Is Ti-Coated PEEK Superior to PEEK for Lumbar and Cervical Fusion Procedures? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
- Non-English studies;
- Non-original articles such as commentaries, systematic reviews, or meta-analysis;
- Studies lacking fusion rates following surgical intervention;
- Study designs other than RCTs;
- Studies lacking PEEK comparison.
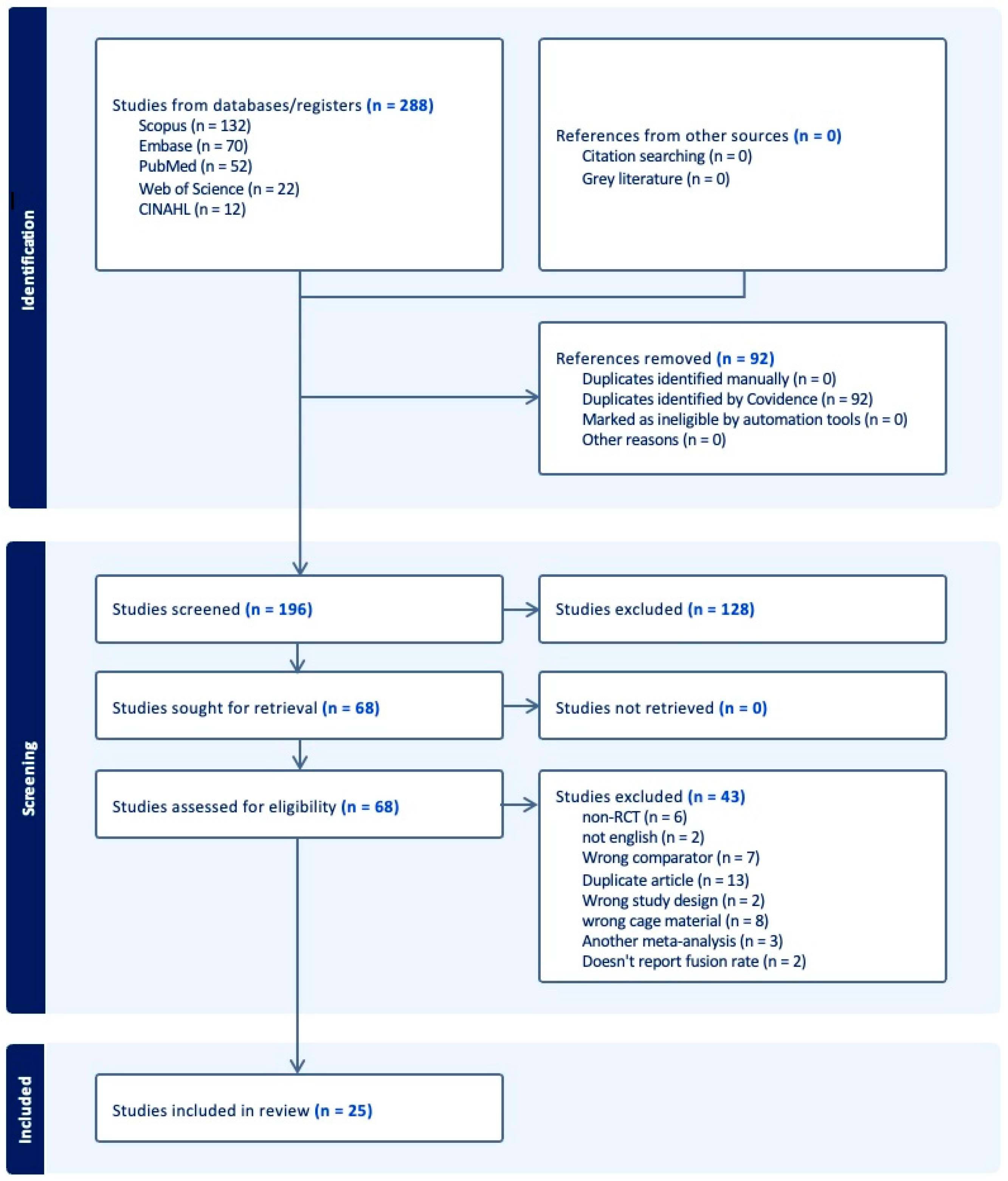
2.3. Article Selection Process
2.4. Data Extraction
2.5. Meta-Analysis
2.6. Article Quality Grading
3. Results
3.1. Study Demographics
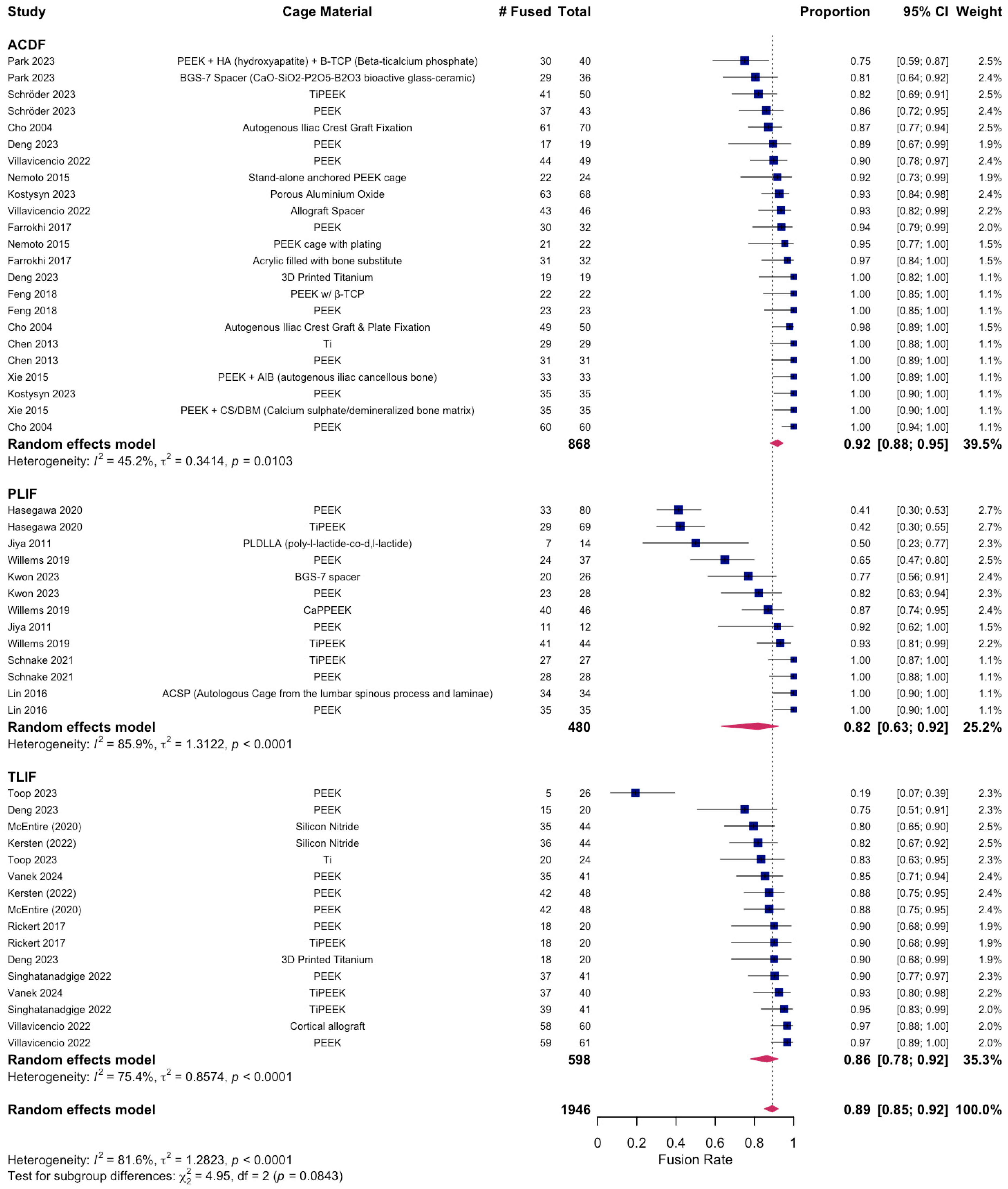
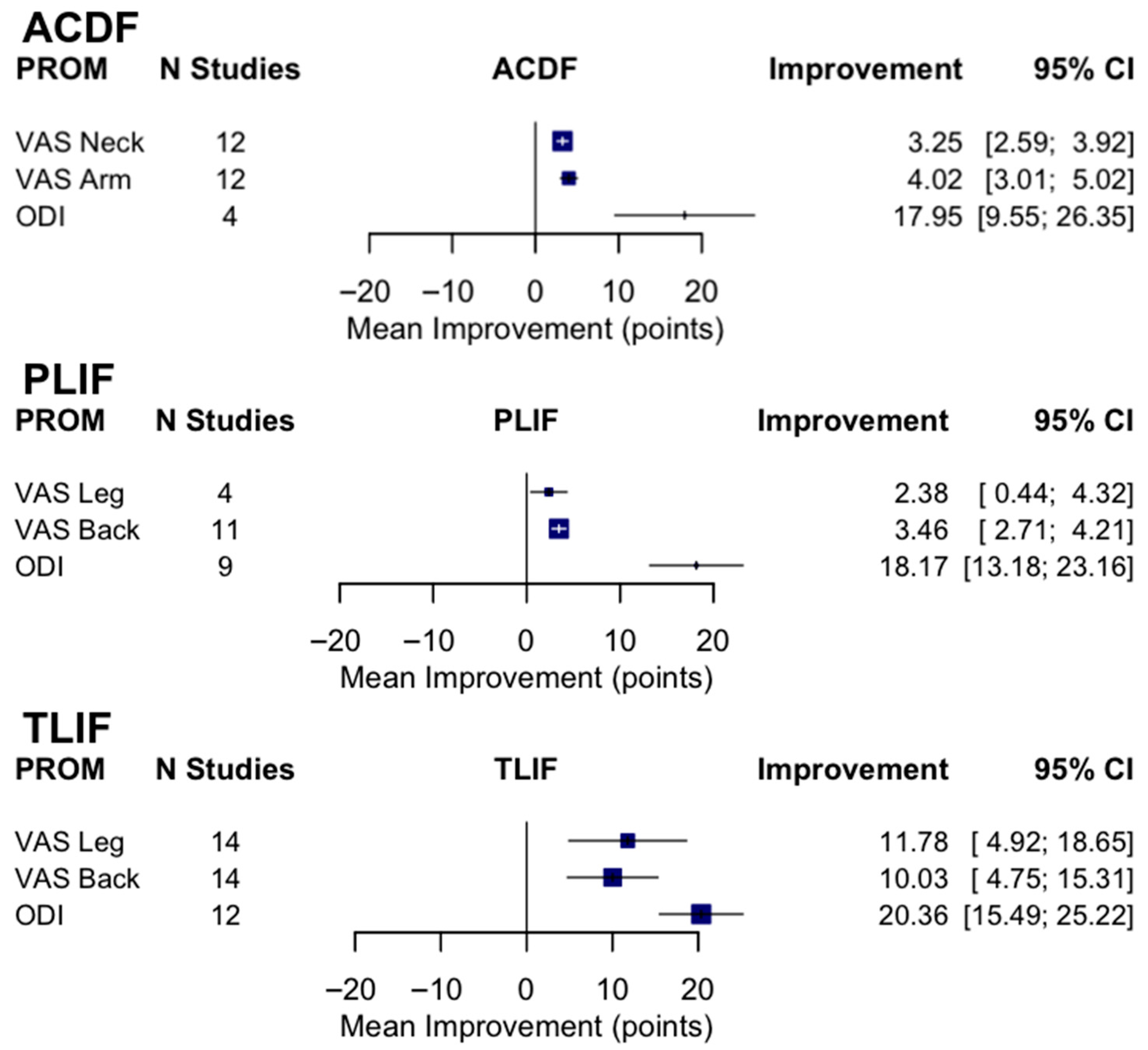
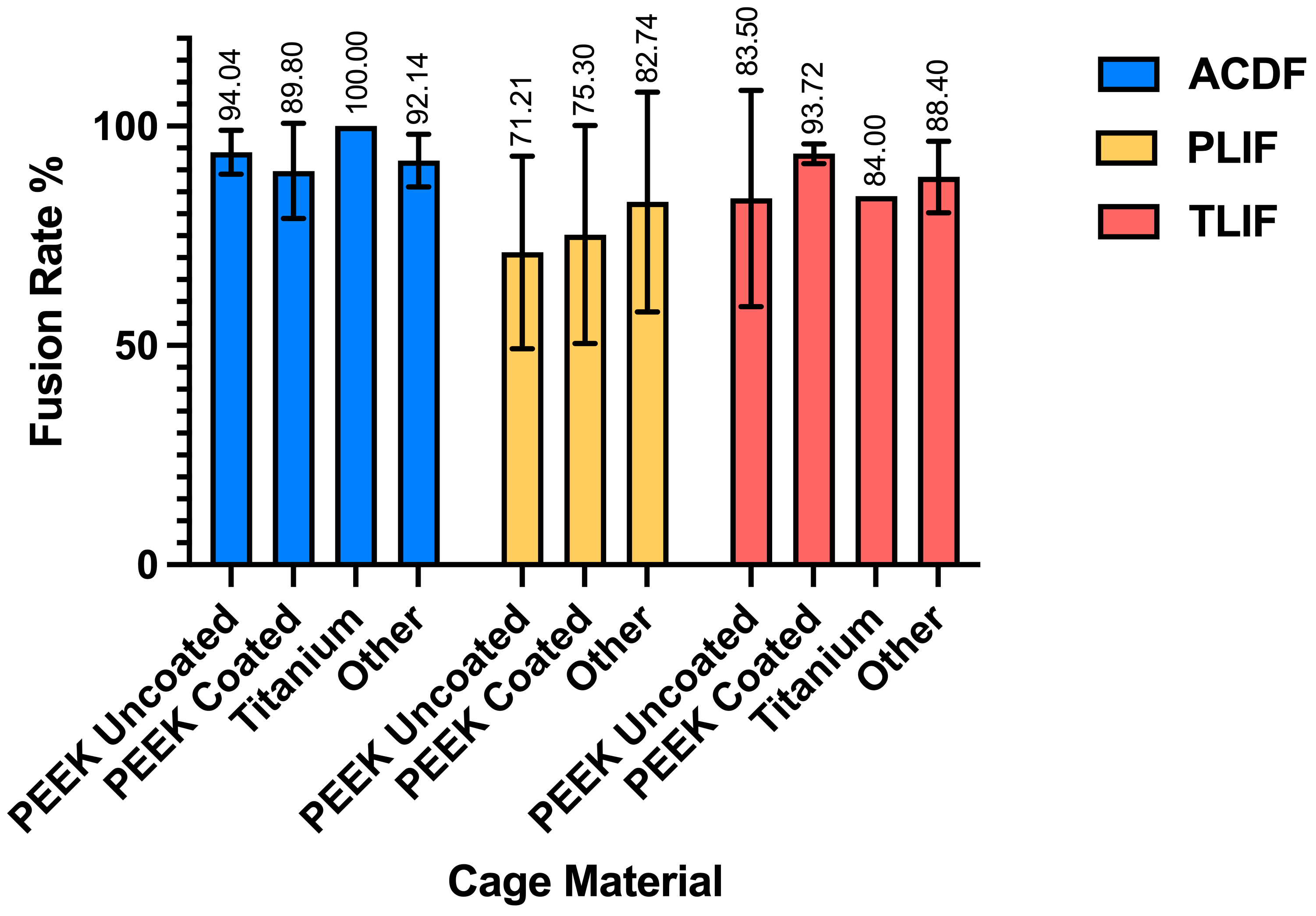
3.2. Postoperative Fusion Outcomes in ACDF
3.3. Postoperative Fusion Outcomes in PLIF
3.4. Postoperative Fusion Outcomes in TLIF
4. Discussion
4.1. Study Trends
4.2. Historical Trends
4.3. Upcoming Materials
4.4. Clinical Implications and Decision-Making Framework
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACDF | Anterior Cervical Discectomy and Fusion |
| PLIF | Posterior Lumbar Interbody Fusion |
| TLIF | Transforaminal Lumbar Interbody Fusion |
| PEEK | Polyetheretherketone |
| TiPEEK | Titanium-coated Polyetheretherketone |
| HA | Hydroxyapatite |
| β-TCP | Beta-Tricalcium Phosphate |
| BGS-7 | CaO-SiO2-P2O5-B2O3 Glass-Ceramic Spacer |
| ODI | Oswestry Disability Index |
| VAS | Visual Analog Scale |
| RCT | Randomized Controlled Trial |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| 3DPT | 3D-Printed Titanium |
| ACSP | Autologous Cage using Spinous Process/Laminae |
| Si3N4 | Silicon Nitride |
References
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef]
- Reid, P.C.; Morr, S.; Kaiser, M.G. State of the union: A review of lumbar fusion indications and techniques for degenerative spine disease. J. Neurosurg. Spine 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Viola, A., III; Appiah, J.; Donnally, C.J., III; Kim, Y.H.; Shenoy, K. Bone Graft Options in Spinal Fusion: A Review of Current Options and the Use of Mesenchymal Cellular Bone Matrices. World Neurosurg. 2022, 158, 182–188. [Google Scholar] [CrossRef]
- Cowan, J.A., Jr.; Dimick, J.B.; Wainess, R.; Upchurch, G.R., Jr.; Chandler, W.F.; La Marca, F. Changes in the utilization of spinal fusion in the United States. Neurosurgery 2006, 59, 15–20; discussion 15–20. [Google Scholar] [CrossRef]
- Cruz, A.; Ropper, A.E.; Xu, D.S.; Bohl, M.; Reece, E.M.; Winocour, S.J.; Buchanan, E.; Kaung, G. Failure in Lumbar Spinal Fusion and Current Management Modalities. Semin. Plast. Surg. 2021, 35, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Mirza, S.K.; Spina, N.; Spiker, W.R.; Lawrence, B.; Brodke, D.S. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine 2019, 44, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion-a literature review. J. Spine Surg. 2020, 6, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Riew, K.D.; Ecker, E.; Dettori, J.R. Anterior cervical discectomy and fusion for the management of axial neck pain in the absence of radiculopathy or myelopathy. Evid.-Based Spine-Care J. 2010, 1, 45–50. [Google Scholar] [CrossRef]
- Chong, E.; Pelletier, M.H.; Mobbs, R.J.; Walsh, W.R. The design evolution of interbody cages in anterior cervical discectomy and fusion: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 99. [Google Scholar] [CrossRef]
- Jain, S.; Eltorai, A.E.; Ruttiman, R.; Daniels, A.H. Advances in Spinal Interbody Cages. Orthop. Surg. 2016, 8, 278–284. [Google Scholar] [CrossRef]
- Johnson, S.E.; Michalopoulos, G.D.; Flanigan, P.M.; Katsos, K.; Ibrahim, S.; Freedman, B.A.; Bydon, M. Interbody cages versus structural bone grafts in lumbar arthrodesis: A systematic review and meta-analysis. J. Neurosurg. Spine 2024, 41, 188–198. [Google Scholar] [CrossRef]
- Patel, D.V.; Yoo, J.S.; Karmarkar, S.S.; Lamoutte, E.H.; Singh, K. Interbody options in lumbar fusion. J. Spine Surg. 2019, 5 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Fogel, G.; Martin, N.; Williams, G.M.; Unger, J.; Yee-Yanagishita, C.; Pelletier, M.; Walsh, W.; Peng, Y.; Jekir, M. Choice of Spinal Interbody Fusion Cage Material and Design Influences Subsidence and Osseointegration Performance. World Neurosurg. 2022, 162, e626–e634. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kwon, O.H.; Park, J.Y. Comparison Between 3-Dimensional-Printed Titanium and Polyetheretherketone Cages: 1-Year Outcome After Minimally Invasive Transforaminal Interbody Fusion. Neurospine 2022, 19, 524–532. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Al Dosari, M.A.A.; Al Kuwari, A.; Khan, N.M. The outcomes of stand alone polyetheretherketone cages in anterior cervical discectomy and fusion. Int. Orthop. 2021, 45, 173–180. [Google Scholar] [CrossRef]
- Li, G.; Yang, L.; Wu, G.; Qian, Z.; Li, H. An update of interbody cages for spine fusion surgeries: From shape design to materials. Expert Rev. Med. Devices 2022, 19, 977–989. [Google Scholar] [CrossRef]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef]
- Laubach, M.; Kobbe, P.; Hutmacher, D.W. Biodegradable interbody cages for lumbar spine fusion: Current concepts and future directions. Biomaterials 2022, 288, 121699. [Google Scholar] [CrossRef]
- Muthiah, N.; Yolcu, Y.U.; Alan, N.; Agarwal, N.; Hamilton, D.K.; Ozpinar, A. Evolution of polyetheretherketone (PEEK) and titanium interbody devices for spinal procedures: A comprehensive review of the literature. Eur. Spine J. 2022, 31, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.C.; Chou, P.H.; Lin, H.H.; Wang, S.T.; Chang, M.C. Outcome of Ti/PEEK Versus PEEK Cages in Minimally Invasive Transforaminal Lumbar Interbody Fusion. Glob. Spine J. 2023, 13, 472–478. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Cho, D.Y.; Lee, W.Y.; Sheu, P.C. Treatment of multilevel cervical fusion with cages. Surg. Neurol. 2004, 62, 378–385; discussion 385. [Google Scholar] [CrossRef] [PubMed]
- Jiya, T.U.; Smit, T.; van Royen, B.J.; Mullender, M. Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-L-lactide-co-D,L-lactide fusion devices. Clinical outcome at a minimum of 2-year follow-up. Eur. Spine J. 2011, 20, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Lu, X.; Yang, L.; Yang, H.; Yuan, W.; Chen, D. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: A prospective, randomized, control study with over 7-year follow-up. Eur. Spine J. 2013, 22, 1539–1546. [Google Scholar] [CrossRef]
- Nemoto, O.; Kitada, A.; Naitou, S.; Tachibana, A.; Ito, Y.; Fujikawa, A. Stand-alone anchored cage versus cage with plating for single-level anterior cervical discectomy and fusion: A prospective, randomized, controlled study with a 2-year follow-up. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, H.; Yuan, J.; Fu, L.; Yang, J.; Zhang, P. A prospective randomized comparison of PEEK cage containing calcium sulphate or demineralized bone matrix with autograft in anterior cervical interbody fusion. Int. Orthop. 2015, 39, 1129–1136. [Google Scholar] [CrossRef]
- Lin, B.; Yu, H.; Chen, Z.; Huang, Z.; Zhang, W. Comparison of the PEEK cage and an autologous cage made from the lumbar spinous process and laminae in posterior lumbar interbody fusion. BMC Musculoskelet. Disord. 2016, 17, 374. [Google Scholar] [CrossRef]
- Arts, M.P.; Wolfs, J.F.C.; Corbin, T.P. Porous silicon nitride spacers versus PEEK cages for anterior cervical discectomy and fusion: Clinical and radiological results of a single-blinded randomized controlled trial. Eur. Spine J. 2017, 26, 2372–2379. [Google Scholar] [CrossRef]
- Farrokhi, M.R.; Nikoo, Z.; Gholami, M.; Hosseini, K. Comparison Between Acrylic Cage and Polyetheretherketone (PEEK) Cage in Single-level Anterior Cervical Discectomy and Fusion: A Randomized Clinical Trial. Clin. Spine Surg. 2017, 30, 38–46. [Google Scholar] [CrossRef]
- Rickert, M.; Fleege, C.; Tarhan, T.; Schreiner, S.; Makowski, M.R.; Rauschmann, M.; Arabmotlagh, M. Transforaminal lumbar interbody fusion using polyetheretherketone oblique cages with and without a titanium coating. Bone Jt. J. 2017, 99-B, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Chang, M.C.; Chou, P.H.; Lin, H.; Wang, S.T.; Liu, C.L. Implantation of an empty polyetheretherketone cage in anterior cervical discectomy and fusion: A prospective randomised controlled study with 2 years follow-up. Eur. Spine J. 2018, 27, 1358–1364. [Google Scholar] [CrossRef]
- Willems, K.; Lauweryns, P.; Verleye, G.; Van Goethem, J. Randomized controlled trial of posterior lumbar interbody fusion with Ti- and cap-nanocoated polyetheretherketone cages: Comparative study of the 1-year radiological and clinical outcome. Int. J. Spine Surg. 2019, 13, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ushirozako, H.; Shigeto, E.; Ohba, T.; Oba, H.; Mukaiyama, K.; Shimizu, S.; Yamato, Y.; Ide, K.; Shibata, S.; et al. The Titanium-coated PEEK Cage Maintains Better Bone Fusion with the Endplate Than the PEEK Cage 6 Months After PLIF Surgery: A Multicenter, Prospective, Randomized Study. Spine 2020, 45, E892–E902. [Google Scholar] [CrossRef]
- McEntire, B.J.; Maslin, G.; Sonny Bal, B. Two-year results of a double-blind multicenter randomized controlled non-inferiority trial of polyetheretherketone (Peek) versus silicon nitride spinal fusion cages in patients with symptomatic degenerative lumbar disc disorders. J. Spine Surg. 2020, 6, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Schnake, K.J.; Fleiter, N.; Hoffmann, C.; Pingel, A.; Scholz, M.; Langheinrich, A.; Kandziora, F. PLIF surgery with titanium-coated PEEK or uncoated PEEK cages: A prospective randomised clinical and radiological study. Eur. Spine J. 2021, 30, 114–121. [Google Scholar] [CrossRef]
- Kersten, R.F.; van Gaalen, S.M.; Arts, M.P.; Roes, K.C.; de Gast, A.; Corbin, T.P.; Oner, F. The SNAP trial: A double blind multi-center randomized controlled trial of a silicon nitride versus a PEEK cage in transforaminal lumbar interbody fusion in patients with symptomatic degenerative lumbar disc disorders: Study protocol. BMC Musculoskelet. Disord. 2014, 15, 57. [Google Scholar] [CrossRef]
- Singhatanadgige, W.; Tangchitcharoen, N.; Kerr, S.J.; Tanasansomboon, T.; Yingsakmongkol, W.; Kotheeranurak, V.; Limthongkul, W. A Comparison of Polyetheretherketone and Titanium-Coated Polyetheretherketone in Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Randomized Clinical Trial. World Neurosurg. 2022, 168, e471–e479. [Google Scholar] [CrossRef]
- Villavicencio, A.T.; Nelson, E.L.; Rajpal, S.; Beasley, K.; Burneikiene, S. Prospective, Randomized, Blinded Clinical Trial Comparing PEEK and Allograft Spacers in Patients Undergoing Anterior Cervical Discectomy and Fusion Surgeries. Spine 2022, 47, 1043–1054. [Google Scholar] [CrossRef]
- Villavicencio, A.T.; Nelson, E.L.; Rajpal, S.; Beasley, K.; Burneikiene, S. Prospective, randomized, double-blinded clinical trial comparing PEEK and allograft spacers in patients undergoing transforaminal lumbar interbody fusion surgeries. Spine J. 2022, 22, 84–94. [Google Scholar] [CrossRef]
- Deng, Z.; Zou, Q.; Wang, L.; Wang, L.; Xiu, P.; Feng, G.; Song, Y.; Yang, X. Comparison between Three-Dimensional Printed Titanium and PEEK Cages for Cervical and Lumbar Interbody Fusion: A Prospective Controlled Trial. Orthop. Surg. 2023, 15, 2889–2900. [Google Scholar] [CrossRef]
- Kostysyn, R.; Ryska, P.; Jandura, J.; Selke-Krulichova, I.; Poczos, P.; Hosszu, T.; Cesak, T. Speed and quality of interbody fusion in porous bioceramic Al2O3 and polyetheretherketone cages for anterior cervical discectomy and fusion: A comparative study. J. Orthop. Surg. Res. 2023, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.T.; Kim, H.J.; Lee, S.; Park, S.M.; Ham, D.W.; Park, H.J.; Kwon, O.; Yeom, J.S. Feasibility and safety of a CaO-SiO2-P2O5-B2O3 bioactive glass ceramic spacer in posterior lumbar interbody fusion compared with polyetheretherketone cage: A prospective randomized controlled trial. Acta Neurochir. 2023, 165, 135–144. [Google Scholar] [CrossRef]
- Park, J.; Park, S.M.; Ham, D.W.; Hong, J.Y.; Kim, H.J.; Yeom, J.S. Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial. J. Clin. Med. 2023, 12, 4069. [Google Scholar] [CrossRef]
- Schröder, J.; Kampulz, T.; Bajaj, S.K.; Hellwig, G.; Winking, M. PEEK Cages versus Titanium-Coated PEEK Cages in Single-Level Anterior Cervical Fusion: A Randomized Controlled Study. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2023, 85, 262–268. [Google Scholar] [CrossRef]
- Toop, N.; Dhaliwal, J.; Gifford, C.S.; Gibbs, D.; Keister, A.; Miracle, S.; Forghani, R.; Grossbach, A.; Farhadi, F. Promotion of higher rates of early fusion using activated titanium versus polyetheretherketone cages in adults undergoing 1- and 2-level transforaminal lumbar interbody fusion procedures: A randomized controlled trial. J. Neurosurg. Spine 2023, 39, 709–718. [Google Scholar] [CrossRef]
- Vanek, P.; Svoboda, N.; Bradac, O.; Malik, J.; Kaiser, R.; Netuka, D. Clinical and radiological results of TLIF surgery with titanium-coated PEEK or uncoated PEEK cages: A prospective single-centre randomised study. Eur. Spine J. 2024, 33, 332–338. [Google Scholar] [CrossRef]
- Chang, S.Y.; Kang, D.-H.; Cho, S.K. Innovative Developments in Lumbar Interbody Cage Materials and Design: A Comprehensive Narrative Review. Asian Spine J. 2024, 18, 444–457. [Google Scholar] [CrossRef]
- Gelfand, B.W.; Paek, S.; Zelenty, W.D.; Paek, S.; Zelenty, W.D.; Girardi, F.P. History and current state of interbody fusion device material science. Semin. Spine Surg. 2022, 34, 100972. [Google Scholar] [CrossRef]
- Phan, K.; Mobbs, R.J. Evolution of Design of Interbody Cages for Anterior Lumbar Interbody Fusion. Orthop. Surg. 2016, 8, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Han, C.M.; Lee, E.J.; Kim, H.E.; Koh, Y.H.; Kim, K.N.; Ha, Y.; Kuh, S. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In vitro and in vivo studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar] [CrossRef]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery-Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef]
- Cottrill, E.; Pennington, Z.; Lankipalle, N.; Ehresman, J.; Valencia, C.; Schilling, A.; Feghali, J.; Perdomo-Pantoja, A.; Theodore, N.; Sciubba, D.; et al. The effect of bioactive glasses on spinal fusion: A cross-disciplinary systematic review and meta-analysis of the preclinical and clinical data. J. Clin. Neurosci. 2020, 78, 34–46. [Google Scholar] [CrossRef]
- Liang, H.; Tu, J.; Wang, B.; Song, Y.; Wang, K.; Zhao, K.; Hua, W.; Li, S.; Tan, L.; Feng, X.; et al. Innovative 3D-printed porous tantalum cage with non-window design to accelerate spinal fusion: A proof-of-concept study. Mater. Today Bio. 2025, 31, 101576. [Google Scholar] [CrossRef]
- Corso, K.A.; Teferra, A.A.; Michielli, A.; Corrado, K.; Marcini, A.; Lotito, M.; Smith, C.; Costa, M.; Ruppenkamp, J.; Wallace, A. Evaluation of Healthcare Outcomes of Patients Treated with 3D-Printed-Titanium and PEEK Cages During Fusion Procedures in the Lumbar Spine. Med. Devices Evid. Res. 2025, 18, 37–51. [Google Scholar] [CrossRef]
- Kienle, A.; Graf, N.; Wilke, H.J. Does impaction of titanium-coated interbody fusion cages into the disc space cause wear debris or delamination? Spine J. 2016, 16, 235–242. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| Author(s), Year | Procedure | Control Group | Control (N) | Case Group | Case (N) | Country | Final Quality Grade |
|---|---|---|---|---|---|---|---|
| Cho et al., 2004 [23] | ACDF | (1) AICG fixation (2) AICG and plate fixation | (1) 70 (2) 50 | PEEK cage | 60 | China | Moderate |
| Jiya et al., 2011 [24] | PLIF | PLDLLA cage | 14 | PEEK cage | 12 | The Netherlands | Moderate |
| Chen et al., 2013 [25] | ACDF | Titanium cage | 29 | PEEK cage | 31 | China | High |
| Nemoto et al., 2015 [26] | ACDF | PEEK cage with plating | 22 | Standalone anchored PEEK cage | 24 | Japan | Moderate |
| Xie et al., 2015 [27] | ACDF | PEEK cage containing IAB | 33 | PEEK cage containing CS/DBM | 35 | China | High |
| Lin et al., 2016 [28] | PLIF | ACSP | 34 | PEEK cage | 35 | China | Moderate |
| Arts et al., 2017 [29] | ACDF | Porous silicon nitride spacer | 52 | PEEK cage containing autograft from osteophyte | 48 | The Netherlands | Moderate |
| Farrokhi et al., 2017 [30] | ACDF | Acrylic cage filled with bone substitute | 32 | PEEK cage | 32 | Iran | Moderate |
| Rickert et al., 2017 [31] | TLIF | TiPEEK cage | 20 | PEEK cage | 20 | Germany | Moderate |
| Feng et al., 2018 [32] | ACDF | PEEK cage containing β-TCP | 22 | PEEK cage | 23 | Taiwan | Moderate |
| Willems et al., 2019 [33] | PLIF | (1) TiPEEK cage (2) CaP-PEEK cage | (1) 44 (2) 46 | PEEK cage | 37 | Belgium | Moderate |
| Hasegawa et al., 2020 [34] | PLIF | TiPEEK cage | 69 | PEEK cage | 80 | Japan | Moderate |
| McEntire et al., 2020 [35] | TLIF | Silicon nitride cage | 44 | PEEK cage | 48 | USA | Moderate |
| Schnake et al., 2021 [36] | PLIF | TiPEEK cage | 27 | PEEK cage | 28 | Germany | Moderate |
| Kersten et al., 2014 [37] | TLIF | Silicon nitride cage | 44 | PEEK cage | 48 | The Netherlands | Moderate |
| Singhatanadgige et al., 2022 [38] | TLIF | TiPEEK cage | 41 | PEEK cage | 41 | Thailand | Moderate |
| Villavicencio et al., 2022 [39] | TLIF | Cortical allograft | 60 | PEEK cage | 61 | USA | High |
| Villavicencio et al., 2022 [40] | ACDF | Cortical allograft spacer | 46 | PEEK lordotic spacer | 49 | USA | Moderate |
| Deng et al., 2023 [41] | (1) ACDF (2) TLIF | 3DPT cage | (1) 19 (2) 20 | PEEK cage | (1) 19 (2) 20 | China | Moderate |
| Kostysyn et al., 2023 [42] | ACDF | Porous aluminum oxide cage | 68 | PEEK cage | 35 | Czech Republic | Moderate |
| Kwon et al., 2023 [43] | PLIF | BGS-7 spacer | 26 | PEEK cage | 28 | South Korea | Moderate |
| Park et al., 2023 [44] | ACDF | BGS-7 spacer | 36 | PEEK cage containing HA and β-TCP | 40 | South Korea | Moderate |
| Schröder et al., 2023 [45] | ACDF | TiPEEK cage | 50 | PEEK cage | 43 | Germany | Moderate |
| Toop et al., 2023 [46] | TLIF | Titanium cage | 24 | PEEK cage | 26 | USA | Moderate |
| Vanek et al., 2024 [47] | TLIF | TiPEEK cage | 40 | PEEK cage | 41 | Czech Republic | High |
| Surgery Type | Patients (N) | Males | Females | FWM Age (Years) (N = 25) | FWM Follow-Up Time (Months) (N = 25) | FWM Operative Time (Minutes) (N = 12) | FWM Hospital Stay (Days) (N = 9) | FWM Blood Loss (mL) (N = 12) | FWM Postop Fusion Rate (%) (N = 25) |
|---|---|---|---|---|---|---|---|---|---|
| ACDF | 968 | 530 | 438 | 51.64 ± 5.68 | 30.08 ± 24.01 | 155.05 ± 99.70 | 3.17 ± 2.97 | 85.91 ± 34.42 | 92.60 ± 6.88 |
| PLIF | 480 | 228 | 252 | 56.88 ± 8.62 | 14.38 ± 5.76 | — | — | — | 73.55 ± 22.45 |
| TLIF | 598 | 238 | 360 | 58.58 ± 5.00 | 18.84 ± 8.05 | 178.28 ± 38.08 | 13.46 ± 17.15 | 291.78 ± 129.96 | 86.62 ± 18.16 |
| Patients (N) | Age (Years) | Follow-Up (Months) | Blood Loss (mL) | Operative Time (Minutes) | Hospital Stay (Days) | Fusion Rate (%) | ||
|---|---|---|---|---|---|---|---|---|
| ACDF | ||||||||
| PEEK Uncoated | 364 | 51.25 +/− 6.90 | 24.62 +/− 27.89 | 70.47 +/− 27.94 | 152.95 +/− 109.091 | 2.25 +/− 3.36 | 94.04 +/− 5.04 | |
| PEEK Coated | 202 | 51.53 +/− 6.15 | 21.62 +/− 4.90 | 77.37 +/− 26.41 | 79.32 +/− 26.66 | 6.74 +/− 0.35 | 89.80 +/− 10.83 | |
| Titanium | 29 | 45.7 +/− 7.2 | 97.2 +/− 0 | — | — | — | 100 +/− 0 | |
| Other (3D-Printed, Acrylic, Porous Aluminum, BGS-7, Autogenous Iliac, Allograft Spacer, Porous Silicon Nitride) | 373 | 52.1 +/− 3.93 | 27.52 +/− 16.04 | 102.52 +/− 46.28 | 188.91 +/− 107.78 | 2 +/− 3.03 | 92.14 +/− 5.99 | |
| PLIF | ||||||||
| PEEK Uncoated | 220 | 58.11 +/− 10.24 | 14.56 +/− 6.20 | — | — | — | 71.21 +/− 21.93 | |
| PEEK Coated | 186 | 56.60 +/− 8.56 | 12 +/− 0 | — | — | — | 75.30 +/− 24.82 | |
| Other (Autologous, PLDLLA, BGS-7) | 74 | 53.91 +/− 8.55 | 19.78 +/− 6.93 | — | — | — | 82.74 +/− 25.05 | |
| TLIF | ||||||||
| PEEK Uncoated | 305 | 59.35 +/− 5.87 | 18.89 +/− 8.33 | 260.56 +/− 73.00 | 171.38 +/− 59.45 | 13.89 +/− 18.58 | 83.50 +/− 24.66 | |
| PEEK Coated | 101 | 63.71 +/− 0 | 16.75 +/− 6.93 | — | — | — | 93.72 +/− 2.23 | |
| Titanium | 24 | 61 +/− 0 | 6 +/− 0 | — | 227.9 +/− 0 | 3.9 +/− 0 | 84 +/− 0 | |
| Other (Silicon Nitride, 3D-Printed, Cortical Allograft) | 168 | 56.99 +/− 2.24 | 21.86 +/− 9.0 | 341.37 +/− 150.85 | 169.5 +/− 31.56 | 14.30 +/− 19.77 | 88.40 +/− 8.15 |
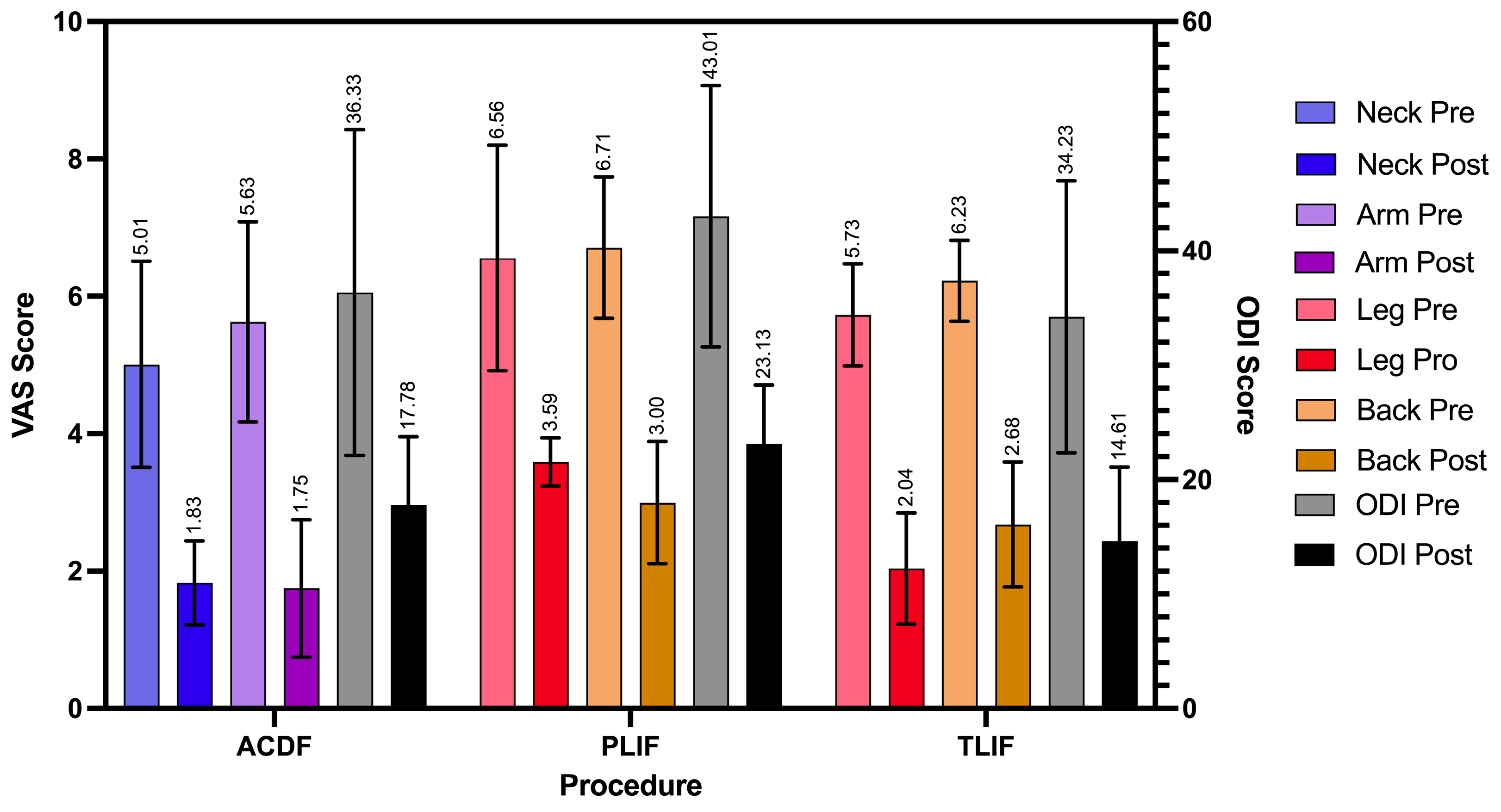
| Surgery Type | VAS Neck Pre/Postop (N = 6) | VAS Arm Pre/Postop (N = 6) | VAS Leg Pre/Postop (N = 9) | VAS Back Pre/Postop (N = 12) | ODI Preop (N = 12) | ODI Postop (N = 12) |
|---|---|---|---|---|---|---|
| ACDF | 5.01 ± 1.50, 1.83 ± 0.61 | 5.63 ± 1.46, 1.75 ± 1.00 | — | — | 36.33 ± 14.23 | 17.78 ± 5.96 |
| PLIF | — | — | 6.56 ± 1.64, 3.59 ± 0.35 | 6.71 ± 1.03, 3.00 ± 0.89 | 43.01 ± 11.42 | 23.13 ± 5.15 |
| TLIF | — | — | 5.73 ± 0.74, 2.04 ± 0.81 | 6.23 ± 0.59, 2.68 ± 0.91 | 34.23 ± 11.88 | 14.61 ± 6.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kincaid, J.; Kim, R.J.; Verma, A.; Turlip, R.W.; Liu, D.D.; Chauhan, D.; Dagli, M.M.; Chung, R.J.; Ahmad, H.S.; Ghenbot, Y.; et al. Is Ti-Coated PEEK Superior to PEEK for Lumbar and Cervical Fusion Procedures? A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 7696. https://doi.org/10.3390/jcm14217696
Kincaid J, Kim RJ, Verma A, Turlip RW, Liu DD, Chauhan D, Dagli MM, Chung RJ, Ahmad HS, Ghenbot Y, et al. Is Ti-Coated PEEK Superior to PEEK for Lumbar and Cervical Fusion Procedures? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(21):7696. https://doi.org/10.3390/jcm14217696
Chicago/Turabian StyleKincaid, Julia, Richelle J. Kim, Akash Verma, Ryan W. Turlip, David D. Liu, Daksh Chauhan, Mert Marcel Dagli, Richard J. Chung, Hasan S. Ahmad, Yohannes Ghenbot, and et al. 2025. "Is Ti-Coated PEEK Superior to PEEK for Lumbar and Cervical Fusion Procedures? A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 21: 7696. https://doi.org/10.3390/jcm14217696
APA StyleKincaid, J., Kim, R. J., Verma, A., Turlip, R. W., Liu, D. D., Chauhan, D., Dagli, M. M., Chung, R. J., Ahmad, H. S., Ghenbot, Y., Gu, B., & Yoon, J. W. (2025). Is Ti-Coated PEEK Superior to PEEK for Lumbar and Cervical Fusion Procedures? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(21), 7696. https://doi.org/10.3390/jcm14217696







