi Chtourou, Raul Ioan Muntean, and Mohamed Amine Bouzid. 2025. "Neuromuscular Electrical Stimulation During Hemodialysis Enhances Exercise Capacity in Patients with End-Stage Renal Disease: A Pilot Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 21: 7702. https://doi.org/10.3390/jcm14217702
APA StyleMachfer, A., Ammar, A., Ceylan, H. İ., Zghal, F., Daab, W., Amor, H. I. H., Chtourou, H., Muntean, R. I., & Bouzid, M. A. (2025). Neuromuscular Electrical Stimulation During Hemodialysis Enhances Exercise Capacity in Patients with End-Stage Renal Disease: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine, 14(21), 7702. https://doi.org/10.3390/jcm14217702








