Neuropsychological Assessments to Explore the Cognitive Impact of Cochlear Implants: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question and Added Value of This Study
2.2. Search Strategy and Eligibility Criteria
- Participants: Adult patients aged 50 or older with postlingual severe-to-profound HL who were CI users or met the criteria for implantation.
- Intervention: Multi-electrode implant.
- Comparators: No constrictions are imposed. However, in the case of inter-group analysis, CI users were compared with unaided HL patients, HA users, and NH.
- Outcomes: gain in cognitive performance assessed through longitudinal neuropsychological evaluations conducted before and/or after cochlear implantation. Additionally, differences in cognitive performance across groups, and potential correlations between cognitive function, speech intelligibility, and QoL.
- Timeframe: Studies published in the last 10 years (2015–2025).
2.3. Study and Variable Extraction
2.4. Assessment of Study Quality and Risk of Bias
2.5. Descriptive and Qualitative Analysis
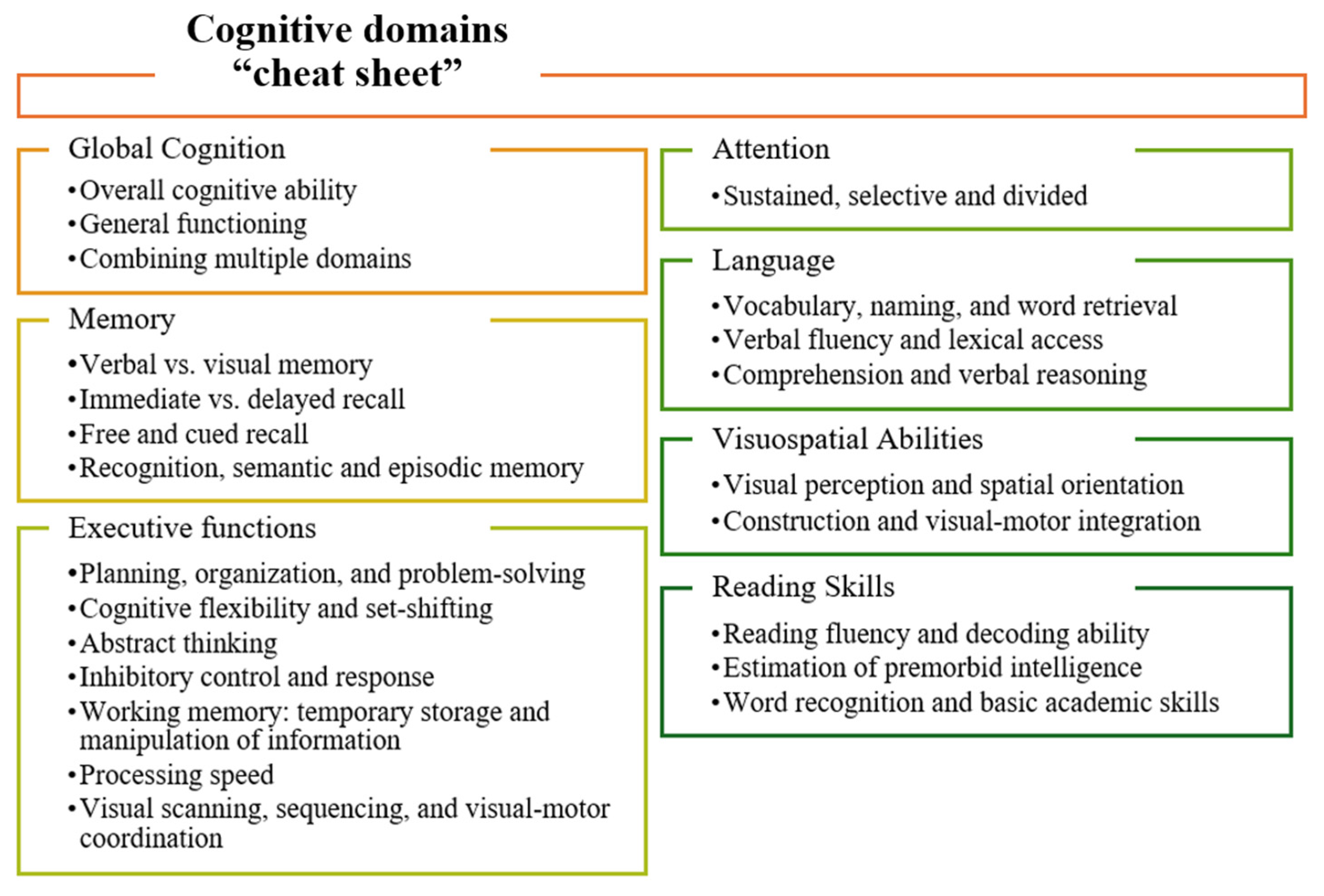
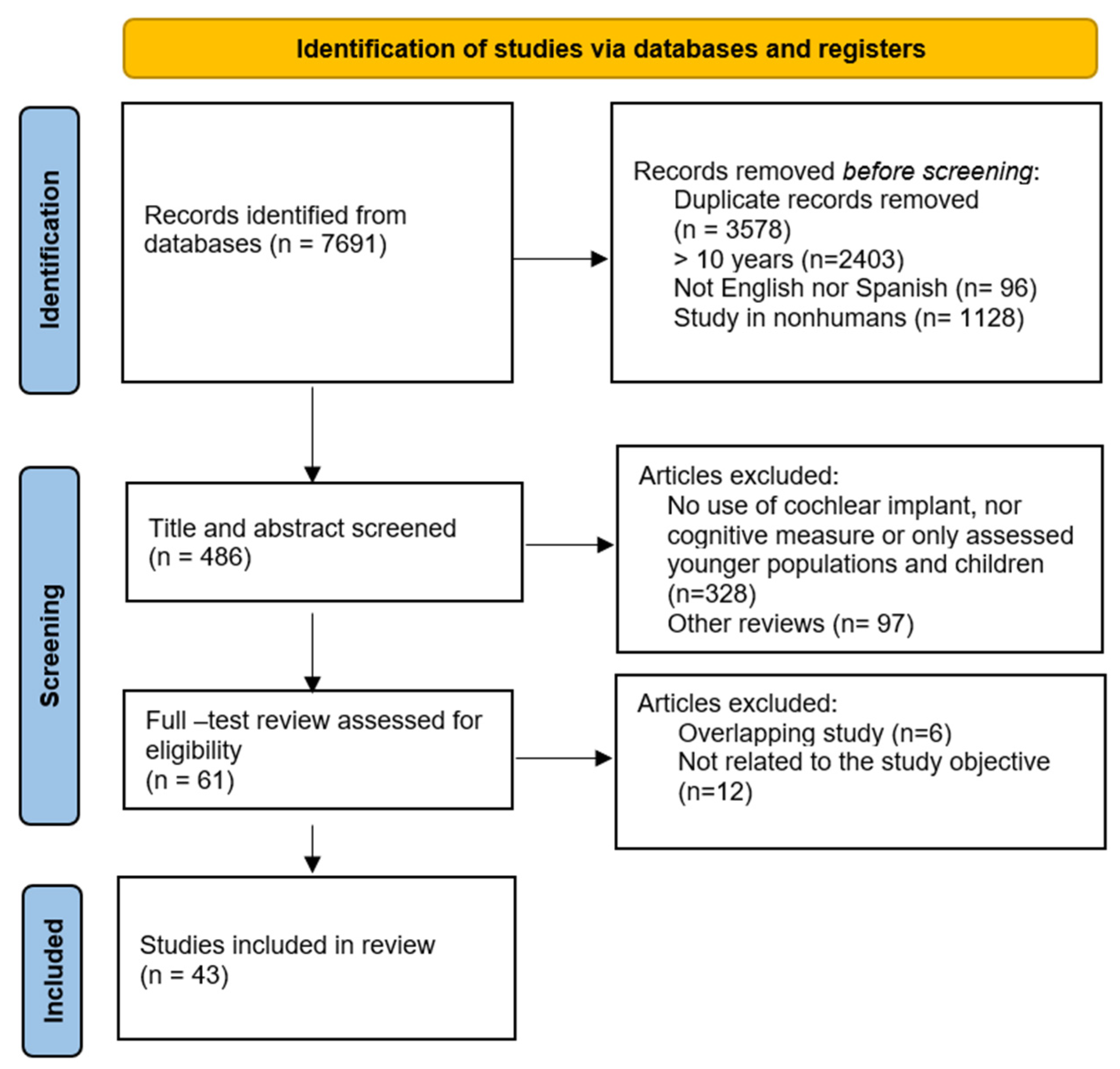
3. Results
| Test | N | Were There Significant Improvements After CI and Over Time? | Did They Correlate with Speech Intelligibility Outcomes? | Did They Correlate with QoL or Other Health Variables? | References |
|---|---|---|---|---|---|
| Global Cognition | |||||
| AlaCog | 60 | Yes (6-m)
Verbal fluency OSPAN | No | No | Völter et al., 2018 [56] |
| 71 | Yes (12-m)
| Yes
| No | Völter et al., 2021 [57] | |
| 71 | Yes (6-m)
| No | Yes (baseline)
| Völter et al., 2022 [58] | |
| 75 | Yes (24-m)
| No | No | Völter et al., 2023 [59] | |
| CANTAB | 23 | Yes (6-m)
| Yes
| N/A | Jayakody et al., 2017 [60] |
| CERAD | 29 | No | No | N/A | Huber et al., 2021 [61] |
| CODEX | 18 | Yes (12-m) | No | N/A | Ambert-Dahan et al., 2017 [62] |
| Cogstate Brief Battery | 59 | Yes (18-m)
| No | No | Sarant et al., 2019 [63] |
| 101 | Yes (54-m)
| No | No | Sarant et al., 2024 [64] | |
| MoCA | 15 | Yes (12-m) | Yes
| Yes GDS (β = −1.07, R2 =0.68) | Castiglione et al., 2016 [65] |
| 18 | Yes (12-m) | No | N/A | Ambert-Dahan et al., 2017 [62] | |
| 77 | Yes (6-m) | Yes
| N/A | Vasil et al., 2021 [66] | |
| MMSE | 93 | Yes (6-m) | No | No | Mosnier et al., 2015 [28] |
| 16 | No | No | N/A | Sonnet et al., 2017 [67] | |
| 70 | Yes (7-y) | No | N/A | Mosnier et al., 2018 [68] | |
| 25 | Yes (6-m) | Yes
| No | Anzivino et al., 2019 [69] | |
| 44 | No | Yes
| Yes
| Sorrentino et al., 2020 [70] | |
| 37 | No | No | No | Gurgel et al., 2022 [55] | |
| 53 | No | No | No | Herzog et al., 2022 [71] | |
| 21 | Yes (1-y) | N/A | Yes
| Ohta et al., 2022 [72] | |
| 21 | No | No | N/A | Zucca et al., 2022 [73] | |
| 98 | No | N/A | No | Mosnier et al., 2024 [74] | |
| 15 | No | No | N/A | Schauwecker et al., 2024 [75] | |
| 30 | No | No | N/A | Yoshida et al., 2025 [76] | |
| SAGE | 55 | No | N/A | N/A | Young et al., 2023 [39] |
| RBANS | 7 | Yes (3.7-y)
| Yes (2-y) WRS in quiet (List learning: ↑6.65% [2.19–11.12]) | N/A | Cosetti et al., 2016 [53] |
| 20 | Yes (12-m)
| No | No | Claes et al., 2018 [77] | |
| 24 | Yes (12-m)
| No | N/A | Mertens et al., 2021 [78] | |
| 63 | Yes (12-m)
| Yes (pre-op)
| Yes (pre-op)
| Calvino et al., 2022 [79] | |
| 21 | Yes (12-m)
| Yes SRS in noise (r = −0.48) | No | Andries et al., 2023 [80] | |
| 25 | Yes (1-year)
| No | N/A | Vandenbroeke et al., 2024 [81] | |
| WASI | 7 | Yes (3.7-year)
| Yes (2-year and 3-year) WRS in quiet (WASI-VIQ: ↑12.20% [6.14–18.26], ↑12.43% [5.68–19.17]; WASI-FSIQ: ↑10.68% [4.03–17.34], ↑11.60% [4.70–18.50]; Vocabulary: ↑10.22% [4.75–15.69], ↑9.85% [3.40–16.29]) | N/A | Cosetti et al., 2016 [53] |
| Memory | |||||
| 5-Word Test | 93 | Yes (6-m) | No | No | Mosnier et al., 2015 [28] |
| 16 | No | No | N/A | Sonnet et al., 2017 [67] | |
| 70 | No | No | N/A | Mosnier et al., 2018 [68] | |
| BVMT | 37 | Yes (12-m) | No | No | Gurgel et al., 2022 [55] |
| Hopkins Verbal Learning Test–Revised (HVLT-R) | 37 | Yes (12-m) | No | No | Gurgel et al., 2022 [55] |
| Rey Auditory Verbal Learning Test (RAVLT) | 25 | Yes (6-m) | Yes WRS in noise | No | Anzivino et al., 2019 [69] |
| 21 | No | No | N/A | Zucca et al., 2022 [73] | |
| Executive Function | |||||
| Arithmetic | 21 | No | No | No | Knopke et al., 2021 [82] |
| 33 | Yes (2-year) | No | No | Haeussler et al., 2023 [83] | |
| Digit Span | 25 | No | No | No | Anzivino et al., 2019 [69] |
| 37 | Yes (12-m) | No | No | Gurgel et al., 2022 [55] | |
| 21 | No | No | No | Knopke et al., 2021 [82] | |
| 21 | No | No | N/A | Zucca et al., 2022 [73] | |
| 33 | No | No | No | Haeussler et al., 2023 [83] | |
| 15 | No | Yes (1-m)
| N/A | Schauwecker et al., 2024 [75] | |
| Digit Symbol Coding | 21 | No | No | No | Knopke et al., 2021 [82] |
| 33 | No | No | No | Haeussler et al., 2023 [83] | |
| 98 | Yes (6-m) | No | No | Mosnier et al., 2015 [28] | |
| Hayling Sentence Completion Test | 37 | No | No | No | Gurgel et al., 2022 [55] |
| Raven’s Progressive Matrices | 19 | No | No | N/A | Zhan et al., 2020 [84] |
| Stroop Word Color Test | 29 | No | No | N/A | Huber et al., 2021 [61] |
| 25 | Yes (12-m) | No | No | Anzivino et al., 2019 [69] | |
| 37 | Yes (12-m) | No | No | Gurgel et al., 2022 [55] | |
| 19 | Yes (6-m)
| Yes
| N/A | Zhan et al., 2020 [84] | |
| 15 | No | No | N/A | Schauwecker et al., 2024 [75] | |
| Symbol Search | 21 | No | No | No | Knopke et al., 2021 [82] |
| 33 | Yes (12-m) | No | No | Haeussler et al., 2023 [83] | |
| Trail Making Test A&B (TMT) | 93 | Yes (6-m)
| No | No | Mosnier et al., 2015 [28] |
| 7 | No | No | N/A | Cosetti et al., 2016 [53] | |
| 16 | No | Yes (6-m; TMT B) WRS in quiet (r = −0.72) | N/A | Sonnet et al., 2017 [67] | |
| 70 | Yes (7-year) | No | No | Mosnier et al., 2018 [68] | |
| 25 | No | No | No | Anzivino et al., 2019 [69] | |
| 37 | No | No | No | Gurgel et al., 2022 [55] | |
| 21 | No | Yes (12-m) WRS in quiet (TMT A, R2 = 0.24, β = − 0.49) | N/A | Zucca et al., 2022 [73] | |
| 98 | No | No | No | Mosnier et al., 2024 [74] | |
| Timed up and go (TUG) | 98 | No | No | No | Mosnier et al., 2024 [74] |
| Visual symbol Span | 19 | Yes (6-m) | Yes
| N/A | Zhan et al., 2020 [84] |
| Visual digit Span | 19 | No | No | N/A | Zhan et al., 2020 [84] |
| Visual Object Span | 19 | No | No | N/A | Zhan et al., 2020 [84] |
| RCPM | 30 | No | Yes WRS in quiet (r = 0.367) | N/A | Yoshida et al., 2025 [76] |
| Attention | |||||
| d2 Test of Attention | 93 | Yes (6-m) speed (12-m) number of errors | No | No | Mosnier et al., 2015 [28] |
| 70 | Yes (7-year) | No | N/A | Mosnier et al., 2018 [68] | |
| 37 | Yes | No | No | Gurgel et al., 2022 [55] | |
| Multiple Features Target Cancelation | 25 | No | No | No | Anzivino et al., 2019 [69] |
| Language | |||||
| Boston Naming Test (BNT) | 7 | No | Yes (2-year and 3-year)
| N/A | Cosetti et al., 2016 [53] |
| Cardebat’s fluencies | 43 | Yes (3-m) | No | N/A | Baranger et al., 2023 [54] |
| Controlled Oral Word Association Tests | 7 | No | No | N/A | Cosetti et al., 2016 [53] |
| Test de Dénomination Orale d’images (DO80) | 16 | No | No | N/A | Sonnet et al., 2017 [67] |
| Phonemic and semantic fluency tasks | 93 | No | Yes
| No | Mosnier et al., 2015 [28] |
| 70 | Yes (7-year) | No | N/A | Mosnier et al., 2018 [68] | |
| 25 | No | No | No | Anzivino et al., 2019 [69] | |
| 21 | No | No | N/A | Zucca et al., 2022 [73] | |
| Visuospatial Abilities | |||||
| Clock Drawing Test (CDT) | 93 | Yes (12-m) | No | No | Mosnier et al., 2015 [28] |
| 29 | Yes (12-m) | Yes (3-m)
| N/A | Huber et al., 2021 [61] | |
| 70 | Yes (7-year) | No | N/A | Mosnier et al., 2018 [68] | |
| 21 | No | No | N/A | Zucca et al., 2022 [73] | |
| Corsi Block-tapping Test | 25 | No | No | No | Anzivino et al., 2019 [69] |
| 21 | No | No | N/A | Zucca et al., 2022 [73] | |
| Rey-Osterrieth Complex Figure Test | 16 | No | No | N/A | Sonnet et al., 2017 [67] |
| 25 | No | No | No | Anzivino et al., 2019 [69] | |
| Spatial span | 37 | No | No | No | Gurgel et al., 2022 [55] |
| Kohs block design | 30 | No | Yes WRS in quiet (r = 0.538) | N/A | Yoshida et al., 2025 [76] |
| Reading skills | |||||
| Test of Premorbid Functioning (TOPF) | 7 | No | Yes (2-year) WRS in quiet (↑11.91% [3.83–19.99]) | N/A | Cosetti et al., 2016 [53] |
| Test of Word Reading Efficiency (TOWRE) | 15 | No | No | N/A | Schauwecker et al., 2024 [75] |
| ReaCT Kyoto | 30 | No | No | N/A | Yoshida et al., 2025 [76] |
4. Discussion
4.1. Impact of Cochlear Implantation on Cognitive Function
4.1.1. Global Cognition
4.1.2. Memory
4.1.3. Executive Function
4.1.4. Attention
4.1.5. Language
4.1.6. Visuospatial Abilities
4.1.7. Reading Skills
4.2. Cognitive Differences Across Profile Groups
| Test | N | Were There Significant Differences in Cognitive Performance Across Groups? | Did They Correlate with Speech Intelligibility Outcomes? | Did They Correlate with QoL or Other Health Variables? | References |
|---|---|---|---|---|---|
| Global Cognition | |||||
| MoCA | 45 | Yes | No | No | Castiglione et al., 2016 [65] |
| NIH Toolbox Cognition Battery | 20 | No | Yes
| N/A | Schvartz-Leyzac et al., 2023 [91] |
| MMSE | 31 | N/A | No | No | Moberly et al., 2018 [30] |
| RBANS | 142 | Yes
| Yes
| Education (CI: r = 0.332) | Claes et al., 2018 [92] |
| 30 | N/A | Yes (Attention)
| No | Giallini et al., 2023 [93] | |
| Memory | |||||
| CVLT-II | 102 | Yes | No | No | Kramer et. al., 2018 [94] |
| Non-Verbal Learning Test (NVLT) | 61 | No | No | Yes
| Huber et al., 2023 [95] |
| Executive Function | |||||
| Auditory Stroop Task | 93 | Yes | No | N/A | Ceuleers et al., 2024 [96] |
| Categorization Working Memory Task (CWMT) | 30 | N/A | Yes
| No | Giallini et al., 2023 [93] |
| Digit Span | 31 | N/A | Yes
| No | Moberly et al., 2018 [30] |
| 30 | N/A | Yes (Forward)
| No | Giallini et al., 2023 [93] | |
| Go/No-Go Test | 61 | No | No | Yes
| Huber et al., 2023 [95] |
| Letter-Number Sequencing Task | 93 | Yes | No | N/A | Ceuleers et al., 2024 [96] |
| N-Back | 61 | No | No | No | Huber et al., 2023 [95] |
| Raven’s Progressive Matrices | 102 | Yes | No | Yes
| Kramer et. al., 2018 [94] |
| 31 | N/A | Yes
| No | Moberly et al., 2018 [30] | |
| 97 | Yes | Yes
| N/A | Moberly et al., 2025 [97] | |
| Stroop Word Color Test | 102 | No | No | No | Kramer et. al., 2018 [94] |
| 31 | N/A | No | No | Moberly et al., 2018 [30] | |
| 97 | No | Yes
| N/A | Moberly et al., 2025 [97] | |
| Trail Making Test A&B (TMT) | 17 | N/A | Yes
| No | Hua et al., 2017 [98] |
| 61 | No | No | Yes (B)
| Huber et al., 2023 [95] | |
| Visual symbol Span | 102 | Yes | No | No | Kramer et. al., 2018 [94] |
| Visual digit Span | 102 | No | No | Yes Socioeconomic status (r = 0.23) | Kramer et. al., 2018 [94] |
| 97 | Yes | No | N/A | Moberly et al., 2025 [97] | |
| Visual Object Span | 102 | No | No | No | Kramer et. al., 2018 [94] |
| Reading Span Test | 17 | N/A | Yes
| N/A | Hua et al., 2017 [98] |
| Attention | |||||
| Letter Detection Test | 93 | Yes | No | N/A | Ceuleers et al., 2024 [96] |
| Language | |||||
| Regensburg Word Test (RWT) | 61 | No | No | Yes (phonemic)
| Huber et al., 2023 [95] |
| WordFAM | 97 | Yes | Yes
| N/A | Moberly et al., 2025 [97] |
| Reading skills | |||||
| Test of Word Reading Efficiency (TOWRE) | 102 | Yes | No | Yes (Non-words and words)
| Kramer et. al., 2018 [94] |
| 31 | N/A | No | No | Moberly et al., 2018 [30] | |
| 97 | Yes | Yes CI users
| N/A | Moberly et al., 2025 [97] | |
| Wide Range Achievement Test (WRAT) | 31 | N/A | No | No | Moberly et al., 2018 [30] |
4.2.1. Global Cognition
4.2.2. Memory
4.2.3. Executive Function
4.2.4. Attention
4.2.5. Language
4.2.6. Reading Skills
4.3. Speech Perception Outcomes and Cognitive Influences
4.3.1. Global Cognition
4.3.2. Memory
4.3.3. Executive Function
4.3.4. Attention
4.3.5. Language
4.3.6. Visuospatial Abilities
4.3.7. Reading Skills
4.4. Factors Influencing Cognitive and Auditory Outcomes
4.4.1. Age
4.4.2. Education
4.4.3. Rehabilitation
4.4.4. Other Influencing Factors
4.5. Long-Term Cognitive Stability and Decline
4.6. The Impact of Cochlear Implants on Mood Disorders and Quality of Life
4.7. Limitations of This Study
4.8. Future Challenges and Directions
4.8.1. Interplay Between Hearing Loss and Dementia
4.8.2. Definition of Normal Cognition
4.8.3. Need for Standardized Cognitive Tests
4.8.4. Statistical Reporting Variability
4.8.5. Neurobiological and Computational Perspectives
4.9. Clinical Recommendations
- Apply adapted cognitive assessments: To minimize auditory bias, use cognitive tools adapted or validated for individuals with HL. For example: RBANS-H (Repeatable Battery for the Assessment of Neuropsychological Status- Hearing Impaired, MoCA-HI (MoCA for Hearing Impaired) [124], HI-ACE-III (Addenbrooke’s Cognitive Examination with auditory-free instructions) [125], and CANTAB (Cambridge Neuropsychological Test Automated Battery).
- Promote longitudinal follow-up: Monitor auditory and cognitive outcomes beyond the first year post-implantation to assess the durability of cognitive benefits and detect late-emerging changes.
- Encourage multidisciplinary/interdisciplinary programs: Teams including audiologists, neuropsychologists, engineers, nurses, researchers, and clinicians are essential in providing holistic care and integrated treatment planning.
- Develop and implement prevention programs: Identifying and addressing modifiable risk factors, such as vascular dysfunction, social isolation, and sensory decline, may help reduce the risk of dementia and improve QoL.
- Increase sample sizes and research collaboration: Multicenter studies with larger and more representative samples are needed to strengthen the reliability and generalizability of findings.
- Address cognitive impairment in candidate selection and rehabilitation: Given the high prevalence of cognitive impairment in older CI candidates, use adapted tests and rehabilitation tools to support personalized outcomes in these patients.
- Include auditory-cognitive training interventions: Incorporate auditory-cognitive training into rehabilitation programs to promote neuroplasticity and cognitive recovery.
- Set realistic expectations: Counsel patients and families about the expected auditory and cognitive benefits of CI, provide practical strategies for daily functioning, and emphasize that improvements in auditory input may help maintain or enhance cognitive abilities and personal autonomy over time.
- Screening for mood disorders: HL aggravates depression, anxiety, and loneliness, which may improve after CI rehabilitation. Mood disorders can impact both auditory and cognitive outcomes. Regular screening, psychoeducation, and mental health support can help prevent the negative interaction between mood disorders and HL.
- Use multimodal evaluation approaches: Combine behavioral, electrophysiological (e.g., CAEP), neuroimaging (MRI/fMRI), and genetic data to better understand the neural mechanisms relating HL and cognition.
- Adopt computational audiology tools: Employ artificial intelligence and data-driven methods to integrate multimodal datasets, and identify patients at risk of cognitive decline based on their hearing profile and other clinical variables.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Goman, A.M.; Lin, F.R. Prevalence of Hearing Loss by Severity in the United States. Am. J. Public. Health 2016, 106, 1820–1822. [Google Scholar] [CrossRef]
- Lin, F.R.; Thorpe, R.; Gordon-Salant, S.; Ferrucci, L. Hearing Loss Prevalence and Risk Factors among Older Adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 582–590. [Google Scholar] [CrossRef]
- Goodwin, M.V.; Hogervorst, E.; Hardy, R.; Stephan, B.C.M.; Maidment, D.W. How Are Hearing Loss and Physical Activity Related? Analysis from the English Longitudinal Study of Ageing. Prev. Med. 2023, 173, 107609. [Google Scholar] [CrossRef]
- Huang, A.R.; Reed, N.S.; Deal, J.A.; Arnold, M.; Burgard, S.; Chisolm, T.; Couper, D.; Glynn, N.W.; Gmelin, T.; Goman, A.M.; et al. Depression and Health-Related Quality of Life Among Older Adults with Hearing Loss in the ACHIEVE Study. J. Appl. Gerontol. 2024, 43, 550–561. [Google Scholar] [CrossRef]
- Maharani, A.; Pendleton, N.; Leroi, I. Hearing Impairment, Loneliness, Social Isolation, and Cognitive Function: Longitudinal Analysis Using English Longitudinal Study on Ageing. Am. J. Geriatr. Psychiatry 2019, 27, 1348–1356. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, C. Hearing Impairment and Cognitive Function: Mediating Role of Social Isolation and Depression. Am. J. Alzheimers Dis. Other Dement. 2024, 39, 15333175241227318. [Google Scholar] [CrossRef]
- Arjmandi, M.K.; Neils-Strunjas, J.; Nemati, S.; Fridriksson, J.; Newman-Norlund, S.; Newman-Norlund, R.; Bonilha, L. Age-Related Hearing Loss, Cognitive Decline, and Social Interaction: Testing a Framework. J. Speech Lang. Hear. Res. 2024, 67, 2743–2760. [Google Scholar] [CrossRef]
- WHO. Dementia; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.M.; An, Y.; Ferrucci, L.; Deal, J.A.; Lin, F.R.; Resnick, S.M. Temporal Sequence of Hearing Impairment and Cognition in the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Armstrong, N.M.; Agrawal, Y.; Gross, A.L.; Schrack, J.A.; Lin, F.R.; Ferrucci, L.; Resnick, S.M.; Deal, J.A.; Powell, D.S. Associations of Audiometric Hearing and Speech-in-Noise Performance with Cognitive Decline among Older Adults: The Baltimore Longitudinal Study of Aging (BLSA). Front. Neurol. 2022, 13, 1029851. [Google Scholar] [CrossRef]
- Boisvert, I.; Reis, M.; Au, A.; Cowan, R.; Dowell, R.C. Cochlear Implantation Outcomes in Adults: A Scoping Review. PLoS ONE 2020, 15, e0232421. [Google Scholar] [CrossRef]
- Harris, M.S.; Doerfer, K.; Moberly, A.C. Discussing Age-Related Hearing Loss and Cognitive Decline with Patients. JAMA Otolaryngol. Head. Neck Surg. 2019, 145, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Lindenberger, U.; Baltes, P.B. Sensory Functioning and Intelligence in Old Age: A Strong Connection. Psychol. Aging 1994, 9, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Wayne, R.V.; Johnsrude, I.S. A Review of Causal Mechanisms Underlying the Link between Age-Related Hearing Loss and Cognitive Decline. Ageing Res. Rev. 2015, 23, 154–166. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of Age-Related Hearing Loss with Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-Analysis. JAMA Otolaryngol.–Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Uchida, Y.; Sugiura, S.; Nishita, Y.; Saji, N.; Sone, M.; Ueda, H. Age-Related Hearing Loss and Cognitive Decline—The Potential Mechanisms Linking the Two. Auris Nasus Larynx 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Dhanda, N.; Hall, A.; Martin, J. Does Social Isolation Mediate the Association between Hearing Loss and Cognition in Adults? A Systematic Review and Meta-Analysis of Longitudinal Studies. Front. Public. Health 2024, 12, 1347794. [Google Scholar] [CrossRef]
- Contrera, K.J.; Sung, Y.K.; Betz, J.; Li, L.; Lin, F.R. Change in Loneliness after Intervention with Cochlear Implants or Hearing Aids. Laryngoscope 2017, 127, 1885–1889. [Google Scholar] [CrossRef]
- Hörnsten, C.; Lövheim, H.; Nordström, P.; Gustafson, Y. The Prevalence of Stroke and Depression and Factors Associated with Depression in Elderly People with and without Stroke. BMC Geriatr. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Babajanian, E.E.; Gurgel, R.K. Cognitive and Behavioral Effects of Hearing Loss. Curr. Opin. Otolaryngol. Head. Neck Surg. 2022, 30, 339–343. [Google Scholar] [CrossRef]
- Powell, D.S.; Oh, E.S.; Reed, N.S.; Lin, F.R.; Deal, J.A. Hearing Loss and Cognition: What We Know and Where We Need to Go. Front. Aging Neurosci. 2022, 13, 769405. [Google Scholar] [CrossRef]
- Deal, J.A.; Goman, A.M.; Albert, M.S.; Arnold, M.L.; Burgard, S.; Chisolm, T.; Couper, D.; Glynn, N.W.; Gmelin, T.; Hayden, K.M.; et al. Hearing Treatment for Reducing Cognitive Decline: Design and Methods of the Aging and Cognitive Health Evaluation in Elders Randomized Controlled Trial. Alzheimers Dement. 2018, 4, 499–507. [Google Scholar] [CrossRef]
- Azeem, A.; Julleekeea, A.; Knight, B.; Sohail, I.; Bruyns-Haylett, M.; Sastre, M. Hearing Loss and Its Link to Cognitive Impairment and Dementia. Front. Dement. 2023, 2, 1199319. [Google Scholar] [CrossRef]
- Naples, J.G.; Ruckenstein, M.J. Cochlear Implant. Otolaryngol. Clin. N. Am. 2020, 53, 87–102. [Google Scholar] [CrossRef]
- Mosnier, I.; Bebear, J.-P.; Marx, M.; Fraysse, B.; Truy, E.; Lina-Granade, G.; Mondain, M.; Sterkers-Artières, F.; Bordure, P.; Robier, A.; et al. Improvement of Cognitive Function after Cochlear Implantation in Elderly Patients. JAMA Otolaryngol. Head. Neck Surg. 2015, 141, 442–450. [Google Scholar] [CrossRef]
- An, S.; Jo, E.; Jun, S.B.; Sung, J.E. Effects of Cochlear Implantation on Cognitive Decline in Older Adults: A Systematic Review and Meta-Analysis. Heliyon 2023, 9, e19703. [Google Scholar] [CrossRef] [PubMed]
- Moberly, A.C.; Castellanos, I.; Mattingly, J.K. Neurocognitive Factors Contributing to Cochlear Implant Candidacy. Otol. Neurotol. 2018, 39, e1010–e1018. [Google Scholar] [CrossRef] [PubMed]
- Pichora-Fuller, M.K.; Singh, G. Effects of Age on Auditory and Cognitive Processing: Implications for Hearing Aid Fitting and Audiologic Rehabilitation. Trends Amplif. 2006, 10, 29–59. [Google Scholar] [CrossRef]
- Blamey, P.; Artieres, F.; Başkent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors Affecting Auditory Performance of Postlinguistically Deaf Adults Using Cochlear Implants: An Update with 2251 Patients. Audiol. Neurootol. 2013, 18, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhoof, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors Affecting Open-Set Word Recognition in Adults with Cochlear Implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef]
- Goudey, B.; Plant, K.; Kiral, I.; Jimeno-Yepes, A.; Swan, A.; Gambhir, M.; Büchner, A.; Kludt, E.; Eikelboom, R.H.; Sucher, C.; et al. A MultiCenter Analysis of Factors Associated with Hearing Outcome for 2,735 Adults with Cochlear Implants. Trends Hear. 2021, 25, 23312165211037525. [Google Scholar] [CrossRef]
- Yeo, B.S.Y.; Song, H.J.J.M.D.; Toh, E.M.S.; Ng, L.S.; Ho, C.S.H.; Ho, R.; Merchant, R.A.; Tan, B.K.J.; Loh, W.S. Association of Hearing Aids and Cochlear Implants with Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. JAMA Neurol. 2023, 80, 134–141. [Google Scholar] [CrossRef]
- Pisoni, D.B.; Kronenberger, W.G.; Harris, M.S.; Moberly, A.C. Three Challenges for Future Research on Cochlear Implants. World J. Otorhinolaryngol.-Head. Neck Surg. 2017, 3, 240–254. [Google Scholar] [CrossRef]
- Raymond, M.J.; Ma, C.; Schvartz-Leyzac, K.C.; Camposeo, E.L.; Nguyen, S.A.; Meyer, T.A.; McRackan, T.R. Association of Cognitive Impairment Screening Scores with Improvements in Speech Recognition and Quality of Life After Cochlear Implantation. JAMA Otolaryngol.-Head Neck Surg. 2023, 149, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Moberly, A.C.; Pisoni, D.B.; Tamati, T.N. Audiovisual Processing Skills Before Cochlear Implantation Predict Post-Operative Speech Recognition in Adults. Ear Hear. 2024, 45, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Fechtner, L.; Kim, C.; Nayak, N.; Kellermeyer, B.; Ortega, C.; Rende, S.; Rosenberg, S.; Wazen, J. Long-Term Cognition and Speech Recognition Outcomes after Cochlear Implantation in the Elderly. Am. J. Otolaryngol. 2023, 45, 104071. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.E.; Naples, J.G.; Hwa, T.; Larrow, D.C.; Campbell, F.M.; Qiu, M.; Castellanos, I.; Moberly, A.C. Emerging Relations among Cognitive Constructs and Cochlear Implant Outcomes: A Systematic Review and Meta-Analysis. Otolaryngol.–Head. Neck Surg. 2023, 169, 792–810. [Google Scholar] [CrossRef]
- Schroeder, R.W.; Martin, P.K.; Walling, A. Neuropsychological Evaluations in Adults. afp 2019, 99, 101–108. [Google Scholar]
- Dawes, P.; Cross, H.; Millman, R.; Leroi, I.; Voelter, C. Do People with Cognitive Impairment Benefit from Cochlear Implants? A Scoping Review. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 4565–4573. [Google Scholar] [CrossRef]
- Patro, A.; Lawrence, P.J.; Tamati, T.N.; Ning, X.; Moberly, A.C. Using Machine Learning and Multifaceted Preoperative Measures to Predict Adult Cochlear Implant Outcomes: A Prospective Pilot Study. Ear Hear. 2024, 46, 543–549. [Google Scholar] [CrossRef]
- Zhang, Y.; Callejon-Leblic, M.A.; Picazo-Reina, A.M.; Blanco-Trejo, S.; Patou, F.; Sanchez-Gomez, S. Impact of SNR, Peripheral Auditory Sensitivity, and Central Cognitive Profile on the Psychometric Relation between Pupillary Response and Speech Performance in CI Users. Front. Neurosci. 2023, 17, 1307777. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Claes, A.J.; Van de Heyning, P.; Gilles, A.; Van Rompaey, V.; Mertens, G. Cognitive Outcomes after Cochlear Implantation in Older Adults: A Systematic Review. Cochlear Implant. Int. 2018, 19, 239–254. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; Goddard, O.; et al. OCEBM Levels of Evidence. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 7 April 2025).
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Volume 2, pp. 1–12. [Google Scholar]
- Beckers, L.; Tromp, N.; Philips, B.; Mylanus, E.; Huinck, W. Exploring Neurocognitive Factors and Brain Activation in Adult Cochlear Implant Recipients Associated with Speech Perception Outcomes-A Scoping Review. Front. Neurosci. 2023, 17, 1046669. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying Neurocognitive Disorders: The DSM-5 Approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Cosetti, M.K.; Pinkston, J.B.; Flores, J.M.; Friedmann, D.R.; Jones, C.B.; Roland, J.T.; Waltzman, S.B. Neurocognitive Testing and Cochlear Implantation: Insights into Performance in Older Adults. Clin. Interv. Aging 2016, 11, 603–613. [Google Scholar] [CrossRef]
- Baranger, M.; Manera, V.; Sérignac, C.; Derreumaux, A.; Cancian, E.; Vandersteen, C.; Gros, A.; Guevara, N. Evaluation of the Cognitive Function of Adults with Severe Hearing Loss Pre- and Post-Cochlear Implantation Using Verbal Fluency Testing. J. Clin. Med. 2023, 12, 3792. [Google Scholar] [CrossRef]
- Gurgel, R.K.; Duff, K.; Foster, N.L.; Urano, K.A.; deTorres, A. Evaluating the Impact of Cochlear Implantation on Cognitive Function in Older Adults. Laryngoscope 2022, 132, S1–S15. [Google Scholar] [CrossRef]
- Völter, C.; Götze, L.; Dazert, S.; Falkenstein, M.; Thomas, J.P. Can Cochlear Implantation Improve Neurocognition in the Aging Population? Clin. Interv. Aging 2018, 13, 701–712. [Google Scholar] [CrossRef]
- Völter, C.; Götze, L.; Haubitz, I.; Müther, J.; Dazert, S.; Thomas, J.P. Impact of Cochlear Implantation on Neurocognitive Subdomains in Adult Cochlear Implant Recipients. Audiol. Neurootol. 2021, 26, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Völter, C.; Götze, L.; Bajewski, M.; Dazert, S.; Thomas, J.P. Cognition and Cognitive Reserve in Cochlear Implant Recipients. Front. Aging Neurosci. 2022, 14, 838214. [Google Scholar] [CrossRef] [PubMed]
- Völter, C.; Goetze, L.; Dazert, S.; Thomas, J.P.; Kamin, S.T. Longitudinal Trajectories of Memory among Middle-Aged and Older People with Hearing Loss: The Influence of Cochlear Implant Use on Cognitive Functioning. Front. Aging Neurosci. 2023, 15, 1220184. [Google Scholar] [CrossRef]
- Jayakody, D.M.P.; Peter, L.F.; Nel, E.; Martins, R.N.; Atlas, M.D.; Sohrabi, H.R. Impact of Cochlear Implantation on Cognitive Functions of Older Adults: Pilot Test Results. Otol. Neurotol. 2017, 38, e289–e295. [Google Scholar] [CrossRef]
- Huber, M.; Roesch, S.; Pletzer, B.; Lukaschyk, J.; Lesinski-Schiedat, A.; Illg, A. Can Cochlear Implantation in Older Adults Reverse Cognitive Decline Due to Hearing Loss? Ear Hear. 2021, 42, 1560–1576. [Google Scholar] [CrossRef]
- Ambert-Dahan, E.; Routier, S.; Marot, L.; Bouccara, D.; Sterkers, O.; Ferrary, E.; Mosnier, I. Cognitive Evaluation of Cochlear Implanted Adults Using CODEX and MoCA Screening Tests. Otol. Neurotol. 2017, 38, e282–e284. [Google Scholar] [CrossRef]
- Sarant, J.; Harris, D.; Busby, P.; Maruff, P.; Schembri, A.; Dowell, R.; Briggs, R. The Effect of Cochlear Implants on Cognitive Function in Older Adults: Initial Baseline and 18-Month Follow Up Results for a Prospective International Longitudinal Study. Front. Neurosci. 2019, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Sarant, J.Z.; Busby, P.A.; Schembri, A.J.; Briggs, R.J.S.; Masters, C.L.; Harris, D.C. COCHLEA: Longitudinal Cognitive Performance of Older Adults with Hearing Loss and Cochlear Implants at 4.5-Year Follow-Up. Brain Sci. 2024, 14, 1279. [Google Scholar] [CrossRef]
- Castiglione, A.; Benatti, A.; Velardita, C.; Favaro, D.; Padoan, E.; Severi, D.; Pagliaro, M.; Bovo, R.; Vallesi, A.; Gabelli, C.; et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiol. Neurootol. 2016, 21 (Suppl. S1), 21–28. [Google Scholar] [CrossRef]
- Vasil, K.J.; Ray, C.; Lewis, J.; Stefancin, E.; Tamati, T.N.; Moberly, A.C. How Does Cochlear Implantation Lead to Improvements on a Cognitive Screening Measure? J. Speech Lang. Hear. Res. 2021, 64, 1053–1061. [Google Scholar] [CrossRef]
- Sonnet, M.-H.; Montaut-Verient, B.; Niemier, J.-Y.; Hoen, M.; Ribeyre, L.; Parietti-Winkler, C. Cognitive Abilities and Quality of Life After Cochlear Implantation in the Elderly. Otol. Neurotol. 2017, 38, e296–e301. [Google Scholar] [CrossRef]
- Mosnier, I.; Vanier, A.; Bonnard, D.; Lina-Granade, G.; Truy, E.; Bordure, P.; Godey, B.; Marx, M.; Lescanne, E.; Venail, F.; et al. Long-Term Cognitive Prognosis of Profoundly Deaf Older Adults After Hearing Rehabilitation Using Cochlear Implants. J. Am. Geriatr. Soc. 2018, 66, 1553–1561. [Google Scholar] [CrossRef]
- Anzivino, R.; Conti, G.; Di Nardo, W.; Fetoni, A.R.; Picciotti, P.M.; Marra, C.; Guglielmi, V.; Fortunato, S.; Forli, F.; Paludetti, G.; et al. Prospective Evaluation of Cognitive Functions After Rehabilitation with Cochlear Implant or Hearing Aids: Preliminary Results of a Multicentric Study on Elderly Patients. Am. J. Audiol. 2019, 28, 762–774. [Google Scholar] [CrossRef]
- Sorrentino, T.; Donati, G.; Nassif, N.; Pasini, S.; Redaelli de Zinis, L.O. Cognitive Function and Quality of Life in Older Adult Patients with Cochlear Implants. Int. J. Audiol. 2020, 59, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Herzog, J.A.; Buchman, C.A.; Kallogjeri, D.; Chen, S.; Wick, C.; Durakovic, N.; Shew, M.A. Cognitive Assessment in Elderly Cochlear Implant Recipients: Long-Term Analysis. Laryngoscope 2022, 133, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Imai, T.; Maekawa, Y.; Morihana, T.; Osaki, Y.; Sato, T.; Okazaki, S.; Hanamoto, M.; Suwa, K.; Takeya, Y.; et al. The Effect of Cochlear Implants on Cognitive Function in Older Adults: A Prospective, Longitudinal 2-Year Follow-up Study. Auris Nasus Larynx 2022, 49, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Zucca, M.; Albera, A.; Albera, R.; Montuschi, C.; Della Gatta, B.; Canale, A.; Rainero, I. Cochlear Implant Results in Older Adults with Post-Lingual Deafness: The Role of “Top-Down” Neurocognitive Mechanisms. Int. J. Environ. Res. Public. Health 2022, 19, 1343. [Google Scholar] [CrossRef]
- Mosnier, I.; Belmin, J.; Cuda, D.; Manrique Huarte, R.; Marx, M.; Ramos Macias, A.; Khnifes, R.; Hilly, O.; Bovo, R.; James, C.J.; et al. Cognitive Processing Speed Improvement after Cochlear Implantation. Front. Aging Neurosci. 2024, 16, 1444330. [Google Scholar] [CrossRef]
- Schauwecker, N.; Tamati, T.N.; Moberly, A.C. Predicting Early Cochlear Implant Performance: Can Cognitive Testing Help? Otol. Neurotol. Open 2024, 4, e050. [Google Scholar] [CrossRef]
- Yoshida, T.; Kobayashi, M.; Hara, D.; Taniguchi, R.; Fukunaga, Y.; Sone, M. Cognitive Function and Speech Outcomes after Cochlear Implantation in Older Adults. Front. Neurol. 2025, 16, 1630946. [Google Scholar] [CrossRef]
- Claes, A.J.; Van de Heyning, P.; Gilles, A.; Van Rompaey, V.; Mertens, G. Cognitive Performance of Severely Hearing-Impaired Older Adults Before and After Cochlear Implantation: Preliminary Results of a Prospective, Longitudinal Cohort Study Using the RBANS-H. Otol. Neurotol. 2018, 39, e765–e773. [Google Scholar] [CrossRef]
- Mertens, G.; Andries, E.; Claes, A.J.; Topsakal, V.; Van de Heyning, P.; Van Rompaey, V.; Calvino, M.; Cuadrado, I.S.; Muñoz, E.; Gavilán, J.; et al. Cognitive Improvement after Cochlear Implantation in Older Adults with Severe or Profound Hearing Impairment: A Prospective, Longitudinal, Controlled, Multicenter Study. Ear Hear. 2021, 42, 606. [Google Scholar] [CrossRef]
- Calvino, M.; Sánchez-Cuadrado, I.; Gavilán, J.; Lassaletta, L. The Effect of Risk Factors on Cognition in Adult Cochlear Implant Candidates with Severe to Profound Hearing Loss. Front. Psychol. 2022, 13, 837366. [Google Scholar] [CrossRef]
- Andries, E.; Bosmans, J.; Engelborghs, S.; Cras, P.; Vanderveken, O.M.; Lammers, M.J.W.; van de Heyning, P.H.; Van Rompaey, V.; Mertens, G. Evaluation of Cognitive Functioning Before and After Cochlear Implantation in Adults Aged 55 Years and Older at Risk for Mild Cognitive Impairment. JAMA Otolaryngol.-Head Neck Surg. 2023, 149, 310–316. [Google Scholar] [CrossRef]
- Vandenbroeke, T.; Andries, E.; Lammers, M.J.; Van de Heyning, P.; Hofkens-Van den Brandt, A.; Vanderveken, O.; Van Rompaey, V.; Mertens, G. Cognitive Changes Up to 4 Years After Cochlear Implantation in Older Adults: A Prospective Longitudinal Study Using the RBANS-H. Ear Hear. 2024, 46, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Knopke, S.; Schubert, A.; Häussler, S.M.; Gräbel, S.; Szczepek, A.J.; Olze, H. Improvement of Working Memory and Processing Speed in Patients over 70 with Bilateral Hearing Impairment Following Unilateral Cochlear Implantation. J. Clin. Med. 2021, 10, 3421. [Google Scholar] [CrossRef] [PubMed]
- Haeussler, S.M.; Stankow, E.; Knopke, S.; Szczepek, A.J.; Olze, H. Sustained Cognitive Improvement in Patients over 65 Two Years after Cochlear Implantation. Brain Sci. 2023, 13, 1673. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.Y.; Lewis, J.H.; Vasil, K.J.; Tamati, T.N.; Harris, M.S.; Pisoni, D.B.; Kronenberger, W.G.; Ray, C.; Moberly, A.C. Cognitive Functions in Adults Receiving Cochlear Implants: Predictors of Speech Recognition and Changes After Implantation. Otol. Neurotol. 2020, 41, e322–e329. [Google Scholar] [CrossRef]
- Kral, A.; Sharma, A. Crossmodal Plasticity in Hearing Loss. Trends Neurosci. 2023, 46, 377–393. [Google Scholar] [CrossRef]
- Fliegelman, J. What Role Does Age-Associated Neuroplasticity Play in the Efficacy of Cochlear Implantation? Sci. J. Lander Coll. Arts Sci. 2020, 14, 14–21. [Google Scholar]
- Han, J.-H.; Lee, H.-J.; Kang, H.; Oh, S.-H.; Lee, D.S. Brain Plasticity Can Predict the Cochlear Implant Outcome in Adult-Onset Deafness. Front. Hum. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef]
- Shinn-Cunningham, B.G.; Best, V. Selective Attention in Normal and Impaired Hearing. Trends Amplif. 2008, 12, 283–299. [Google Scholar] [CrossRef]
- King, J.B.; Jones, K.G.; Goldberg, E.; Rollins, M.; MacNamee, K.; Moffit, C.; Naidu, S.R.; Ferguson, M.A.; Garcia-Leavitt, E.; Amaro, J.; et al. Increased Functional Connectivity After Listening to Favored Music in Adults with Alzheimer Dementia. J. Prev. Alzheimer’s Dis. 2019, 6, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Mishkin, M. A New Neural Framework for Visuospatial Processing. Nat. Rev. Neurosci. 2011, 12, 217–230. [Google Scholar] [CrossRef]
- Schvartz-Leyzac, K.C.; Giordani, B.; Pfingst, B.E. Association of Aging and Cognition with Complex Speech Understanding in Cochlear-Implanted Adults Use of a Modified National Institutes of Health (NIH) Toolbox Cognitive Assessment. JAMA Otolaryngol.-Head Neck Surg. 2023, 149, 239–246. [Google Scholar] [CrossRef]
- Claes, A.J.; Van de Heyning, P.; Gilles, A.; Hofkens-Van den Brandt, A.; Van Rompaey, V.; Mertens, G. Impaired Cognitive Functioning in Cochlear Implant Recipients Over the Age of 55 Years: A Cross-Sectional Study Using the Repeatable Battery for the Assessment of Neuropsychological Status for Hearing-Impaired Individuals (RBANS-H). Front. Neurosci. 2018, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Giallini, I.; Inguscio, B.M.S.; Nicastri, M.; Portanova, G.; Ciofalo, A.; Pace, A.; Greco, A.; D’Alessandro, H.D.; Mancini, P. Neuropsychological Functions and Audiological Findings in Elderly Cochlear Implant Users: The Role of Attention in Postoperative Performance. Audiol. Res. 2023, 13, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; Vasil, K.J.; Adunka, O.F.; Pisoni, D.B.; Moberly, A.C. Cognitive Functions in Adult Cochlear Implant Users, Cochlear Implant Candidates, and Normal-Hearing Listeners. Laryngoscope Investig. Otolaryngol. 2018, 3, 304–310. [Google Scholar] [CrossRef]
- Huber, M.; Reuter, L.; Weitgasser, L.; Pletzer, B.; Rösch, S.; Illg, A. Hearing Loss, Depression, and Cognition in Younger and Older Adult CI Candidates. Front. Neurol. 2023, 14, 1272210. [Google Scholar] [CrossRef]
- Ceuleers, D.; Keppler, H.; Degeest, S.; Baudonck, N.; Swinnen, F.; Kestens, K.; Dhooge, I. Auditory, Visual, and Cognitive Abilities in Normal-Hearing Adults, Hearing Aid Users, and Cochlear Implant Users. EAR Hear. 2024, 45, 679–694. [Google Scholar] [CrossRef]
- Moberly, A.C.; Du, L.; Tamati, T.N. Individual Differences in the Recognition of Spectrally Degraded Speech: Associations with Neurocognitive Functions in Adult Cochlear Implant Users and with Noise-Vocoded Simulations. Trends Hear. 2025, 29, 23312165241312449. [Google Scholar] [CrossRef]
- Hua, H.; Johansson, B.; Magnusson, L.; Lyxell, B.; Ellis, R.J. Speech Recognition and Cognitive Skills in Bimodal Cochlear Implant Users. J. Speech Lang. Hear. Res. 2017, 60, 2752–2763. [Google Scholar] [CrossRef]
- Pronk, M.; Lissenberg-Witte, B.I.; van der Aa, H.P.A.; Comijs, H.C.; Smits, C.; Lemke, U.; Zekveld, A.A.; Kramer, S.E. Longitudinal Relationships Between Decline in Speech-in-Noise Recognition Ability and Cognitive Functioning: The Longitudinal Aging Study Amsterdam. J. Speech Lang. Hear. Res. 2019, 62, 1167–1187. [Google Scholar] [CrossRef]
- Humes, L.E. Factors Underlying Individual Differences in Speech-Recognition Threshold (SRT) in Noise Among Older Adults. Front. Aging Neurosci. 2021, 13, 702739. [Google Scholar] [CrossRef]
- Fulton, S.E.; Lister, J.J.; Bush, A.L.H.; Edwards, J.D.; Andel, R. Mechanisms of the Hearing–Cognition Relationship. Semin. Hear. 2015, 36, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Rosemann, S.; Gießing, C.; Özyurt, J.; Carroll, R.; Puschmann, S.; Thiel, C.M. The Contribution of Cognitive Factors to Individual Differences in Understanding Noise-Vocoded Speech in Young and Older Adults. Front. Hum. Neurosci. 2017, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Lövdén, M.; Fratiglioni, L.; Glymour, M.M.; Lindenberger, U.; Tucker-Drob, E.M. Education and Cognitive Functioning Across the Life Span. Psychol. Sci. Public. Interest. 2020, 21, 6–41. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive Reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Schaie, K.W. What Can We Learn from Longitudinal Studies of Adult Development? Res. Human. Dev. 2005, 2, 133–158. [Google Scholar] [CrossRef]
- Lin, F.R.; Pike, J.R.; Albert, M.S.; Arnold, M.; Burgard, S.; Chisolm, T.; Couper, D.; Deal, J.A.; Goman, A.M.; Glynn, N.W.; et al. Hearing Intervention versus Health Education Control to Reduce Cognitive Decline in Older Adults with Hearing Loss in the USA (ACHIEVE): A Multicentre, Randomised Controlled Trial. Lancet 2023, 402, 786–797. [Google Scholar] [CrossRef]
- Ciorba, A.; Bianchini, C.; Pelucchi, S.; Pastore, A. The Impact of Hearing Loss on the Quality of Life of Elderly Adults. Clin. Interv. Aging 2012, 7, 159–163. [Google Scholar] [CrossRef]
- Sung, Y.; Li, L.; Blake, C.; Betz, J.; Lin, F.R. Association of Hearing Loss and Loneliness in Older Adults. J. Aging Health 2016, 28, 979–994. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, S.H.; Yeo, S.G. Association of Nutritional Factors with Hearing Loss. Nutrients 2019, 11, 307. [Google Scholar] [CrossRef]
- Myrstad, C.; Engdahl, B.L.; Costafreda, S.G.; Krokstad, S.; Lin, F.; Livingston, G.; Strand, B.H.; Ohre, B.; Selbaek, G. Hearing Impairment and Risk of Dementia in The HUNT Study (HUNT4 70+): A Norwegian Cohort Study. eClinicalMedicine 2023, 66, 102319. [Google Scholar] [CrossRef]
- Gallacher, J.; Ilubaera, V.; Ben-Shlomo, Y.; Bayer, A.; Fish, M.; Babisch, W.; Elwood, P. Auditory Threshold, Phonologic Demand, and Incident Dementia. Neurology 2012, 79, 1583–1590. [Google Scholar] [CrossRef]
- Bekele Okuba, T.; Lystad, R.P.; Boisvert, I.; McMaugh, A.; Moore, R.C.; Walsan, R.; Mitchell, R.J. Cochlear Implantation Impact on Health Service Utilisation and Social Outcomes: A Systematic Review. BMC Health Serv. Res. 2023, 23, 929. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, K.; Pichora-Fuller, M.K.; Chasteen, A.L.; Marchuk, V.; Singh, G.; Smith, S.L. Effects of Hearing and Vision Impairments on the Montreal Cognitive Assessment. Aging Neuropsychol. Cogn. 2015, 22, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Wasmann, J.-W.A.; Lanting, C.P.; Huinck, W.J.; Mylanus, E.A.M.; van der Laak, J.W.M.; Govaerts, P.J.; Swanepoel, D.W.; Moore, D.R.; Barbour, D.L. Computational Audiology: New Approaches to Advance Hearing Health Care in the Digital Age. Ear Hear. 2021, 42, 1499–1507. [Google Scholar] [CrossRef]
- Callejón-Leblic, M.A.; Blanco-Trejo, S.; Villarreal-Garza, B.; Picazo-Reina, A.M.; Tena-García, B.; Lara-Delgado, A.; Lazo-Maestre, M.; López-Benítez, F.; Escobar-Reyero, F.; Álvarez-Cendrero, M.; et al. A Multimodal Database for the Collection of Interdisciplinary Audiological Research Data in Spain. Auditio 2024, 8, e109. [Google Scholar] [CrossRef]
- Gebru, T.; Morgenstern, J.; Vecchione, B.; Vaughan, J.W.; Wallach, H.; III, H.D.; Crawford, K. Datasheets for Datasets. Commun. ACM 2021, 64, 86–92. [Google Scholar] [CrossRef]
- Fitzhugh, M.C.; Pa, J. Exploring Gray Matter Volume Atrophy Associations with Hearing in the UK Biobank. Alzheimer’s Dement. 2023, 19, e074985. [Google Scholar] [CrossRef]
- Giroud, N.; Pichora-Fuller, M.; Mick, P.; Wittich, W.; Al-Yawer, F.; Rehan, S.; Orange, J.; Phillips, N. Hearing Loss Is Associated with Gray Matter Differences in Older Adults at Risk for and with Alzheimer’s Disease. Aging Brain 2021, 1, 100018. [Google Scholar] [CrossRef]
- Wang, H.-F.; Zhang, W.; Rolls, E.T.; Li, Y.; Wang, L.; Ma, Y.-H.; Kang, J.; Feng, J.; Yu, J.-T.; Cheng, W. Hearing Impairment Is Associated with Cognitive Decline, Brain Atrophy and Tau Pathology. eBioMedicine 2022, 86, 104336. [Google Scholar] [CrossRef] [PubMed]
- Khandalavala, K.R.; Marinelli, J.P.; Lohse, C.M.; Przybelski, S.A.; Petersen, R.C.; Vassilaki, M.; Vemuri, P.; Carlson, M.L. Neuroimaging Characteristics of Hearing Loss in the Mayo Clinic Study of Aging. Otolaryngol.–Head. Neck Surg. 2024, 170, 886–895. [Google Scholar] [CrossRef]
- Vandenbroeke, T.; Andries, E.; Lammers, M.J.W.; Hofkens-Van den Brandt, A.; Mertens, G.; Van Rompaey, V. Cochlear Implantation and Cognitive Function in the Older Adult Population: Current State of the Art and Future Perspectives. Braz. J. Otorhinolaryngol. 2025, 91, 101544. [Google Scholar] [CrossRef]
- Deal, J.A.; Jiang, K.; Rawlings, A.; Sharrett, A.R.; Reed, N.S.; Knopman, D.; Mosley, T.; Wong, D.; Zhou, Y.; Lin, F.R.; et al. Hearing, β-Amyloid Deposition and Cognitive Test Performance in Black and White Older Adults: The ARIC-PET Study. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2023, 78, 2105–2110. [Google Scholar] [CrossRef]
- Volter, C.; Fricke, H.; Gotze, L.; Labrenz, F.; Tokic, M.; Wirth, R.; Nasreddine, Z.; Dawes, P. Evaluation of the Non-Auditory Neurocognitive Test MoCA-HI for Hearing-Impaired. Front. Neurol. 2022, 13, 1022292. [Google Scholar] [CrossRef]
- North, C.; Heatley, M.H.; Utoomprurkporn, N.; Bamiou, D.E.; Costafreda, S.G.; Stott, J. Adaption and Preliminary Validation of the Addenbrooke’s Cognitive Examination-III as a Screening Test for Mild Cognitive Impairment and Dementia in Hearing-Impaired Individuals. Eur. J. Neurol. 2021, 28, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal-Garza, B.; Callejón-Leblic, M.A. Neuropsychological Assessments to Explore the Cognitive Impact of Cochlear Implants: A Scoping Review. J. Clin. Med. 2025, 14, 7628. https://doi.org/10.3390/jcm14217628
Villarreal-Garza B, Callejón-Leblic MA. Neuropsychological Assessments to Explore the Cognitive Impact of Cochlear Implants: A Scoping Review. Journal of Clinical Medicine. 2025; 14(21):7628. https://doi.org/10.3390/jcm14217628
Chicago/Turabian StyleVillarreal-Garza, Brenda, and María Amparo Callejón-Leblic. 2025. "Neuropsychological Assessments to Explore the Cognitive Impact of Cochlear Implants: A Scoping Review" Journal of Clinical Medicine 14, no. 21: 7628. https://doi.org/10.3390/jcm14217628
APA StyleVillarreal-Garza, B., & Callejón-Leblic, M. A. (2025). Neuropsychological Assessments to Explore the Cognitive Impact of Cochlear Implants: A Scoping Review. Journal of Clinical Medicine, 14(21), 7628. https://doi.org/10.3390/jcm14217628







