What to Measure? Development of a Core Outcome Set to Assess Remote Technologies for Cochlear Implant Users
Abstract
1. Introduction
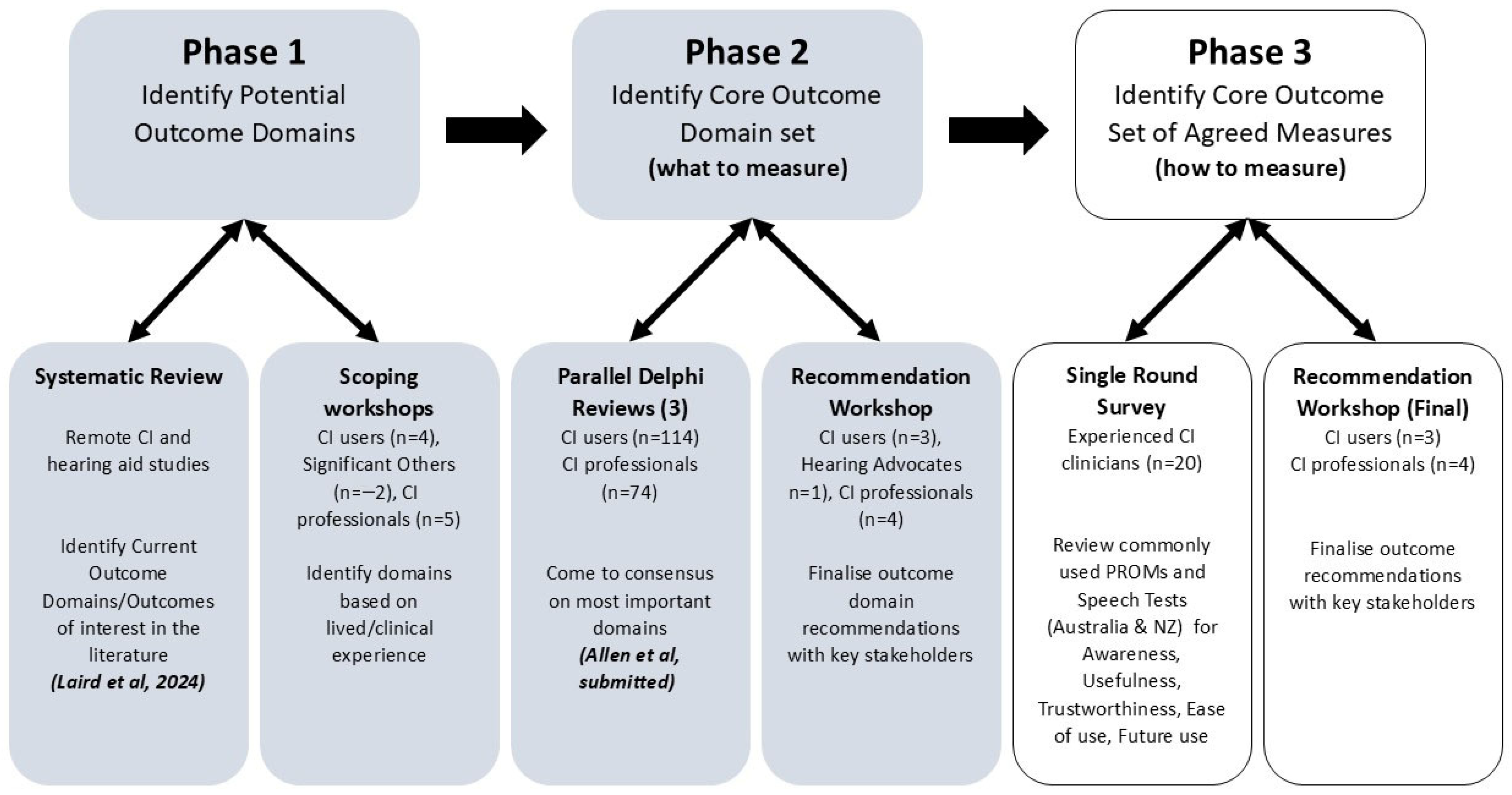
2. Materials and Methods
2.1. Single-Round Online Survey
- This measure is easy to use in clinical practice (ease of use);
- This measure gives results that are trustworthy/believable;
- This measure gives results that are useful in clinical practice;
- I would use this measure in clinical practice if it were recommended to me.
2.2. Online Final Recommendation Workshops
- What domains should be included in future iterations of the COS?
- (CI professionals only) Which outcome measures or subdomains should be recommended as a minimum standard?
- How should we prioritise outcome measures within each subdomain?
3. Results
3.1. Single-Round Online Survey
3.1.1. Familiarity Ratings (Speech Perception Outcome Measures and PROMs)
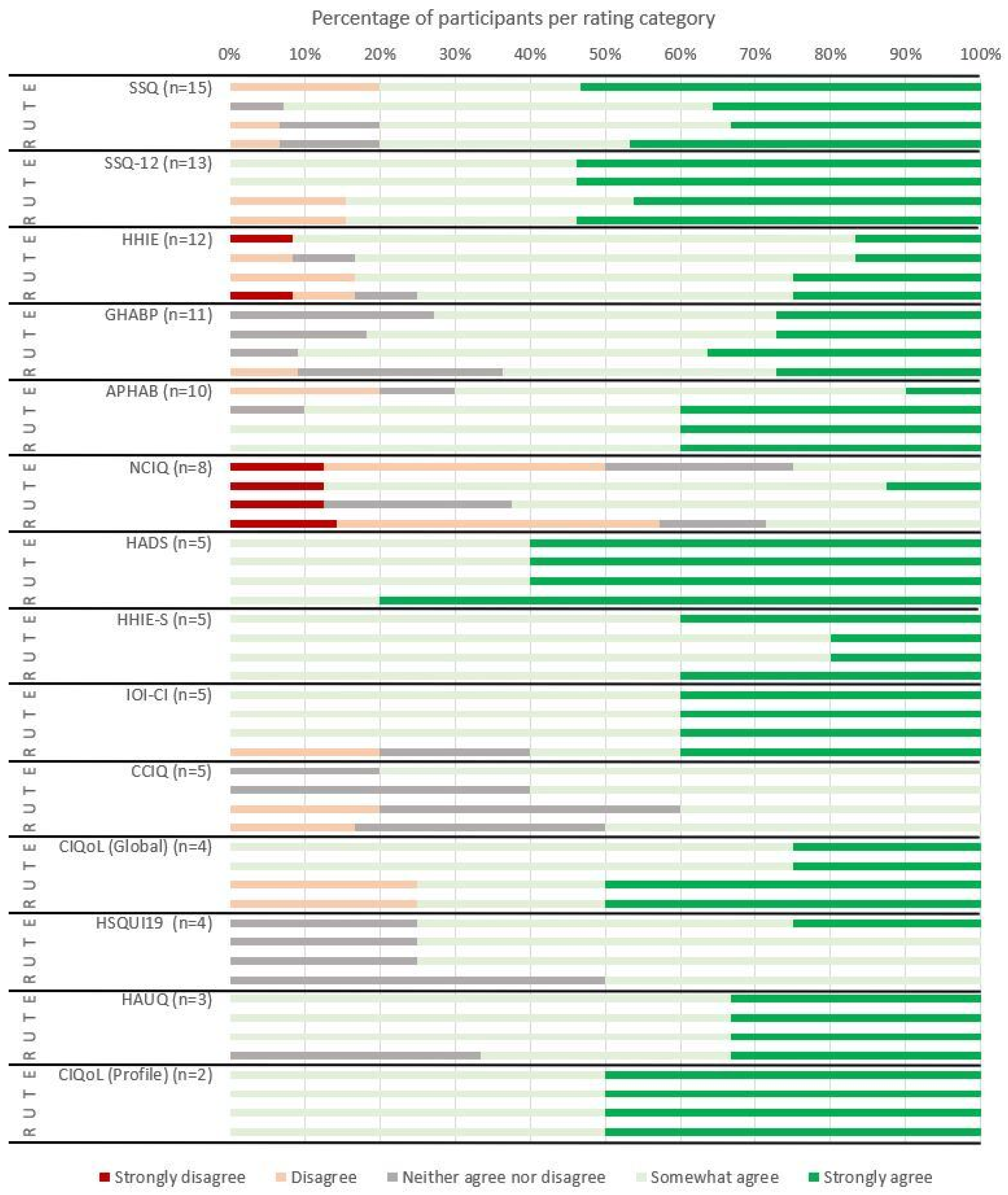
3.1.2. Ease of Use, Trustworthiness, Usefulness, and Likely Recommendation to Use Ratings
3.1.3. Free Text Responses
3.2. Final Recommendation Workshops
4. Discussion
- Lack of consensus between CI users and CI professionals on the most important domains for the patient supra-domain [33] means that the implementation of a concise COS is problematic if one is to measure the most important domains within each supra-domain.
- Lack of well-designed and/or well-validated outcome measures for some of the domains rated as most important to assess. Rigorous development and assessment of novel outcome measures is, therefore, required.
- Current clinical practice trends in Australia and New Zealand, observed in our online survey of CI clinicians, indicate that CI services rely predominantly on a relatively small pool of specific speech perception outcome measures as the primary measure of CI outcomes. Clinicians are far less familiar with most PROMs that align with the CODS.
- Several of the outcome measures identified and explored in the outcomes survey have proprietary test materials, technical requirements, and licencing costs associated with them.
4.1. Recommended Interim, Pragmatic COS
4.1.1. Service Outcomes
4.1.2. Clinically Measured Outcomes
4.1.3. Patient-Reported Outcome Measures (PROMs)
4.1.4. Future Considerations for Validation, Revision, and Expansion of the COS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Cochlear Implant |
| CODS | Core Outcome Domain Set |
| COS | Core Outcome Set |
| PROM | Patient Reported Outcome Measure |
| WHO | World Health Organisation |
| ICF | International classification of Functioning, Disability, and Health framework |
| COMET | Core Outcome Measures in Effectiveness Trials |
References
- Ferguson, M.A.; Eikelboom, R.H.; Sucher, C.M.; Maidment, D.W.; Bennett, R.J. Remote Technologies to Enhance Service Delivery for Adults: Clinical Research Perspectives. Semin. Hear. 2023, 44, 328–350. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, S.; Kim, D.; Shin, Y. A Review of Contemporary Teleaudiology: Literature Review, Technology, and Considerations for Practicing. J. Audiol. Otol. 2021, 25, 1–7. [Google Scholar] [CrossRef]
- Luryi, A.L.; Tower, J.I.; Preston, J.; Burkland, A.; Trueheart, C.E.; Hildrew, D.M. Cochlear Implant Mapping Through Telemedicine-A Feasibility Study. Otol. Neurotol. 2020, 41, e330–e333. [Google Scholar] [CrossRef]
- Maruthurkkara, S.; Allen, A.; Cullington, H.; Muff, J.; Arora, K.; Johnson, S. Remote check test battery for cochlear implant recipients: Proof of concept study. Int. J. Audiol. 2022, 61, 443–452. [Google Scholar] [CrossRef]
- Schepers, K.; Steinhoff, H.-J.; Ebenhoch, H.; Böck, K.; Bauer, K.; Rupprecht, L.; Möltner, A.; Morettini, S.; Hagen, R. Remote programming of cochlear implants in users of all ages. Acta Otolaryngol. 2019, 139, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Cullington, H.; Kitterick, P.; Weal, M.; Margol-Gromada, M. Feasibility of personalised remote long-term follow-up of people with cochlear implants: A randomised controlled trial. BMJ Open 2018, 8, e019640. [Google Scholar] [CrossRef] [PubMed]
- Maruthurkkara, S.; Case, S.; Rottier, R. Evaluation of Remote Check: A Clinical Tool for Asynchronous Monitoring and Triage of Cochlear Implant Recipients. Ear Hear. 2022, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.; Smits, C.; Govaerts, P.J.; Doorn, I.; Vanpoucke, F. Empowering Senior Cochlear Implant Users at Home via a Tablet Computer Application. Am. J. Audiol. 2018, 27, 417–430. [Google Scholar] [CrossRef]
- Carner, M.; Bianconi, L.; Fulco, G.; Confuorto, G.; Soloperto, D.; Molteni, G.; Sacchetto, L. Personal experience with the remote check telehealth in cochlear implant users: From COVID-19 emergency to routine service. Eur. Arch. Otorhinolaryngol. 2023, 280, 5293–5298. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Saoji, A.A.; DeJong, M.D.; Tombers, N.M.; Driscoll, C.L.W.; Neff, B.A.; Haynes, D.S.; Carlson, M.L. Implementation Strategy for Highly-Coordinated Cochlear Implant Care With Remote Programming: The Complete Cochlear Implant Care Model. Otol. Neurotol. 2022, 43, e916–e923. [Google Scholar] [CrossRef]
- Nittari, G.; Savva, D.; Tomassoni, D.; Tayebati, S.K.; Amenta, F. Telemedicine in the COVID-19 Era: A Narrative Review Based on Current Evidence. Int. J. Environ. Res. Public Health 2022, 19, 5101. [Google Scholar] [CrossRef]
- Chong-White, N.; Incerti, P.; Poulos, M.; Tagudin, J. Exploring teleaudiology adoption, perceptions and challenges among audiologists before and during the COVID-19 pandemic. BMC Digit. Health 2023, 1, 24. [Google Scholar] [CrossRef]
- Lilies, A.; Darnton, P.; Kryl, D.; Sibley, A.; Chandler, J.; Barton, S.; Benson, T.; Robertson, A. Independent Evaluation of CHOICE 2021; Darnton, P., Ed.; Health Innovation Wessex: Southampton, UK, 2021; pp. 1–53. [Google Scholar]
- Sucher, C.; Norman, R.; Chaffey, E.; Bennett, R.; Ferguson, M. Patient preferences for Remote cochlear implant management: A discrete choice experiment. PLoS ONE 2025, 20, e0320421. [Google Scholar] [CrossRef] [PubMed]
- Barreira-Nielsen, C.S.C.; Campos, L.S. Implementation of the hybrid teleaudiology model: Acceptance, feasibility and satisfaction in a cochlear implant program. Audiol. Commun. Res. 2022, 27, e2538. [Google Scholar] [CrossRef]
- Department of Health, Victoria (Ed.) Virtual Care Operational Framework; Department of Health, Victoria: Melbourne, Australia, 2023.
- Granberg, S.; Dahlström, J.; Möller, C.; Kähäri, K.; Danermark, B. The ICF Core Sets for hearing loss-researcher perspective. Part I: Systematic review of outcome measures identified in audiological research. Int. J. Audiol. 2014, 53, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Akeroyd, M.A.; Wright-Whyte, K.; Holman, J.A.; Whitmer, W.M. A comprehensive survey of hearing questionnaires: How many are there, what do they measure, and how have they been validated? Trials 2015, 16, P26. [Google Scholar] [CrossRef]
- Neal, K.; McMahon, C.M.; Hughes, S.E.; Boisvert, I. Listening-based communication ability in adults with hearing loss: A scoping review of existing measures. Front. Psychol. 2022, 13, 786347. [Google Scholar] [CrossRef]
- Danermark, B.; Granberg, S.; Kramer, S.E.; Selb, M.; Möller, C. The creation of a comprehensive and a brief core set for hearing loss using the international classification of functioning, disability and health. Am. J. Audiol. 2013, 22, 323–328. [Google Scholar] [CrossRef]
- Allen, D.; Hickson, L.; Ferguson, M. Defining a Patient-Centred Core Outcome Domain Set for the Assessment of Hearing Rehabilitation With Clients and Professionals. Front. Neurosci. 2022, 16, 787607. [Google Scholar] [CrossRef]
- Andries, E.; Lorens, A.; Skarżyński, P.H.; Skarżyński, H.; Calvino, M.; Gavilán, J.; Lassaletta, L.; Tavora-Vieira, D.; Acharya, A.; Kurz, A.; et al. Implementation of the international classification of functioning, disability and health model in cochlear implant recipients: A multi-center prospective follow-up cohort study. Front. Audiol. Otol. 2023, 1, 1257504. [Google Scholar] [CrossRef]
- Boisvert, I.; Ferguson, M.; van Wieringen, A.; Ricketts, T.A. Editorial: Outcome Measures to Assess the Benefit of Interventions for Adults With Hearing Loss: From Research to Clinical Application. Front. Neurosci. 2022, 16, 955189. [Google Scholar] [CrossRef]
- Clarke, M.; Williamson, P.R. Core outcome sets and systematic reviews. Syst. Rev. 2016, 5, 11. [Google Scholar] [CrossRef]
- COMET. Core Outcome Measures in Effectiveness Trials. 2022. Available online: https://www.comet-initiative.org/ (accessed on 1 October 2022).
- Hall, D.A.; Haider, H.; Kikidis, D.; Mielczarek, M.; Mazurek, B.; Szczepek, A.J.; Cederroth, C.R. Toward a global consensus on outcome measures for clinical trials in tinnitus: Report from the first international meeting of the COMiT Initiative, November 14, 2014, Amsterdam, The Netherlands. Trends Hear. 2015, 19, 2331216515580272. [Google Scholar] [CrossRef] [PubMed]
- Dietz, A.; Heinrich, A.; Törmäkangas, T.; Iso-Mustajärvi, M.; Miettinen, P.; Willberg, T.; Linder, P.H. The effectiveness of cochlear implantation on performance-based and patient-reported outcome measures in Finnish recipients. Front. Neurosci. 2022, 16, 786939. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Sucher, C.; Nakano, K.; Ferguson, M. Systematic review of patient and service outcome measures of remote digital technologies for cochlear implant and hearing aid users. Front. Audiol. Otol. 2024, 2, 1403814. [Google Scholar] [CrossRef]
- Hughes, S.E.; Watkins, A.; Rapport, F.; Boisvert, I.; McMahon, C.M.; Hutchings, H.A. Rasch analysis of the listening effort Questionnaire—Cochlear implant. Ear Hear. 2021, 42, 1699–1711. [Google Scholar] [CrossRef]
- McRackan, T.R.; Hand, B.N.; Cochlear Implant Quality of Life Development Consortium; Velozo, C.A.; Dubno, J.R. Cochlear Implant Quality of Life (CIQoL): Development of a profile instrument (CIQOL-35 Profile) and a global measure (CIQOL-10 Global). J. Speech Lang. Hear. Res. 2019, 62, 3554–3563. [Google Scholar] [CrossRef]
- Maidment, D.; Heyes, R.; Gomez, R.; Coulson, N.S.; Wharrad, H.; Ferguson, M.A. Evaluating a theoretically informed and co-created mHealth educational intervention for first-time hearing aid users: A qualitative interview study. J. Med. Internet Res. 2020, 8, e17193. [Google Scholar]
- Gomez, R.; Habib, A.; Maidment, D.W.; Ferguson, M.A. Smartphone-Connected Hearing Aids Enable and Empower Self-Management of Hearing Loss: A Qualitative Interview Study Underpinned by the Behavior Change Wheel. Ear Hear. 2022, 43, 921–932. [Google Scholar] [CrossRef]
- Sucher, C.; Laird, E.; Allen, D.; Boisvert, I.; Ferguson, M. Development of a Core Outcome Set to evaluate Remote Technologies for Cochlear Implant Users. In Proceedings of the World Congress of Audiology, Paris, France, 19–22 September 2024. [Google Scholar]
- The Python Software Foundation. The Python Language Reference; The Python Software Foundation: Beaverton, OR, USA, 2001. [Google Scholar]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- The Matplotlib Development Team. Matplotlib: Visualisation with Python; Zenodo, Ed.; CERN: Geneva, Switzerland, 2025. [Google Scholar]
- Atkinson, J.; Salmond, C.; Crampton, P.; Viggers, H.; Lacey, K. NZDep2023 Index of Socioeconomic Deprivation: Research Report; Department of Public Health, University of Otago: Wellington, New Zealand, 2024. [Google Scholar]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J Audiol 2004, 43, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Bench, J.; Doyle, J. The BKB/A (Banford-Kowal-Bench/Australian Version) Sentence Lists for Hearing-Impaired Children; La Trobe University: Victoria, Australia, 1979. [Google Scholar]
- Boothroyd, A.; Hanin, L.; Hnath, T. A sentence test of speech perception: Reliability, set equivalence, and short term learning. In CUNY Academic Works; City University of New York: New York, NY, USA, 1985. [Google Scholar]
- Peterson, G.E.; Lehiste, I. Revised CNC lists for auditory tests. J. Speech Hear. Disord. 1962, 27, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, A. Developments in Speech Audiometry. Br. J. Sound 1968, 2, 3–10. [Google Scholar]
- Noble, W.; Jensen, N.S.; Naylor, G.; Bhullar, N.; Akeroyd, M.A. A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: The SSQ12. Int. J. Audiol. 2013, 52, 409–412. [Google Scholar] [CrossRef]
- Ventry, I.M.; Weinstein, B.E. The hearing handicap inventory for the elderly: A new tool. Ear Hear. 1982, 3, 128–134. [Google Scholar] [CrossRef]
- Killion, M.C.; Niquette, P.A.; Gudmundsen, G.I.; Revit, L.J.; Banerjee, S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J. Acoust. Soc. Am. 2004, 116, 2395–2405. [Google Scholar] [CrossRef]
- Etymotic Research. Etymotic BKB-SIN Speech-in-Noise Test User Manual; Interacoustics: Middelfart, Denmark, 2005; pp. 1–27. [Google Scholar]
- Smits, C.; Goverts, S.T.; Festen, J.M. The digits-in-noise test: Assessing auditory speech recognition abilities in noise. J. Acoust. Soc. Am. 2013, 133, 1693–1706. [Google Scholar] [CrossRef]
- Gatehouse, S. A self-report outcome measure for the evaluation of hearing aid fittings and services. Health Bull. 1999, 57, 424–436. [Google Scholar]
- Cox, R.M.; Alexander, G.C. The abbreviated profile of hearing aid benefit. Ear Hear. 1995, 16, 176–186. [Google Scholar] [CrossRef]
- Hinderink, J.B.; Krabbe, P.F.; Van Den Broek, P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: The Nijmegen cochlear implant questionnaire. Otolaryngol. Head Neck Surg. 2000, 123, 756–765. [Google Scholar] [CrossRef]
- King, N.; Nahm, E.A.; Liberatos, P.; Shi, Q.; Kim, A.H. A new comprehensive cochlear implant questionnaire for measuring quality of life after sequential bilateral cochlear implantation. Otol. Neurotol. 2014, 35, 407–413. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Soli, S.D.; Sullivan, J.A. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J. Acoust. Soc. Am. 1994, 95, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Cassarly, C.; Matthews, L.J.; Simpson, A.N.; Dubno, J.R. The Revised Hearing Handicap Inventory and Screening Tool Based on Psychometric Reevaluation of the Hearing Handicap Inventories for the Elderly and Adults. Ear Hear. 2020, 41, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.W.; Hersbach, A.A.; Swanson, B.A. An adaptive Australian Sentence Test in Noise (AuSTIN). Ear Hear. 2013, 34, 592–600. [Google Scholar] [CrossRef]
- Spahr, A.J.; Dorman, M.F. Performance of subjects fit with the Advanced Bionics CII and Nucleus 3G cochlear implant devices. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 624–628. [Google Scholar] [CrossRef]
- Cox, R.; Hyde, M.; Gatehouse, S.; Noble, W.; Dillon, H.; Bentler, R.; Stephens, D.; Arlinger, S.; Beck, L.; Wilkerson, D.; et al. Optimal outcome measures, research priorities, and international cooperation. Ear Hear. 2000, 21, 106S–115S. [Google Scholar] [CrossRef]
- Dillon, H.; Birtles, G.; Lovegrove, R. Measuring the Outcomes of a National Rehabilitation Program: Normative Data for the Client Oriented Scale of Improvement (COSI) and the Hearing Aid User’s Questionnaire (HAUQ). J. Am. Acad. Audiol. 1999, 10, 67–79. [Google Scholar] [CrossRef]
- Amann, E.; Anderson, I. Development and validation of a questionnaire for hearing implant users to self-assess their auditory abilities in everyday communication situations: The Hearing Implant Sound Quality Index (HISQUI19). Acta Otolaryngol. 2014, 134, 915–923. [Google Scholar] [CrossRef] [PubMed]
- The ida Institute. ida Institute Motivational Tools: The Line. Available online: https://idainstitute.com/tools/motivation-tools (accessed on 1 October 2022).
- Hawthorne, G.; Hogan, A. Measuring disability-specific patient benefit in cochlear implant programs: Developing a short form of the Glasgow Health Status Inventory, the Hearing Participation Scale. Int. J. Audiol. 2002, 41, 535–544. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.S.; Mulrow, C.D.; Aguilar, C.; Tuley, M.R. Methods for screening for hearing loss in older adults. Am. J. Med. Sci. 1994, 307, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Luetje, C.M.; Brackman, D.; Balkany, T.J.; Maw, J.; Baker, R.S.; Kelsall, D.; Backous, D.; Miyamoto, R.; Parisier, S.; Arts, A. Phase III clinical trial results with the Vibrant Soundbridge implantable middle ear hearing device: A prospective controlled multicenter study. Otolaryngol. Head Neck Surg. 2002, 126, 97–107. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Antony, M.M.; Bieling, P.J.; Cox, B.J.; Enns, M.W.; Swinson, R.P. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol. Assess. 1998, 10, 176–181. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Kompis, M.; Pfiffner, F.; Krebs, M.; Caversaccio, M.D. Factors influencing the decision for Baha in unilateral deafness: The Bern benefit in single-sided deafness questionnaire. Adv. Otorhinolaryngol. 2011, 71, 103–111. [Google Scholar]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Cox, R.M.; Alexander, G.C. Expectations about hearing aids and their relationship to fitting outcome. J. Am. Acad. Audiol. 2000, 11, 368–382. [Google Scholar] [CrossRef]
- Billinger-Finke, M.; Bräcker, T.; Weber, A.; Amann, E.; Anderson, I.; Batsoulis, C. Development and validation of the audio processor satisfaction questionnaire (APSQ) for hearing implant users. Int. J. Audiol. 2020, 59, 392–397. [Google Scholar] [CrossRef] [PubMed]
- De Jong Gierveld, J.; Kamphuls, F. The Development of a Rasch-Type Loneliness Scale. Appl. Psychol. Meas. 1985, 9, 289–299. [Google Scholar] [CrossRef]
- De Jong Gierveld, J.; Van Tilburg, T. A 6-Item Scale forOverall, Emotional, and Social Loneliness. Confirmatory Tests on Survey Data. Res. Aging 2006, 28, 582–598. [Google Scholar] [CrossRef]
- Terluin, B.; van Marwijk, H.W.; Adèr, H.J.; de Vet, H.C.; Penninx, B.W.; Hermens, M.L.; van Boeijen, C.A.; van Balkom, A.J.; van der Klink, J.J.; AB Stalman, W. The Four-Dimensional Symptom Questionnaire (4DSQ): A validation study of a multidimensional self-report questionnaire to assess distress, depression, anxiety and somatization. BMC Psychiatry 2006, 6, 34. [Google Scholar] [CrossRef]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction with Life Scale. J. Personal. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef]
- Russell, D.; Peplau, L.A.; Cutrona, C.E. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J. Personal. Soc. Psychol. 1980, 39, 472–480. [Google Scholar] [CrossRef]
- Davies, A.R.; Ware, J.E., Jr. GHAA’s Consumer Satisfaction Survey and User’s Manual, 2nd ed; Group Health Association of America: Washington, DC, USA, 1991. [Google Scholar]
- McConnaughy, E.A.; Prochaska, J.O.; Velicer, W.F. Stages of change in psychotherapy: Measurement and sample profiles. Psychother. Theory Res. Pract. 1983, 20, 368–375. [Google Scholar] [CrossRef]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.; Berto, E.; Luzi, C.; Andreoli, A. Development of the Perceived Stress Questionnaire: A new tool for psychosomatic research. J. Psychosom. Res. 1993, 37, 19–32. [Google Scholar] [CrossRef]
- Heffernan, E.; Coulson, N.S.; Ferguson, M.A. Development of the Social Participation Restrictions Questionnaire (SPaRQ) through consultation with adults with hearing loss, researchers, and clinicians: A content evaluation study. Int. J. Audiol. 2018, 57, 791–799. [Google Scholar] [CrossRef]
- Sansoni, J.; Hawthorne, G.; Fleming, G.; Owen, E.; Marosszeky, N. Technical Manual and Instructions for the Revised Incontinence and Patient Satisfaction Tools; Centre for Health Service Development, University of Wollongong: Wollongong, Australia, 2011. [Google Scholar]
- Cox, R.M.; Alexander, G.C. Measuring Satisfaction with Amplification in Daily Life: The SADL scale. Ear Hear. 1999, 20, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Reichheld, F.F. The One Number You Need to Grow, in Harvard Business Review; Harvard Business School Publishing: Brighton, MA, USA, 2003. [Google Scholar]
- Heffernan, E.; Habib, A.; Ferguson, M. Evaluation of the psychometric properties of the social isolation measure (SIM) in adults with hearing loss. Int. J. Audiol. 2019, 58, 45–52. [Google Scholar] [CrossRef] [PubMed]
- CI Task Force. Adult Hearing Standards of Care; Living Guidelines. 2022. Available online: https://adulthearing.com/standards-of-care/ (accessed on 24 March 2025).
- Mokkink, L.B.; Elsman, E.B.M.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures version 2.0. Qual. Life Res. 2024, 33, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, I.; Reis, M.; Au, A.; Cowan, R.; Dowell, R.C. Cochlear implantation outcomes in adults: A scoping review. PLoS ONE 2020, 15, e0232421. [Google Scholar] [CrossRef]
- Duncan, E.A.; Murray, J. The barriers and facilitators to routine outcome measurement by allied health professionals in practice: A systematic review. BMC Health Serv. Res. 2012, 12, 96. [Google Scholar] [CrossRef]
- Hatfield, D.R.; Ogles, B.M. Why some clinicians use outcome measures and others do not. Adm. Policy Ment. Health 2007, 34, 283–291. [Google Scholar] [CrossRef]
- O’Connor, B.; Kerr, C.; Shields, N.; Imms, C. Understanding allied health practitioners’ use of evidence-based assessments for children with cerebral palsy: A mixed methods study. Disabil. Rehabil. 2019, 41, 53–65. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Rivera, S.C.; Roydhouse, J.; Kamudoni, P.; Alder, Y.; Anderson, N.; Baldwin, R.M.; Bhatnagar, V.; Black, J.; Bottomley, A.; et al. Recommendations to address respondent burden associated with patient-reported outcome assessment. Nat. Med. 2024, 30, 650–659. [Google Scholar] [CrossRef]
- Gifford, R.H.; Dorman, M.F. Bimodal Hearing or Bilateral Cochlear Implants? Ask the Patient. Ear Hear. 2019, 40, 501–516. [Google Scholar] [CrossRef]
- Ebrahimi-Madiseh, A.; Eikelboom, R.H.; Jayakody, D.M.; Atlas, M.D. Speech perception scores in cochlear implant recipients: An analysis of ceiling effects in the CUNY sentence test (Quiet) in post-lingually deafened cochlear implant recipients. Cochlear Implants Int. 2016, 17, 75–80. [Google Scholar] [CrossRef]
- Myles, A.J. The clinical use of Arthur Boothroyd (AB) word lists in Australia: Exploring evidence-based practice. Int. J. Audiol. 2017, 56, 870–875. [Google Scholar] [CrossRef]
- ANZ Hearing Health Collaborative. ANZ Hearing Health Collaborative (ANZ HHC): Living Guidelines for Cochlear Implant (CI) Referral, CI Evaluation and Candidacy, and CI Outcome Evaluation in Adults 2025. Available online: https://app.magicapp.org/#/guideline/10161 (accessed on 10 August 2025).
- Sucher, C.; Laird, E.; Hughe, S.; Elks, B.; Ferguson, M. Development of the LivCI: A patient-reported outcome measure of personal factors that affect quality of life, use and acceptance of cochlear implants. In Proceedings of the World Congress of Audiology, Paris, France, 19–22 September 2024. [Google Scholar]
- Bellucci, C.; Hughes, K.; Toomey, E.; Williamson, P.R.; Matvienko-Sikar, K. A survey of knowledge, perceptions and use of core outcome sets among clinical trialists. Trials 2021, 22, 937. [Google Scholar] [CrossRef]
- Blum, E.; Dy, C.J. Why Core Outcome Sets Do Not Get Used—And How Dissemination and Implementation Science can Help. J. Surg. Res. 2025, 314, 415–420. [Google Scholar] [CrossRef]

| Supra-Domain | ||||||
|---|---|---|---|---|---|---|
| Service | Clinical | Patient | ||||
| Domain Priority | CI Users | CI Professionals | CI Users | CI Professionals | CI Users | CI Professionals |
| First | Reliability of remote technology | Usability of remote technology | Speech recognition in noise | Device integrity and status | Participation restriction due to HL | Expectations of hearing health outcomes |
| Second | Usability of the remote technology | Accessibility of the remote service (for CI user) | Speech recognition in quiet | Speech discrimination | Hearing Related Quality of Life AND * Satisfaction with CI | Motivation and Readiness to Act on hearing difficulties |
| Third | Accessibility of the remote service (for CI user) | Reliability of remote technology | Speech discrimination | Device Use | Mental Health and Wellbeing | Acceptability and Tolerability of the CI (for CI user) |
| Number of Participants (%) | Median (Years) | Range (Years) | |
|---|---|---|---|
| Duration of Clinical Audiology Experience | 20 (100%) | 20.0 | 7–41 |
| Duration of CI-specific clinical Audiology Experience | 20 (100%) | 19.0 | 4–40 |
| Experience in Audiology-focused research | 12 (60%) | 7.5 | 0–40 |
| Experience in CI-specific research | 14 (70%) | 12 | 0–40 |
| Clinical Measure/PROM | Never Heard | Never Used | Occasionally Used | Regularly Used | Median Response |
|---|---|---|---|---|---|
| Speech and Spatial Qualities Scale (SSQ) [41] | 0 | 2 | 5 | 13 | Regularly Used |
| Bamford–Kowal–Bench Sentence Test, Australian Version (BKB/A) [42] | 0 | 1 | 6 | 10 | Regularly Used |
| City University of New York Sentence Test (CUNY©) [43] | 0 | 2 | 2 | 13 | Regularly Used |
| Consonant–Nucleus–Consonant Words (CNC Words) [44] | 0 | 1 | 0 | 16 | Regularly Used |
| Arthur Boothroyd Words (AB Words) [45] | 0 | 1 | 1 | 15 | Regularly Used |
| Short-Form Speech and Spatial Qualities Scale (SSQ-12) [46] | 4 | 1 | 1 | 14 | Regularly Used |
| Hearing Handicap Inventory for the Elderly (HHIE) [47] | 2 | 6 | 10 | 2 | Occasionally Used |
| Quick Speech In Noise Test (QuickSIN™) [48] | 2 | 4 | 9 | 2 | Occasionally Used |
| Bamford–Kowal–Bench Sentences In Noise Test (BKB-SIN™) [49] | 1 | 5 | 4 | 7 | Occasionally Used |
| Digits-In-Noise/Digit Triplet Test (DIN/DTT) [50] | 2 | 3 | 7 | 5 | Occasionally Used |
| Glasgow Hearing Aid Benefit Profile (GHABP) [51] | 0 | 9 | 8 | 3 | Occasionally Used |
| Abbreviated Profile of Hearing Aid Benefit (APHAB) [52] | 2 | 7 | 4 | 7 | Occasionally Used |
| Nijmegen Cochlear Implant Questionnaire (NCIQ) [53] | 4 | 6 | 9 | 1 | Never Used |
| Comprehensive Cochlear Implant Questionnaire (CCIQ) [54] | 8 | 5 | 7 | 0 | Never Used |
| General Anxiety Disorder-7 (GAD-7) [55] | 7 | 13 | 0 | 0 | Never Used |
| Hearing In Noise Test (HINT) [56] | 2 | 8 | 6 | 1 | Never Used |
| Revised Hearing Handicap for the Elderly (RHHI) [57] | 7 | 12 | 1 | 0 | Never Used |
| Revised Hearing Handicap for the Elderly—Screening (RHHI-S) [57] | 8 | 11 | 1 | 0 | Never Used |
| Austin Sentence Test (Austin) [58] | 3 | 6 | 4 | 4 | Never Used |
| AzBio Sentence Test (AzBio) [59] | 1 | 12 | 3 | 1 | Never Used |
| International Outcomes Inventory—Cochlear Implants (IOI-CI) [60] | 2 | 11 | 4 | 3 | Never Used |
| Hearing Aid Users Questionnaire (HAUQ) [61] | 9 | 8 | 2 | 1 | Never Used |
| Cochlear Implant Quality of Life Questionnaire—Global (CIQoL-Global) [30] | 9 | 4 | 7 | 0 | Never Used |
| Hearing Implant Sound Quality Index (HISQUI19) [62] | 9 | 7 | 3 | 1 | Never Used |
| IDA Tool—The Line (The Line) [63] | 8 | 8 | 2 | 2 | Never Used |
| Hearing Participation Scale (HPS) [64] | 8 | 11 | 1 | 0 | Never Used |
| Hearing Handicap Inventory for the Elderly—Screening (HHIE-S) [65] | 4 | 9 | 5 | 2 | Never Used |
| Geriatric Depression Scale—Long (GDS-L) [66] | 9 | 11 | 0 | 0 | Never Used |
| Cochlear Implant Quality of Life Questionnaire—Profile (CIQoL-Profile) [30] | 9 | 7 | 4 | 0 | Never Used |
| Hearing Device Satisfaction Scale (HDSS) [67] | 8 | 11 | 1 | 0 | Never Used |
| Beck’s Depression Index (BDI) [68] | 9 | 10 | 1 | 0 | Never Used |
| Depression Anxiety Stress Scale (21 Item) (DASS-21) [69] | 8 | 10 | 2 | 0 | Never Used |
| Depression Anxiety Stress Scale (42 Item) (DASS-42) [69] | 7 | 11 | 2 | 0 | Never Used |
| Hospital Anxiety and Depression Scale (HADS) [70] | 9 | 5 | 5 | 1 | Never Used |
| Bern Benefit in Single-Sided Deafness (BBSS) [71] | 10 | 9 | 1 | 0 | Never Heard |
| WHO Wellbeing Index (WHO-S) [72] | 10 | 10 | 0 | 0 | Never Heard |
| Expected Consequences of Hearing Aid Ownership (ECHO) [73] | 13 | 7 | 0 | 0 | Never Heard |
| Audio Processor Satisfaction Questionnaire (APSQ) [74] | 12 | 7 | 1 | 0 | Never Heard |
| De Jong Gierveld Loneliness scale (11 Item) (DJGLS-11) [75] | 15 | 5 | 0 | 0 | Never Heard |
| De Jong Gierveld Loneliness scale (6 Item) (DJGLS-6) [76] | 15 | 5 | 0 | 0 | Never Heard |
| The Four-Dimensional Symptom Questionnaire (4DSQ) [77] | 16 | 4 | 0 | 0 | Never Heard |
| Satisfaction With Life Scale (SWLS) [78] | 16 | 4 | 0 | 0 | Never Heard |
| UCLA Loneliness Index (Revised) (UCLA) [79] | 16 | 4 | 0 | 0 | Never Heard |
| Visit-Specific Satisfaction Questionnaire (VSQ-9) [80] | 19 | 1 | 0 | 0 | Never Heard |
| University of Rhode Island Change Assessment adapted for hearing loss (URICA-HL) [81] | 13 | 7 | 0 | 0 | Never Heard |
| Perceived Stress Questionnaire (PSQ) [82] | 12 | 8 | 0 | 0 | Never Heard |
| Social Participation Restrictions Questionnaire (SPaRQ) [83] | 12 | 8 | 0 | 0 | Never Heard |
| Short Assessment of Patient Satisfaction (SAPS) [84] | 14 | 5 | 0 | 1 | Never Heard |
| Satisfaction with Amplification in Daily Life (SADL) [85] | 11 | 9 | 0 | 0 | Never Heard |
| Net Promoter Score (NPS) [86] | 12 | 6 | 2 | 0 | Never Heard |
| Social Isolation Measure (SIM) [87] | 14 | 6 | 0 | 0 | Never Heard |
| CI Professionals | CI Users | |
|---|---|---|
| General | Assessment of all three supra-domains is important. | Assessment of all three supra-domains is important. Asynchronous remote assessments must consider the amount of time required for the user to complete.
|
| Service Supra-domain | Preference for simplicity of measurement
| Preference for simplicity of measurement
|
Technology for remote services should be accessible to everyone
| Technology for remote services should be accessible to everyone
| |
| Clinical Supra-domain | System checks are essential
| System checks are essential
|
Preference for a minimalist approach focusing on a few key outcome measures
| Preference for a minimalist approach focusing on a few key outcome measures
| |
Speech perception tests
| Speech perception tests
| |
Historical testing
| Historical testing
| |
Remote test environment
| ||
| Patient Supra-domain | PROMs (patient-reported outcome measures)
| PROMs (patient-reported outcome measures)
|
Mental Health and Wellbeing
| Subjective hearing disability
| |
Satisfaction with CI
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sucher, C.; Allen, D.; Laird, E.; Boisvert, I.; Ferguson, M. What to Measure? Development of a Core Outcome Set to Assess Remote Technologies for Cochlear Implant Users. J. Clin. Med. 2025, 14, 7697. https://doi.org/10.3390/jcm14217697
Sucher C, Allen D, Laird E, Boisvert I, Ferguson M. What to Measure? Development of a Core Outcome Set to Assess Remote Technologies for Cochlear Implant Users. Journal of Clinical Medicine. 2025; 14(21):7697. https://doi.org/10.3390/jcm14217697
Chicago/Turabian StyleSucher, Catherine, David Allen, Emma Laird, Isabelle Boisvert, and Melanie Ferguson. 2025. "What to Measure? Development of a Core Outcome Set to Assess Remote Technologies for Cochlear Implant Users" Journal of Clinical Medicine 14, no. 21: 7697. https://doi.org/10.3390/jcm14217697
APA StyleSucher, C., Allen, D., Laird, E., Boisvert, I., & Ferguson, M. (2025). What to Measure? Development of a Core Outcome Set to Assess Remote Technologies for Cochlear Implant Users. Journal of Clinical Medicine, 14(21), 7697. https://doi.org/10.3390/jcm14217697