Sleep and Athletic Performance: A Multidimensional Review of Physiological and Molecular Mechanisms
Abstract
1. Introduction
2. Methodology
3. The Physiology of Sleep
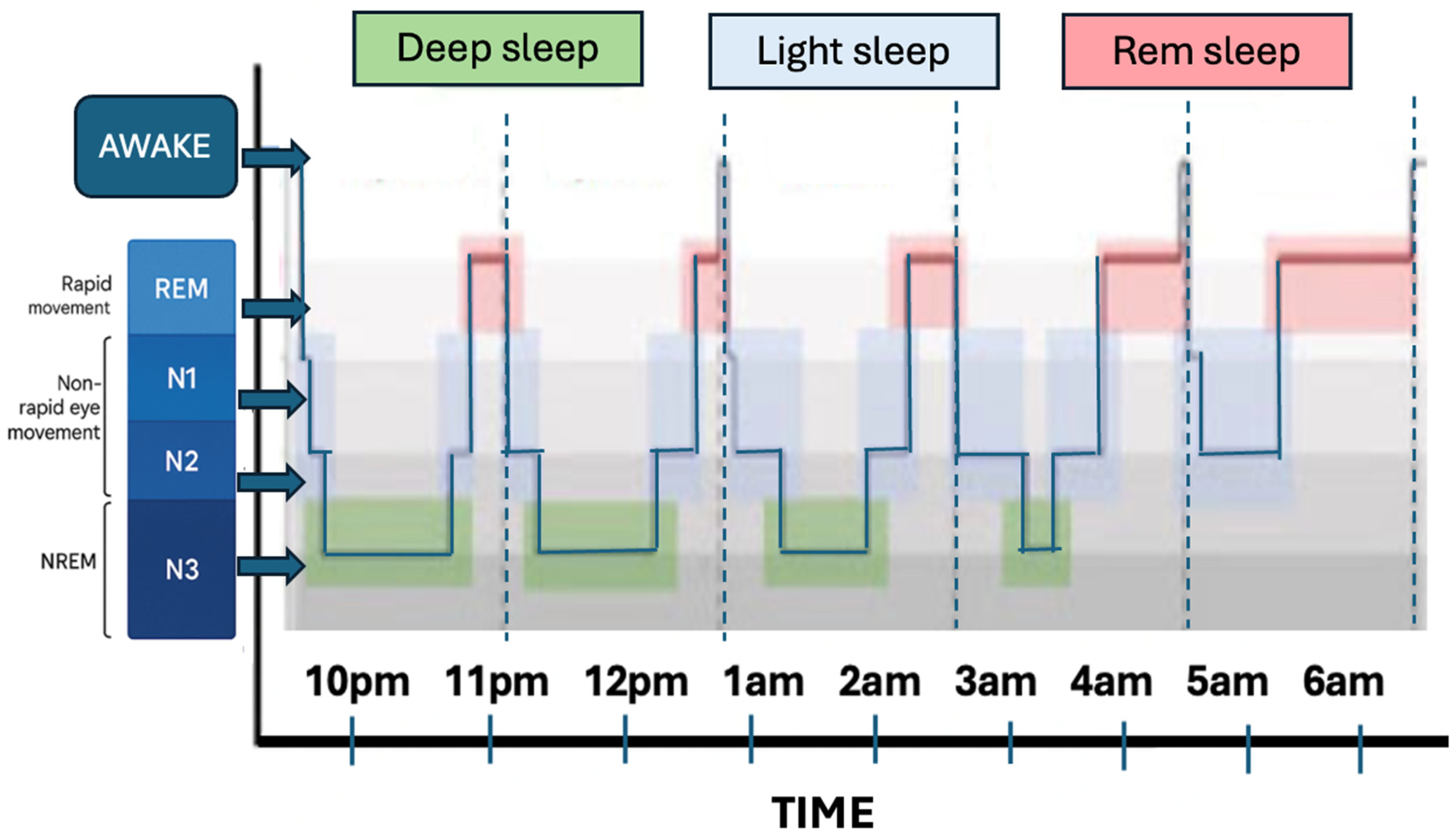
3.1. Neurochemical Mechanisms of Sleep and Wakefulness
3.2. Physiological and Molecular Benefits of Sleep
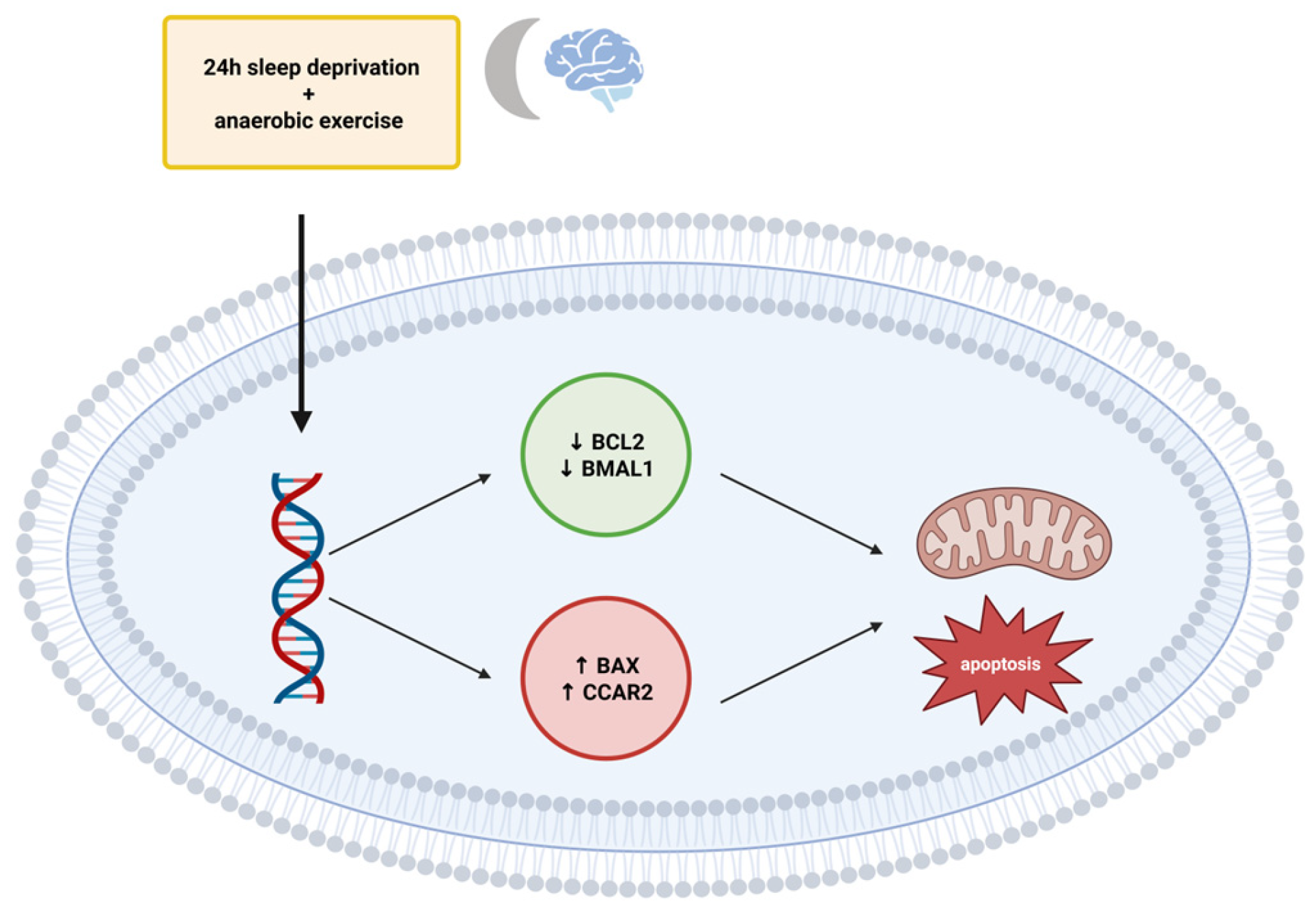
Molecular Consequences of Sleep Loss: Apoptosis and Circadian Disruption
4. Methods and Tools for Assessing Sleep Quality and Duration
5. Sleep and Performance in Athletes: Selected External Factors
- (1)
- Pre-adaptation—gradually shift sleep timing by 30–60 min in the days preceding travel, combined with appropriate light exposure (morning light for eastward travel, evening light for westward travel),
- (2)
- Post-arrival adaptation—adjust sleep, light exposure, meals, and training schedules to the local time zone, avoiding bright light at inappropriate times,
- (3)
- Recovery—allow a minimum of 24 h rest following travel; naps lasting 20–90 min can support physical and cognitive performance,
- (4)
- Sleep hygiene—limit caffeine intake and screen exposure in the evening, utilise eye masks and earplugs, and avoid intensive activities immediately after waking,
- (5)
5.1. Sleep Deprivation and Athletic Performance
5.2. Sleep Disorders (SD)
5.3. Sleep Disturbances as a Risk of Other Diseases
6. Treatment of Sleep Disorders in Athletes
7. Recovery Through Sleep in Athletes
8. Discussion
9. Strengths and Limitations of This Review
10. Summary
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| ACG | Actigraphy |
| ACh | Acetylcholine |
| AHI | Apnea-hypoapnea index |
| AIS | Athens Insomnia Scale |
| ALA | Alpha-linolenic acid |
| AM | Ante meridiem |
| APOE | Apolipoprotein E |
| ASBQ | Athlete Sleep Behavior Questionnaire |
| ASSQ | Athlete Sleep Screening Questionnaire |
| ATP | Adenosine triphosphate |
| BAX | Bcl-2 associated X protein |
| BCL2 | B-cell lymphoma protein 2 |
| BDNF | Brain-derived neurotrophic factor |
| Bglap2 | Osteocalcin |
| BMAL1 | Brain and muscle ARNT-like protein 1 |
| Bsp | Bone sialoprotein |
| CBD | Compound cannabidiol |
| CBT-I | Cognitive Behavioural Therapy for Insomnia |
| CCAR2 | Apoptosis regulator 2 |
| CO2 | Carbon dioxide |
| DFUs | Diabetic foot ulcers |
| DHA | Docosahexaenoic acid |
| Dmp1 | Dentin matrix protein 1 |
| EEG | Electroencephalogram |
| EPA | Eicosapentaenoic acid |
| FODMAP | Fermentable oligosaccharides, monosaccharides, and polyols |
| GABA | Gamma-aminobutyric acid |
| GH | Growth hormone |
| GI | Glycemic Index |
| ID | Insomnia Disorder |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin 6 |
| IOC | International Olympic Committee |
| ISI | Insomnia Severity Index |
| LEA | Low energy availability |
| MCTQ | Munich Chronotype Questionnaire |
| MEQ | Morningness-Eveningness Questionnaire |
| MyoD | Myoblast determination protein |
| NaSSA | Noradrenergic and specific serotonergic antidepressant |
| NCAA | National Collegiate Athletic Association |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| NREM | Non-rapid eye movement |
| OSA | Obstructive sleep apnea |
| PET | Positron emission tomography |
| PGO | Ponto-geniculo-occipital |
| PM | Post meridiem |
| PSG | Polysomnography |
| PSQI | Pittsburgh Sleep Quality Index |
| PUFA | Polyunsaturated fatty acid |
| REM | Rapid eye movement |
| RHT | Retino-hypothalamic tract |
| RLS | Restless legs syndrome |
| SCN | Hypothalamic suprachiasmatic nucleus |
| SD | Sleep disorders |
| SIRT1 | Sirtuin 1 |
| SHY | Synaptic Homeostasis Hypothesi |
| Spp1 | Osteopontin |
| THC | Tetrahydrocannabinol |
| TNF-α | Tumor necrosis factor-α |
| TUE | Therapeutic Use Exemption |
| WADA | World Anti-Doping Agency |
| WASO | Wakefulness after sleep onset |
References
- Scott, H.; Naik, G.; Lechat, B.; Manners, J.; Fitton, J.; Nguyen, D.P.; Hudson, A.L.; Reynolds, A.C.; Sweetman, A.; Escourrou, P.; et al. Are we getting enough sleep? Frequent irregular sleep found in an analysis of over 11 million nights of objective in-home sleep data. Sleep Health 2024, 10, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Antunes, H.; Medeiros, A.; Neto, M.M.; Souza, H.; Tufik, S.; de Mello, M. Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Med. Hypotheses 2011, 77, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016, 3, 67–104. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Chen, Y.; Li, N.; Lou, S.; Shao, Y.; Guo, Y.; Sun, Q.; Ni, Z.; Dong, L.; et al. Sleep disturbances, mental health symptoms, and chronotype in Chinese elite athletes: Insights from the Beijing 2022 winter olympics preparatory period. BMC Psychiatry 2025, 25, 400. [Google Scholar] [CrossRef]
- Massart, R.; Freyburger, M.; Suderman, M.; Paquet, J.; El Helou, J.; Belanger-Nelson, E.; Rachalski, A.; Koumar, O.C.; Carrier, J.; Szyf, M.; et al. The genome-wide landscape of DNA methylation and hydroxymethylation in response to sleep deprivation impacts on synaptic plasticity genes. Transl. Psychiatry 2014, 4, e347. [Google Scholar] [CrossRef]
- Fullagar, H.H.K.; Duffield, R.; Skorski, S.; Coutts, A.J.; Julian, R.; Meyer, T. Sleep and Recovery in Team Sport: Current Sleep-Related Issues Facing Professional Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2015, 10, 950–957. [Google Scholar] [CrossRef]
- Memar, P.; Faradji, F. A Novel Multi-Class EEG-Based Sleep Stage Classification System. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 26, 84–95. [Google Scholar] [CrossRef]
- Sun, H.; Ye, E.; Paixao, L.; Ganglberger, W.; Chu, C.J.; Zhang, C.; Rosand, J.; Mignot, E.; Cash, S.S.; Gozal, D.; et al. The sleep and wake electroencephalogram over the lifespan. Neurobiol. Aging 2023, 124, 60–70. [Google Scholar] [CrossRef]
- Driller, M.W.; Dunican, I.C.; Omond, S.E.T.; Boukhris, O.; Stevenson, S.; Lambing, K.; Bender, A.M. Pyjamas, Polysomnography and Professional Athletes: The Role of Sleep Tracking Technology in Sport. Sports 2023, 11, 14. [Google Scholar] [CrossRef]
- Leach, S.; Krugliakova, E.; Sousouri, G.; Snipes, S.; Skorucak, J.; Schühle, S.; Müller, M.; Ferster, M.L.; Da Poian, G.; Karlen, W.; et al. Acoustically evoked K-complexes together with sleep spindles boost verbal declarative memory consolidation in healthy adults. Sci. Rep. 2024, 14, 19184. [Google Scholar] [CrossRef]
- Jiang, X.; Gonzalez-Martinez, J.; Halgren, E. Coordination of Human Hippocampal Sharpwave Ripples during NREM Sleep with Cortical Theta Bursts, Spindles, Downstates, and Upstates. J. Neurosci. 2019, 39, 8744–8761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fei, X.; Liu, X.; Wang, Y.; Hu, Y.; Peng, W.; Wang, Y.-W.; Zhang, S.; Xu, M. REM sleep is associated with distinct global cortical dynamics and controlled by occipital cortex. Nat. Commun. 2022, 13, 6896. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G. The Ontogenesis of Mammalian Sleep: Form and Function. Curr. Sleep Med. Rep. 2020, 6, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi, L.A.; Hilakivi, I.; Ahtee, L.; Haikala, H.; Attila, M. Effect of neonatal nomifensine exposure on adult behavior and brain monoamines in rats. J. Neural Transm. 1987, 70, 99–116. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef]
- Colavito, V.; Fabene, P.F.; Grassi-Zucconi, G.; Pifferi, F.; Lamberty, Y.; Bentivoglio, M.; Bertini, G. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front. Syst. Neurosci. 2013, 7, 106. [Google Scholar] [CrossRef]
- Nere, A.T.; Hashmi, A.; Cirelli, C.; Tononi, G. Sleep-Dependent Synaptic Down-Selection (I): Modeling the Benefits of Sleep on Memory Consolidation and Integration. Front. Neurol. 2013, 4, 143. [Google Scholar] [CrossRef]
- Sassin, J.F.; Parker, D.C.; Mace, J.W.; Gotlin, R.W.; Johnson, L.C.; Rossman, L.G. Human growth hormone release: Relation to slow-wave sleep and sleep-walking cycles. Science 1969, 165, 513–515. [Google Scholar] [CrossRef]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of Sleep Deprivation and Circadian Misalignment on Cortisol, Inflammatory Markers, and Cytokine Balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Cauter, E.V. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodriguez, E.; Arias-Carrion, O.; Sanguino-Rodriguez, K.; Gonzalez-Arias, M.; Haro, R. Mechanisms of sleep-wake cycle modulation. CNS Neurol. Disord.-Drug Targets 2009, 8, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Baghdoyan, H.A.; Lydic, R. Neuropharmacology of Sleep and Wakefulness. Sleep Med. Clin. 2010, 5, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M. Sleep and circadian rhythms: Evolutionary entanglement and local regulation. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100052. [Google Scholar] [CrossRef]
- Kiehn, J.T.; Faltraco, F.; Palm, D.; Thome, J.; Oster, H. Circadian Clocks in the Regulation of Neurotransmitter Systems. Pharmacopsychiatry 2019, 56, 108–117. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Acebo, C.; Jenni, O.G. Regulation of adolescent sleep: Implications for behavior. Ann. N. Y. Acad. Sci. 2004, 1021, 276–291. [Google Scholar] [CrossRef]
- Gaudreau, H.; Carrier, J.; Montplaisir, J. Age-related modifications of NREM sleep EEG: From childhood to middle age. J. Sleep Res. 2001, 10, 165–172. [Google Scholar] [CrossRef]
- George, N.M.; Davis, J.E. Assessing sleep in adolescents through a better understanding of sleep physiology. Am. J. Nurs. 2013, 113, 26–31. [Google Scholar] [CrossRef][Green Version]
- Chaput, J.-P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appl. Physiol. Nutr. Metab. 2020, 45 (Suppl. 2), S232–S247. [Google Scholar] [CrossRef]
- Chen, L.; Ma, W.; Covassin, N.; Chen, D.; Zha, P.; Wang, C.; Gao, Y.; Tang, W.; Lei, F.; Tang, X.; et al. Association of sleep-disordered breathing and wound healing in patients with diabetic foot ulcers. J. Clin. Sleep Med. 2021, 17, 909–916. [Google Scholar] [CrossRef]
- Luo, B.; Zhou, X.; Tang, Q.; Yin, Y.; Feng, G.; Li, S.; Chen, L. Circadian rhythms affect bone reconstruction by regulating bone energy metabolism. J. Transl. Med. 2021, 19, 410. [Google Scholar] [CrossRef]
- Elkhenany, H.; AlOkda, A.; El-Badawy, A.; El-Badri, N. Tissue regeneration: Impact of sleep on stem cell regenerative capacity. Life Sci. 2018, 214, 51–61. [Google Scholar] [CrossRef]
- Charest, J.; Grandner, M.A. Sleep and Athletic Performance: Impacts on Physical Performance, Mental Performance, Injury Risk and Recovery, and Mental Health. Sleep Med. Clin. 2020, 15, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Sun, M.; Sun, Y.; Jin, L.; Li, S. Effects of Acute Sleep Deprivation on Sporting Performance in Athletes: A Comprehensive Systematic Review and Meta-Analysis. Nat. Sci. Sleep 2024, 16, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Kamareh, M.N.; Samadi, M.; Arabzadeh, E.; Abdollahi, M.; Sheidaei, S.; Malayeri, S.R.; Schlicht, J.; Shirvani, H.; Rostamkhani, F. The effect of 24-hour sleep deprivation and anaerobic exercise on the expression of BAX, BCL2, BMAL1 and CCAR2 genes in peripheral blood mononuclear cells after L-arginine supplementation. Gene 2023, 887, 147732. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.R.; Paavola, K.J.; Schaefer, S.A.; Kaur, B.; Van Meir, E.G.; Hall, R.A. Brain-specific angiogenesis inhibitor-1 signaling, regulation, and enrichment in the postsynaptic density. J. Biol. Chem. 2013, 288, 22248–22256. [Google Scholar] [CrossRef]
- Pu, H.; Bailey, L.C.; Bauer, L.G.; Voronkov, M.; Baxter, M.; Huber, K.V.M.; Khorasanizadeh, S.; Ray, D.; Rastinejad, F. Pharmacological targeting of BMAL1 modulates circadian and immune pathways. Nat. Chem. Biol. 2025, 21, 736–745. [Google Scholar] [CrossRef]
- Ortiz, L.M.; Lombardi, P.; Tillhon, M.; Scovassi, A.I. Berberine, an epiphany against cancer. Molecules 2014, 19, 12349–12367. [Google Scholar] [CrossRef]
- Yang, H.; Durocher, J.J.; Larson, R.A.; Dellavalla, J.P.; Carter, J.R. Total sleep deprivation alters cardiovascular reactivity to acute stressors in humans. J. Appl. Physiol. 2012, 113, 903–908. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanbayashi, T.; Saito, Y.; Takahashi, Y.; Kitajima, T.; Takahashi, K.; Hishikawa, Y.; Shimizu, T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep 2003, 26, 986–989. [Google Scholar] [CrossRef]
- Hori, A.; Su, X.; Sagasaki, S.; Saito, R.; Suijo, K.; Miyata, S.; Hasegawa, D.; Mizuno, M.; Hotta, N. Sleep deprivation elevates resting and exercise blood pressures and augments pressor response at exercise onset. Med. Sci. Sports Exerc. 2025, 57, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Phillips, B.G.; Sigurdsson, G.; Narkiewicz, K.; Pesek, C.A.; Somers, V.K. Effects of sleep deprivation on neural circulatory control. Hypertension 2000, 35, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Haack, M.; Toth, M.; Serrador, J.M.; Meier-Ewert, H.K. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog. Cardiovasc. Dis. 2009, 51, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.-J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Grimmer, T.; Laub, T.; Hapfelmeier, A.; Eisele, T.; Fatke, B.; Hölzle, P.; Lüscher, S.; Parchmann, A.; Rentrop, M.; Schwerthöffer, D.; et al. The overnight reduction of amyloid-β 1–42 plasma levels is diminished by the extent of sleep fragmentation, sAPP-β, and APOE ε4 in psychiatrists on call. Alzheimers Dement. 2020, 16, 759–769. [Google Scholar] [CrossRef]
- Yoo, S.S.; Gujar, N.; Hu, P.; Jolesz, F.A.; Walker, M.P. The human emotional brain without sleep—A prefrontal amygdala disconnect. Curr. Biol. 2007, 17, R877–R878. [Google Scholar] [CrossRef]
- Kang, J.-E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef]
- de Menezes-Júnior, L.A.A.; Carraro, J.C.C.; Machado-Coelho, G.L.L.; Meireles, A.L. The Pittsburgh sleep quality index-2 (PSQI-2): The validity of a two-item sleep quality screener in Brazilian adults. Sleep Breath. 2025, 29, 238. [Google Scholar] [CrossRef] [PubMed]
- Penzel, T.; Fietze, I.; Glos, M. Alternative algorithms and devices in sleep apnoea diagnosis: What we know and what we expect. Curr. Opin. Pulm. Med. 2020, 26, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Lüdtke, S.; Hermann, W.; Kirste, T.; Beneš, H.; Teipel, S. An algorithm for actigraphy-based sleep/wake scoring: Comparison with polysomnography. Clin. Neurophysiol. 2021, 132, 137–145. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Hon, F.; Whyte, J.; Monden, K.R.; Bogner, J.; Dahdah, M.; Wittine, L.; Bell, K.R.; Nakase-Richardson, R. Coherence Between Sleep Detection by Actigraphy and Polysomnography in a Multi-Center, Inpatient Cohort of Individuals with Traumatic Brain Injury. PM&R 2020, 12, 1205–1213. [Google Scholar] [CrossRef]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The Consensus Sleep Diary: Standardizing Prospective Sleep Self-Monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Driller, M.W.; Mah, C.D.; Halson, S.L. Development of the athlete sleep behavior questionnaire: A tool for identifying maladaptive sleep practices in elite athletes. Sleep Sci. 2023, 11, 37–44. [Google Scholar] [CrossRef]
- Katz, G. Jet lag and psychotic disorders. Curr. Psychiatry Rep. 2011, 13, 187–192. [Google Scholar] [CrossRef]
- Song, A.; Severini, T.; Allada, R. How jet lag impairs Major League Baseball performance. Proc. Natl. Acad. Sci. USA 2017, 114, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Trinder, J.; Paxton, S.J.; Montgomery, I.; Fraser, G. Endurance as opposed to power training: Their effect on sleep. Psychophysiology 1985, 22, 642–647. [Google Scholar] [CrossRef]
- Heller, H.C.; Herzog, E.; Brager, A.; Poe, G.; Allada, R.; Scheer, F.A.J.L.; Carskadon, M.; de la Iglesia, H.O.; Jang, R.; Montero, A.; et al. The Negative Effects of Travel on Student Athletes Through Sleep and Circadian Disruption. J. Biol. Rhythm. 2024, 39, 5–19. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res. 2012, 56, 17252. [Google Scholar] [CrossRef]
- Benton, D.; Bloxham, A.; Gaylor, C.; Brennan, A.; Young, H.A. Carbohydrate and sleep: An evaluation of putative mechanisms. Front. Nutr. 2022, 9, 933898. [Google Scholar] [CrossRef]
- Barnard, J.; Roberts, S.; Lastella, M.; Aisbett, B.; Condo, D. The Impact of Dietary Factors on the Sleep of Athletically Trained Populations: A Systematic Review. Nutrients 2022, 14, 3271. [Google Scholar] [CrossRef]
- Moss, K.; Zhang, Y.; Kreutzer, A.; Graybeal, A.J.; Porter, R.R.; Braun-Trocchio, R.; Shah, M. The Relationship Between Dietary Intake and Sleep Quality in Endurance Athletes. Front. Sports Act. Living 2022, 4, 810402. [Google Scholar] [CrossRef] [PubMed]
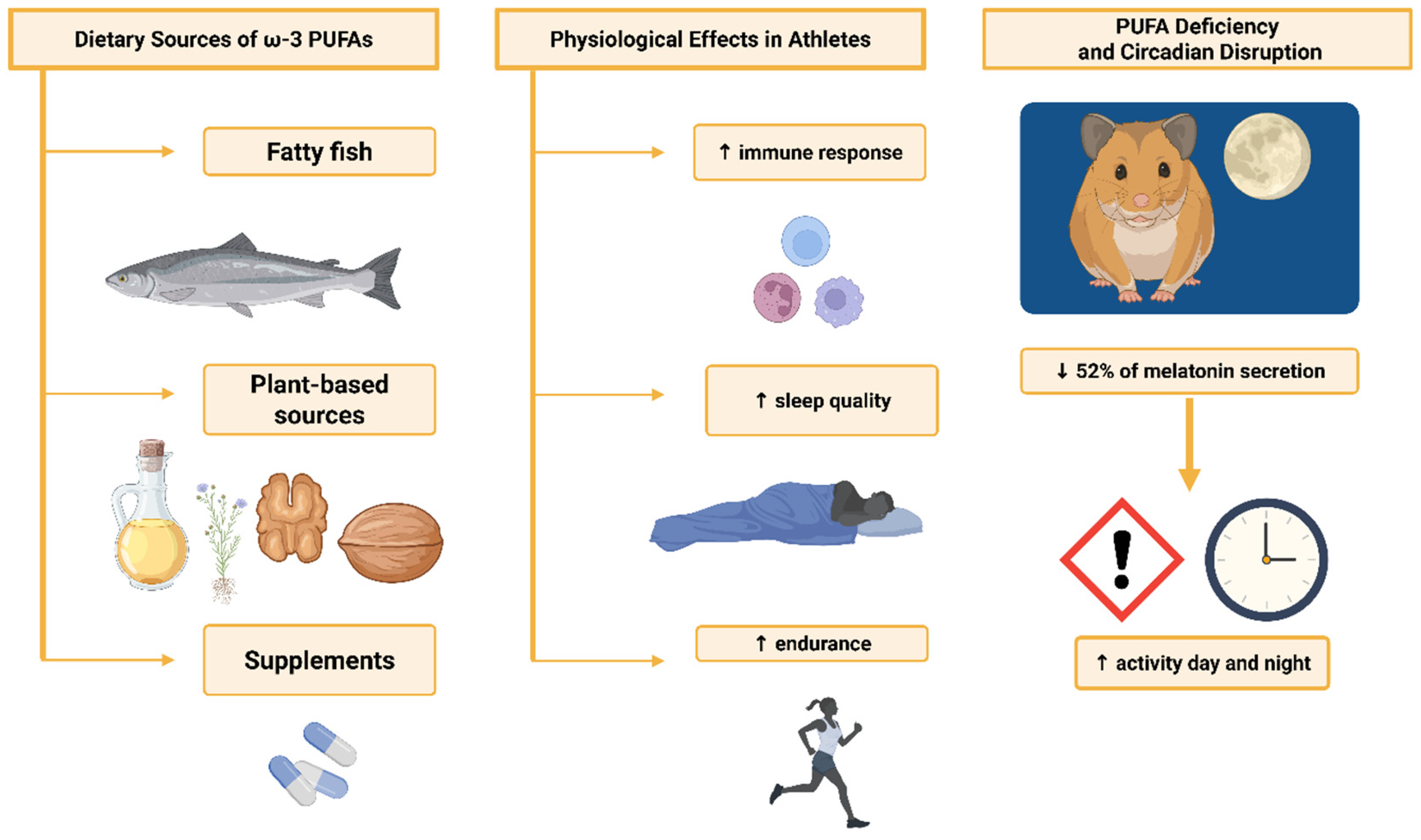
- Lavialle, M.; Champeil-Potokar, G.; Alessandri, J.M.; Balasse, L.; Guesnet, P.; Papillon, C.; Pévet, P.; Vancassel, S.; Vivien-Roels, B.; Denis, I. An (n-3) polyunsaturated fatty acid-deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J. Nutr. 2008, 138, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Heileson, J.L.; Sawan, S.A.; Dickerson, B.L.; Leonard, M.; Kreider, R.B.; Kerksick, C.M.; Cornish, S.M.; Candow, D.G.; Cordingley, D.M.; et al. International Society of Sports Nutrition Position Stand: Long-Chain Omega-3 Polyunsaturated Fatty Acids. J. Int. Soc. Sports Nutr. 2025, 22, 2441775. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.; McCartney, D.; Desbrow, B.; Sabapathy, S.; Bellinger, P.; Roberts, L.; Irwin, C. Effects of Acute Sleep Loss on Physical Performance: A Systematic and Meta-Analytical Review. Sports Med. 2022, 52, 2669–2690. [Google Scholar] [CrossRef] [PubMed]
- Guembri, M.A.; Racil, G.; Dhouibi, M.A.; Coquart, J.; Souissi, N. Evaluation of Age Based-Sleep Quality and Fitness in Adolescent Female Handball Players. Int. J. Environ. Res. Public Health 2022, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, E.J.; Moseley, S.E.; Langan-Evans, C.; Pullinger, S.A.; Robertson, C.M.; Burniston, J.G.; Edwards, B.J. Effects of two nights partial sleep deprivation on an evening submaximal weightlifting performance; are 1 h powernaps useful on the day of competition? Chronobiol. Int. 2019, 36, 407–426. [Google Scholar] [CrossRef]
- Gallagher, C.; Green, C.E.; Kenny, M.L.; Evans, J.R.; McCullagh, G.D.W.; Pullinger, S.A.; Edwards, B.J. Is implementing a post-lunch nap beneficial on evening performance, following two nights partial sleep restriction? Chronobiol. Int. 2023, 40, 1169–1186. [Google Scholar] [CrossRef]
- Mesas, A.E.; de Arenas-Arroyo, S.N.; Martinez-Vizcaino, V.; Garrido-Miguel, M.; Fernández-Rodríguez, R.; Bizzozero-Peroni, B.; Torres-Costoso, A.I. Is daytime napping an effective strategy to improve sport-related cognitive and physical performance and reduce fatigue? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2023, 57, 417–426. [Google Scholar] [CrossRef]
- Boukhris, O.; Trabelsi, K.; Suppiah, H.; Ammar, A.; Clark, C.C.T.; Jahrami, H.; Chtourou, H.; Driller, M. The Impact of Daytime Napping Following Normal Night-Time Sleep on Physical Performance: A Systematic Review, Meta-analysis and Meta-regression. Sports Med. 2024, 54, 323–345. [Google Scholar] [CrossRef]
- Blumert, P.A.; Crum, A.J.; Ernsting, M.; Volek, J.S.; Hollander, D.B.; Haff, E.E.; Haff, G.G. The acute effects of twenty-four hours of sleep loss on the performance of national-caliber male collegiate weightlifters. J. Strength Cond. Res. 2007, 21, 1146–1154. [Google Scholar] [CrossRef]
- Kong, Y.; Yu, B.; Guan, G.; Wang, Y.; He, H. Effects of sleep deprivation on sports performance and perceived exertion in athletes and non-athletes: A systematic review and meta-analysis. Front. Physiol. 2025, 16, 1544286. [Google Scholar] [CrossRef]
- Knufinke, M.; Nieuwenhuys, A.; Maase, K.; Moen, M.H.; Geurts, S.A.E.; Coenen, A.M.L.; Kompier, M.A.J. Effects of Natural Between-Days Variation in Sleep on Elite Athletes’ Psychomotor Vigilance and Sport-Specific Measures of Performance. J. Sports Sci. Med. 2018, 17, 515–524. [Google Scholar]
- Bonnar, D.; Bartel, K.; Kakoschke, N.; Lang, C. Sleep Interventions Designed to Improve Athletic Performance and Recovery: A Systematic Review of Current Approaches. Sports Med. 2018, 48, 683–703. [Google Scholar] [CrossRef]
- Vitale, K.C.; Owens, R.; Hopkins, S.R.; Malhotra, A. Sleep Hygiene for Optimizing Recovery in Athletes: Review and Recommendations. Int. J. Sports Med. 2019, 40, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Halson, S.L.; Weakley, J.; Hawley, J.A. Sleep, circadian biology and skeletal muscle interactions: Implications for metabolic health. Sleep Med. Rev. 2022, 66, 101700. [Google Scholar] [CrossRef] [PubMed]
- Lamon, S.; Morabito, A.; Arentson-Lantz, E.; Knowles, O.; Vincent, G.E.; Condo, D.; Alexander, S.E.; Garnham, A.; Paddon-Jones, D.; Aisbett, B. The effect of acute sleep deprivation on skeletal muscle protein synthesis and the hormonal environment. Physiol. Rep. 2021, 9, e14660. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.D.; Roberson, P.A.; Saunders, M.J.; Hargens, T.A.; Womack, C.J.; Luden, N.D. One night of sleep restriction following heavy exercise impairs 3-km cycling time-trial performance in the morning. Appl. Physiol. Nutr. Metab. 2017, 42, 909–915. [Google Scholar] [CrossRef]
- Dáttilo, M.; Antunes, H.K.M.; Galbes, N.M.N.; Mônico-Neto, M.; Souza, H.D.S.; Quaresma, M.V.L.D.S.; Lee, K.S.; Ugrinowitsch, C.; Tufik, S.; DE Mello, M.T. Effects of Sleep Deprivation on Acute Skeletal Muscle Recovery after Exercise. Med. Sci. Sports Exerc. 2020, 52, 507–514. [Google Scholar] [CrossRef]
- Chennaoui, M.; Vanneau, T.; Trignol, A.; Arnal, P.; Gomez-Merino, D.; Baudot, C.; Perez, J.; Pochettino, S.; Eirale, C.; Chalabi, H. How does sleep help recovery from exercise-induced muscle injuries? J. Sci. Med. Sport 2021, 24, 982–987. [Google Scholar] [CrossRef]
- Grandou, C.; Wallace, L.; Fullagar, H.H.K.; Duffield, R.; Burley, S. The Effects of Sleep Loss on Military Physical Performance. Sports Med. 2019, 49, 1159–1172. [Google Scholar] [CrossRef]
- Smithies, T.D.; Toth, A.J.; Dunican, I.C.; Caldwell, J.A.; Kowal, M.; Campbell, M.J. The effect of sleep restriction on cognitive performance in elite cognitive performers: A systematic review. Sleep 2021, 44, zsab008. [Google Scholar] [CrossRef]
- Palmer, C.A.; Bower, J.L.; Cho, K.W.; Clementi, M.A.; Lau, S.; Oosterhoff, B.; Alfano, C.A. Sleep loss and emotion: A systematic review and meta-analysis of over 50 years of experimental research. Psychol. Bull. 2024, 150, 440–463. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- van Straten, A.; Weinreich, K.J.; Fábián, B.; Reesen, J.; Grigori, S.; Luik, A.I.; Harrer, M.; Lancee, J. The Prevalence of Insomnia Disorder in the General Population: A Meta-Analysis. J. Sleep Res. 2025, 34, e70089. [Google Scholar] [CrossRef]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A Behavioral Perspective on Insomnia Treatment. Psychiatr. Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Fernández-Mendoza, J.; Vela-Bueno, A.; Vgontzas, A.N.; Ramos-Platón, M.J.; Olavarrieta-Bernardino, S.; Bixler, E.O.; De la Cruz-Troca, J.J. Cognitive-Emotional Hyperarousal as a Premorbid Characteristic of Individuals Vulnerable to Insomnia. Biopsychosoc. Sci. Med. 2010, 72, 397. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.R.; Watanabe, K.; Stringer, S.; Skene, N.; Bryois, J.; Hammerschlag, A.R.; de Leeuw, C.A.; Benjamins, J.S.; Muñoz-Manchado, A.B.; Nagel, M.; et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 2019, 51, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.M.; Lee, H.; Baxter, T.; Reddy, S.Y.; Barr, T.; Kim, H.-S.; Wang, D.; Mysliwiec, V. A Diagnosis of Insomnia Is Associated With Differential Expression of Sleep-Regulating Genes in Military Personnel. Biol. Res. Nurs. 2015, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M. Insomnia research: What have we learned and what else do we need to know? J. Sleep Res. 2023, 32, e14081. [Google Scholar] [CrossRef]
- Peyron, C.; Faraco, J.; Rogers, W.; Ripley, B.; Overeem, S.; Charnay, Y.; Nevsimalova, S.; Aldrich, M.; Reynolds, D.; Albin, R.; et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000, 6, 991–997. [Google Scholar] [CrossRef]
- Nishino, S.; Ripley, B.; Overeem, S.; Lammers, G.J.; Mignot, E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000, 355, 39–40. [Google Scholar] [CrossRef]
- Mignot, E.; Lin, X.; Arrigoni, J.; Macaubas, C.; Olive, F.; Hallmayer, J.; Underhill, P.; Guilleminault, C.; Dement, W.C.; Grumet, F.C. DQB1*0602 and DQA1*0102 (DQ1) Are Better Markers Than DR2 for Narcolepsy in Caucasian and Black Americans. Sleep 1994, 17 (Suppl. 8), S60–S67. [Google Scholar] [CrossRef]
- Irfan, M.; Schenck, C.H.; Howell, M.J. Non–Rapid Eye Movement Sleep and Overlap Parasomnias. Continuum 2017, 23, 1035–1050. [Google Scholar] [CrossRef]
- Rodriguez, C.L.; Foldvary-Schaefer, N. Clinical neurophysiology of NREM parasomnias. Handb. Clin. Neurol. 2019, 161, 397–410. [Google Scholar] [CrossRef]
- Heidbreder, A.; Frauscher, B.; Mitterling, T.; Boentert, M.; Schirmacher, A.; Hörtnagl, P.; Schennach, H.; Massoth, C.; Happe, S.; Mayer, G.; et al. Not Only Sleepwalking But NREM Parasomnia Irrespective of the Type Is Associated with HLA DQB1*05:01. J. Clin. Sleep Med. 2016, 12, 565–570. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Messineo, L.; Bakker, J.P.; Cronin, J.; Yee, J.; White, D.P. Obstructive sleep apnea and obesity: A review of epidemiology, pathophysiology and the effect of weight-loss treatments. Sleep Med. Rev. 2024, 78, 101996. [Google Scholar] [CrossRef]
- Ondo, W.G. Restless Legs Syndrome: Pathophysiology and Treatment. Curr. Treat. Options Neurol. 2014, 16, 317. [Google Scholar] [CrossRef]
- Goulart, L.I.; Delgado Rodrigues, R.N.; Prieto Peres, M.F. Restless legs syndrome and pain disorders: What’s in common? Curr. Pain Headache Rep. 2014, 18, 416. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P. Restless Leg Syndrome/Willis-Ekbom Disease Pathophysiology. Sleep Med. Clin. 2015, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jin, X.; Shan, Z.; Li, S.; Huang, H.; Li, P.; Peng, X.; Peng, Z.; Yu, K.; Bao, W.; et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005947. [Google Scholar] [CrossRef] [PubMed]
- Blazhkova, A.; Rehan, D.; Rzym, K.; Solisch, S.; Susłow, A.; Szczęsna, E.; Szwed, A. The Impact of Sleep Disorders on Cardiovascular Risk. J. Educ. Health Sport 2025, 81, 59851. [Google Scholar] [CrossRef]
- Naik, A.; Forrest, K.M.; Paul, O.; Issah, Y.; Valekunja, U.K.; Tang, S.Y.; Reddy, A.B.; Hennessy, E.J.; Brooks, T.G.; Chaudhry, F.; et al. Circadian regulation of lung repair and regeneration. JCI Insight 2023, 8, e164720. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Gan, Y.; Qu, X.; Lu, Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry 2016, 16, 375. [Google Scholar] [CrossRef]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef] [PubMed]
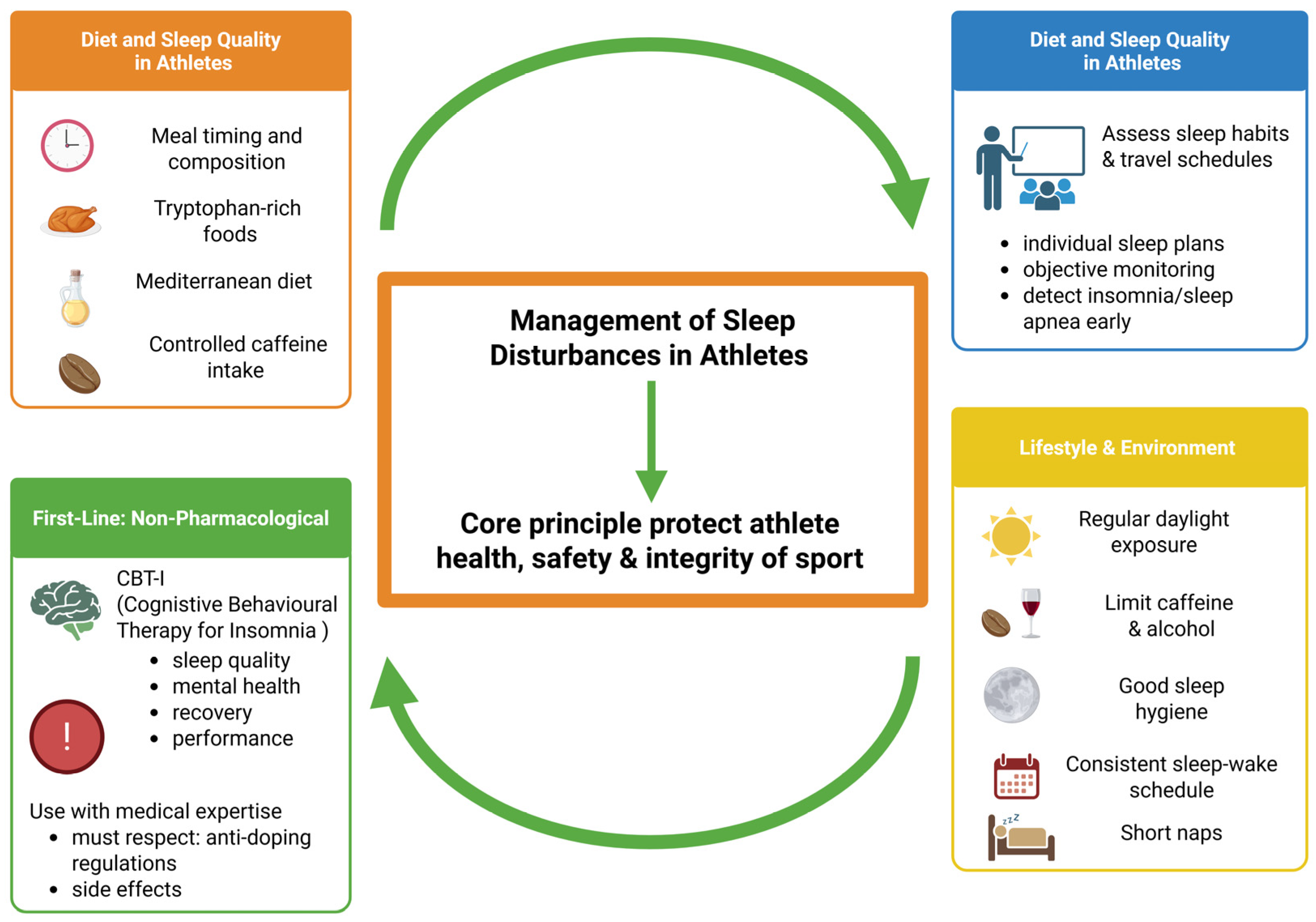
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2020, 55, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Iso, Y. Are sleep disorders a cause of sudden death during sports activities? Eur. Soc. Cardiol. 2021, 19. [Google Scholar]
- Van Cauter, E.; Spiegel, K.; Tasali, E.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9 (Suppl. S1), S23–S28. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- World Anti-Doping Agency. The 2024 Prohibited List. Montreal: WADA. 2024. Available online: https://www.wada-ama.org/en/resources/world-anti-doping-code-and-international-standards/prohibited-list (accessed on 25 July 2025).
- McDuff, D.R.; Garvin, J.; Greenberg, G.; O’Connor, F.G. Use of medications for sleep in elite athletes: A narrative review. Br. J. Sports Med. 2019, 53, 746–750. [Google Scholar] [CrossRef]
- Gmel, G.; Baggio, S.; Mohler-Kuo, M.; Daeppen, J.-B.; Studer, J. Nonmedical use of prescription medications by young men: Impact of cannabis use and sport. Prev. Med. Rep. 2021, 23, 101438. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.M.; Sospedra, I.; Ortiz, C.M.; Baladía, E.; Gil-Izquierdo, A.; Ortiz-Moncada, R. Intended or inadvertent doping? A review of the presence of doping substances in dietary supplements used in sports. Nutrients 2017, 9, 1093. [Google Scholar] [CrossRef]
- Anderson, C.; Sullivan, J.P. Sleep aids and stimulants in sport: Implications for athlete health and performance. Curr. Sports Med. Rep. 2019, 18, 319–325. [Google Scholar] [CrossRef]
- Pallesen, S.; Gundersen, H.S.; Bjorvatn, B. The role of melatonin in treating sleep disorders in athletes. Sleep Med. Clin. 2021, 16, 65–75. [Google Scholar] [CrossRef]
- World Anti-Doping Agency. Therapeutic Use Exemptions (TUEs). Montreal: WADA. 2024. Available online: https://www.wada-ama.org/en/athletes-support-personnel/therapeutic-use-exemptions-tues (accessed on 25 July 2025).
- Polska Agencja Antydopingowa. Lista Substancji i Metod Zabronionych 2025. Available online: https://antydoping.pl/lista-substancji-i-metod-zabronionych-2025/ (accessed on 25 July 2025).
- Saletu, B.; Grunberger, J.; Linzmayer, L. Comparative effects of trazodone and nitrazepam on human sleep and awakening qualities: A double-blind, placebo-controlled study. Int. J. Clin. Pharmacol. Res. 1991, 11, 83–93. [Google Scholar]
- Mendelson, W.B. A review of the evidence for the efficacy and safety of trazodone in insomnia. J. Clin. Psychiatry 2005, 66, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.J.; McCall, W.V.; Liguori, A. Cognitive, psychomotor and polysomnographic effects of trazodone in primary insomniacs. J. Sleep Res. 2011, 20, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Generali, J.A.; Cada, D.J. Trazodone: Insomnia (adults). Hosp. Pharm. 2015, 50, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Delrio, G. Pharmacokinetic and pharmacodynamic characteristics of a controlled-release formulation of trazodone versus the conventional formulation in healthy volunteers. Ital. J. Neurol. Sci. 1993, 14, 443–449. [Google Scholar] [CrossRef]
- Aslan, S.; Isik, E.; Cosar, B. The effects of mirtazapine on sleep: A placebo-controlled, double-blind study in young healthy volunteers. Sleep 2002, 25, 666–668. [Google Scholar] [CrossRef]
- Bakker, M.H.; Hugtenburg, J.G.; van Straten, A.; van der Horst, H.E.; Slottje, P. Effectiveness of low-dose amitriptyline and mirtazapine for insomnia disorder: Study protocol of a randomised, double-blind, placebo-controlled trial in general practice (the DREAMING study). BMJ Open 2021, 11, e047142. [Google Scholar] [CrossRef]
- Ramaekers, J.G.; Muntjewerff, N.D.; Van Veggel, L.M.A.; Uiterwijk, M.M.C.; O’Hanlon, J.F. Effects of nocturnal doses of mirtazapine and mianserin on sleep and on daytime psychomotor and driving performance in young, healthy volunteers. Hum. Psychopharmacol. Clin. Exp. 1998, 13 (Suppl. 2), S87–S97. [Google Scholar] [CrossRef]
- Mah, C.D. Perfect Nighttime Routine = Perfect Performance? Strategies to Improve Sleep and Recovery. Collegiate Strength and Conditioning Coaches Association (CSCCa). 2016. Available online: https://nhssca.us/wp-content/uploads/2017/10/Cheri_Mah_Handout.pdf (accessed on 18 August 2025).
- Doherty, R.; Madigan, S.; Warrington, G.; Ellis, J. Sleep and Nutrition Interactions: Implications for Athletes. Nutrients 2019, 11, 822. [Google Scholar] [CrossRef]
- Michalak, B.; Kopiczko, A.; Gajda, R.; Adamczyk, J.G. Recovery effect of self-myofascial release treatment using different type of a foam rollers. Sci. Rep. 2024, 14, 15762. [Google Scholar] [CrossRef] [PubMed]
- Braun-Trocchio, R.; Graybeal, A.J.; Kreutzer, A.; Warfield, E.; Renteria, J.; Harrison, K.; Williams, A.; Moss, K.; Shah, M. Recovery Strategies in Endurance Athletes. J. Funct. Morphol. Kinesiol. 2022, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Pearcey, G.E.; Bradbury-Squires, D.J.; Kawamoto, J.E.; Drinkwater, E.J.; Behm, D.G.; Button, D.C. Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J. Athl. Train. 2015, 50, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.A.; Costa, J.A.; Marques, E.A.; Brito, J.; Lastella, M.; Figueiredo, P. The Impact of Sleep Interventions on Athletic Performance: A Systematic Review. Sports Med. Open 2023, 9, 58. [Google Scholar] [CrossRef]
- Roberts, L.A.; Nosaka, K.; Coombes, J.S.; Peake, J.M. Cold water immersion enhances recovery of submaximal muscle function after resistance exercise. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R998–R1008. [Google Scholar] [CrossRef]
- Di Corrado, D.; Tortella, P.; Coco, M.; Guarnera, M.; Tusak, M.; Parisi, M.C. Mental imagery and stress: The mediating role of self-efficacy in competitive martial arts athletes. Front. Psychol. 2025, 16, 1517718. [Google Scholar] [CrossRef]
- Ahokas, E.K.; Hanstock, H.G.; Kyröläinen, H.; Ihalainen, J.K. Effects of repeated use of post-exercise infrared sauna on neuromuscular performance and muscle hypertrophy. Front. Sports Act. Living 2025, 7, 1462901. [Google Scholar] [CrossRef]
- Peacock, C.A.; Mena, M.; Sanders, G.J.; Silver, T.A.; Kalman, D.; Antonio, J. Sleep Data, Physical Performance, and Injuries in Preparation for Professional Mixed Martial Arts. Sports 2018, 7, 1. [Google Scholar] [CrossRef]
- Wiewelhove, T.; Döweling, A.; Schneider, C.; Hottenrott, L.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. A Meta-Analysis of the Effects of Foam Rolling on Performance and Recovery. Front. Physiol. 2019, 10, 376. [Google Scholar] [CrossRef]
- Moore, E.; Fuller, J.T.; Bellenger, C.R.; Saunders, S.; Halson, S.L.; Broatch, J.R.; Buckley, J.D. Effects of Cold-Water Immersion Compared with Other Recovery Modalities on Athletic Performance Following Acute Strenuous Exercise in Physically Active Participants: A Systematic Review, Meta-Analysis, and Meta-Regression. Sports Med. 2023, 53, 687–705. [Google Scholar] [CrossRef]
- Versey, N.G.; Halson, S.L.; Dawson, B.T. Water immersion recovery for athletes: Effect on exercise performance and practical recommendations. Sports Med. 2013, 43, 1101–1130. [Google Scholar] [CrossRef] [PubMed]
- Kirby, N.V.; Lucas, S.J.E.; Armstrong, O.J.; Weaver, S.R.; Lucas, R.A.I. Intermittent post-exercise sauna bathing improves markers of exercise capacity in hot and temperate conditions in trained middle-distance runners. Eur. J. Appl. Physiol. 2020, 121, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Volgemute, K.; Vazne, Z.; Malinauskas, R. The benefits of guided imagery on athletic performance: A mixed-methods approach. Front. Psychol. 2025, 16, 1500194. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.L.; Hainline, B.; Aron, C.M.; Baron, D.; Baum, A.L.; Bindra, A.; Budgett, R.; Campriani, N.; Castaldelli-Maia, J.M.; Currie, A.; et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br. J. Sports Med. 2019, 53, 667–699. [Google Scholar] [CrossRef]
- Nobari, H.; Banihashemi, M.; Saedmocheshi, S.; Prieto-González, P.; Oliveira, R. Overview of the impact of sleep monitoring on optimal performance, immune system function and injury risk reduction in athletes: A narrative review. Sci. Prog. 2023, 106, 368504231206265. [Google Scholar] [CrossRef]
- Coelho Silva, A.C.; Silva, A.; Edwards, B.J.; Tod, D.; Amaral, A.S.; de Alcântara Borba, D.; Grade, I.; de Mello, M.T. Sleep extension in athletes: What we know so far. Sleep Med. 2021, 77, 128–135. [Google Scholar] [CrossRef]
- Arnal, P.J.; Lapole, T.; Erblang, M.; Guillard, M.; Bourrilhon, C.; Léger, D.; Chennaoui, M.; Millet, G.Y. Sleep Extension before Sleep Loss: Effects on Performance and Neuromuscular Function. Med. Sci. Sports Exerc. 2016, 48, 1595–1603. [Google Scholar] [CrossRef]
- Van Ryswyk, E.; Weeks, R.; Bandick, L.; O’Keefe, M.; Vakulin, A.; Catcheside, P.; Barger, L.; Potter, A.; Poulos, N.; Wallace, J.; et al. A novel sleep optimisation programme to improve athletes’ well-being and performance. Eur. J. Sport Sci. 2017, 17, 144–151. [Google Scholar] [CrossRef]
- Schwartz, J.; Simon, R.D., Jr. Sleep extension improves serving accuracy: A study with college varsity tennis players. Physiol. Behav. 2015, 151, 541–544. [Google Scholar] [CrossRef]
- Mah, C.D.; Kezirian, E.J.; Marcello, B.M.; Dement, W.C. Poor sleep quality and insufficient sleep of a collegiate student-athlete population. Sleep Health 2018, 4, 251–257. [Google Scholar] [CrossRef]
- Mah, C.D.; Sparks, A.J.; Samaan, M.A.; Souza, R.B.; Luke, A. Sleep restriction impairs maximal jump performance and joint coordination in elite athletes. J. Sports Sci. 2019, 37, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Efron, B.; Mah, C.D.; Malhotra, A. The impact of circadian misalignment on athletic performance in professional football players. Sleep 2013, 36, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.D.; Mah, K.E.; Kezirian, E.J.; Dement, W.C. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep 2011, 34, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.D. Extended sleep and the effects on mood and athletic performance in collegiate swimmers. Sleep 2008, 31, A128. [Google Scholar]
- Mah, C.D.; Mah, K.E.; Dement, W.C. Athletic performance improvements and sleep extension in collegiate tennis players. Sleep 2009, 32, A155. [Google Scholar]
- Kisiolek, J.N.; Smith, K.A.; Baur, D.A.; Willingham, B.D.; Morrissey, M.C.; Leyh, S.M. Sleep duration correlates with performance in ultra-endurance triathlon. Int. J. Sports Physiol. Perform. 2021, 17, 226–233. [Google Scholar] [CrossRef]
- Mah, C.D.; Anguera, J.A.; Gazzaley, A.; Luke, A. 0749 Sleep loading improves visual search response time and reduces fatigue in professional baseball players. Sleep 2017, 40, A278. [Google Scholar] [CrossRef]
- Driller, M.W.; Mah, C.D.; Halson, S. The revised athlete sleep behavior questionnaire (ASBQ-2): Aligning with the latest in sleep science research. Int. J. Sports Sci. Coach. 2025, 17479541251374806. [Google Scholar] [CrossRef]
- Driller, M.W.; Halson, S.L.; Mah, C.D.; Suppiah, H.; Lastella, M.; Miller, D.J. Teamwork Makes the Dream Work: Who Should Be Managing Athletes on Matters Related to Sleep? Sports Med. 2025, 55, 2065–2071. [Google Scholar] [CrossRef]
- Strelau, J.; Doliński, D. (Eds.) Psychologia. Podręcznik Akademicki; GWP: Gdańsk, Poland, 2008; Volume 1, pp. 588–598. [Google Scholar]
- Davies, K.; Evans, G. Perceived stress, coping strategies, and psychological well-being in collegiate athletes. Sport, Exerc. Perform. Psychol. 2024, 13, 345–358. [Google Scholar] [CrossRef]
- Roden, L.; Rudner, T.; Rae, D. Impact of Chronotype on Athletic Performance: Current Perspectives. ChronoPhysiology Ther. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Battaglini, M.P.; Filho, D.M.P.; Calais, S.L.; Miyazaki, M.C.O.S.; Neiva, C.M.; Espada, M.C.; de Moraes, M.G.; Verardi, C.E.L. Analysis of Progressive Muscle Relaxation on Psychophysiological Variables in Basketball Athletes. Int. J. Environ. Res. Public Health 2022, 19, 17065. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Haraldsdottir, K.; Watson, D. Mindfulness in Athletes. Curr. Sports Med. Rep. 2021, 20, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Vveinhardt, J.; Kaspare, M. The Relationship between Mindfulness Practices and the Psychological State and Performance of Kyokushin Karate Athletes. Int. J. Environ. Res. Public Health 2022, 19, 4001. [Google Scholar] [CrossRef]
- Nien, J.-T.; Chen, N.-C.; Kee, Y.-H.; Wu, C.-H.; Ahn, J.; Yu, C.-Y.; Chi, L.; Chang, Y.-K. Athletes with meditation experience counteract the detrimental effect of mental fatigue on endurance performance and neurocognitive functions. J. Sports Sci. 2024, 42, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, L.; Ma, C.; Wen, H.; Zhao, X. Effects of long-term mindfulness meditation training on attentional capacity in professional male fencer athletes. Sci. Rep. 2025, 15, 13040. [Google Scholar] [CrossRef]
- Halson, S.L. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44 (Suppl. S1), S13–S23. [Google Scholar] [CrossRef]
- Patel, A.; Cheung, J. The effect of mediterranean diet and chrononutrition on sleep quality: A scoping review. Nutr. J. 2025, 24, 31. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Møller, S.S.; Faber, J.; Sundgot-Borgen, J.; Sjödin, A. Low-energy density and high fiber intake are dietary concerns in female endurance athletes. Scand. J. Med. Sci. Sports 2016, 26, 1060–1071. [Google Scholar] [CrossRef]
- Scrivin, R.; Costa, R.J.S.; Pelly, F.; Lis, D.; Slater, G. An exploratory study of the management strategies reported by endurance athletes with exercise-associated gastrointestinal symptoms. Front. Nutr. 2022, 9, 1003445. [Google Scholar] [CrossRef]


| Age Group | Total Sleep Duration | Sleep Characteristics | Changes in Architecture | Reference |
|---|---|---|---|---|
| Infants (0–12 months) | ~16–18 h/day in newborns, decreasing to 14–15 h by 12 months | Irregular sleep bouts of 2.5–4 h; categories include quiet sleep (NREM-like), active sleep (REM-like), and indeterminate sleep. Newborns first enter REM, unlike adults. By 2–3 months, the circadian rhythms start emerging; by 6 months, longer nighttime sleep (~6 h) develops. | High proportion of REM-like active sleep; immature circadian regulation initially. | [27] |
| Toddlers and Preschoolers (1–6 years) | Decreases gradually from ~13 h/day at age 2 to ~11 h/day by age 5 | By age 6, circadian preferences (chronotypes) become more pronounced. Children have longer REM latency compared to adolescents. | More time spent in deep slow-wave sleep (N3); well-consolidated sleep. | [28] |
| Adolescents (10–18 years) | ~9–10 h/day | Puberty-related hormonal shifts reduce slow-wave sleep duration and increase time spent in N2. Excessive daytime sleepiness is common during mid-adolescence. | Decline in N3 (deep sleep), longer N2 stage; circadian phase often delayed. | [29] |
| Adults & Older Adults | Total sleep time gradually decreases with age | Sleep becomes more fragmented; older adults often experience advanced sleep phase syndrome (earlier sleep onset and awakening by ~1–1.5 h). | Reduced N3 slow-wave sleep, shorter REM periods, and more nighttime awakenings. | [30] |
| Sleep-Related Factor | Impact on Athletic Performance |
|---|---|
| Total sleep deprivation (24 h) | Increase in cortisol (+21%); decrease in testosterone (−24%); reduced muscle protein synthesis (−18%); elevated blood pressure; reduced anaerobic power and endurance; fatigue and mood disturbances |
| Partial sleep restriction (≤6 h/24 h) | Decrease in strength, power, endurance, and technical skills; poorer results in speed and HIIT tests; most pronounced decline in the afternoon; each additional missing hour = −0.4% in performance outcomes |
| Sleep deficiency in young athletes (under 14, under 17) | 61–64% reported poor sleep quality (PSQI ≥ 5); 8% showed insomnia symptoms; increased risk of musculoskeletal injuries (1.7-fold higher with ≤8 h sleep) |
| Naps (30–90 min) | Can restore strength and power to baseline after partial sleep restriction; improve cognitive function and reaction speed; naps shorter than 30 min = risk of sleep inertia |
| Sleep extension | Improved sprint performance, greater accuracy in passes/serves (tennis, basketball), enhance swimming efficiency; prolonged time to exhaustion; better reaction and alertness; reduced fatigue |
| Sleep quality (latency, NREM/REM phases) | Shorter sleep latency and less light sleep → better results in jumps, sprints, power tests; no significant correlation with N3 or REM |
| Sleep disorders (insomnia, OSA, RLS) | Increased risk of injuries, reduced endurance and immunity, poorer mental health; prevalence of sleep disorders exceeds 50% among elite and youth athletes |
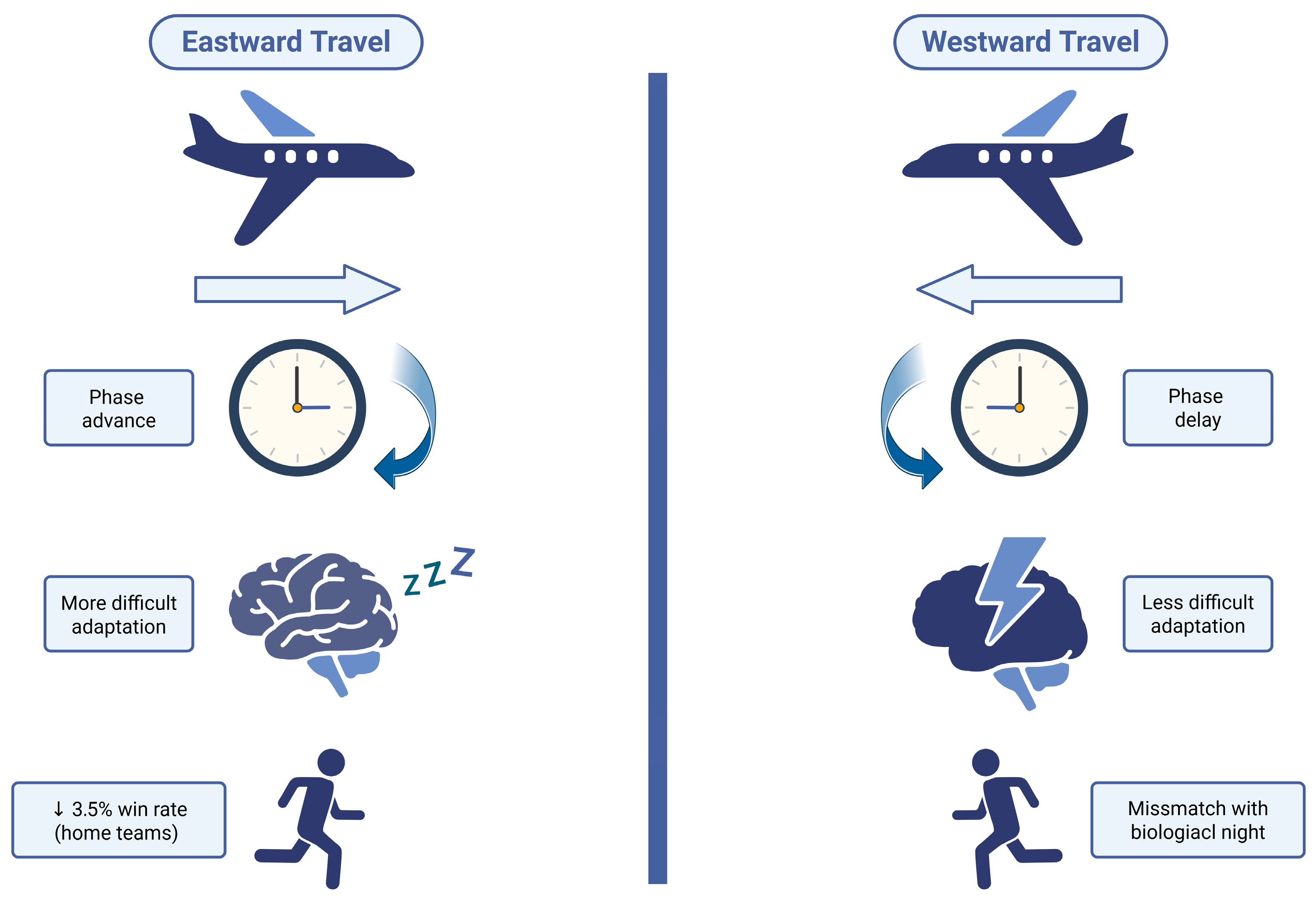
| Jet lag and circadian rhythm disruption | Reduced concentration, fatigue, irritability; poorer performance of MLB teams after eastward travel (−3.5% fewer home wins); higher risk of injuries and mood disturbances |
| Diet and substances |
|
| Category | Trial/Reference | Design & Participants | Intervention | Outcomes Measured | Key Findings (Short) | Citation |
|---|---|---|---|---|---|---|
| Nutritional/physiological | L-arginine supplementation vs. placebo | Randomised controlled; 20 men (26–35 yr) | 1 g/day L-arginine vs. placebo for 8 wk; tested after 24 h sleep deprivation & anaerobic exercise | Gene expression (BAX, BCL2, BMAL1, CCAR2) | L-arginine increased BCL2 & BMAL1 and decreased BAX & CCAR2 vs. placebo | [36] |
| Behavioural (napping) | Partial sleep restriction + 1-h nap (Brotherton) | Randomised cross-over; 15 resistance-trained men | Three conditions: normal sleep; two nights with 3-h sleep restriction; same sleep restriction + 1-h midday nap | Grip strength, bench/leg press, mood & alertness | Sleep restriction reduced strength; a 1-h nap restored performance and improved mood | [74] |
| Behavioural (napping) | 60-min nap after sleep loss | Randomised controlled; 30 strength-trained men | Two nights of 3-h or 5-h sleep; 60-min nap vs. no nap | Alertness, vigor, strength metrics (peak velocity) | A 60-min nap improved alertness & peak velocity after 3-h sleep loss; benefits were smaller after 5-h sleep loss | [75] |
| Behavioural (napping) | Nap duration study | Randomised order; 17 physically active men | 5-h sleep then nap opportunities of 0, 25, 35 or 45 min | 5-m shuttle-run best & total distance; perceived exertion | 25- & 45-min naps improved best distance; all nap durations improved total distance; 45-min nap reduced perceived exertion | [76] |
| Behavioural (sleep extension) | Sleep extension in varsity basketball players | Within-subject; 11 male collegiate players | Baseline sleep 2–4 wk then sleep extension 5–7 wk (target ≈10 h/night) | Sprint time, free-throw & 3-point accuracy, reaction time, mood | Sleep extension increased nightly sleep (~111 min) and improved sprint times & shooting accuracy; reaction time and mood also improved | [135] |
| Pharmacological (antidepressant) | Trazodone in primary insomniacs | Randomised, double-blind, crossover; 16 primary insomniacs | Trazodone 50 mg nightly vs. placebo for 7 days | Sleep architecture & maintenance; cognitive & psychomotor tests | Improved sleep maintenance & slow-wave sleep; small impairments in short-term memory, verbal learning, equilibrium & muscle endurance | [129] |
| Pharmacological (antidepressant) | Trazodone in Alzheimer’s disease | Parallel randomised controlled; 30 patients with Alzheimer’s disease & sleep disorders | Trazodone 50 mg nightly vs. placebo for 2 wk | Total nocturnal sleep time, sleep efficiency, cognitive function & daytime sleepiness | Increased sleep time (~42.5 min) and improved sleep efficiency without worsening cognition or daytime wakefulness | [128] |
| Pharmacological (antidepressant) | Mirtazapine (Sleep 2002) | Randomised, double-blind, placebo-controlled; 20 young healthy volunteers | Single 30 mg dose of mirtazapine vs. placebo | Sleep efficiency, slow-wave sleep, awakenings, REM sleep, psychomotor performance | Increased sleep efficiency & slow-wave sleep; reduced stage 1 sleep & awakenings; no effect on REM; sedation and psychomotor slowing reported | [132] |
| Pharmacological (antidepressant) | Low-dose mirtazapine (DREAMING trial) | Pragmatic randomised double-blind trial; 80 adults with insomnia disorder | Mirtazapine 7.5–15 mg/day or amitriptyline 10–20 mg/day vs. placebo for 16 wk | Insomnia Severity Index (ISI); rates of improvement & recovery | At 6 wk, mirtazapine reduced ISI by ≈6 points vs. placebo and 52 % improved vs. 14 % on placebo; benefits not maintained at 12 wk | [133] |
| Pharmacological (antidepressant) | Mirtazapine & mianserin cross-over | Randomised double-blind cross-over; 18 healthy volunteers | Mirtazapine 15→30 mg and mianserin 30→60 mg nocte vs. placebo for 15 days | Psychomotor & driving performance; sleep duration; side-effects | Day 2: slight psychomotor & driving impairment; tolerance incomplete by day 16; both drugs increased sleep duration; participants reported persistent lethargy & drowsiness | [134] |
| Type of Sport | Recommended Sleep Duration [136] | Napping | Sleep Hygiene and Routines | Specific Recommendations/Recovery Considerations |
|---|---|---|---|---|
| Endurance (e.g., long-distance running, triathlon, cycling) | 8–10 h per night (up to 9+ h during intense training periods) | Short naps of 20–30 min, preferably early afternoon (13:00–15:00); long naps >60 min and late afternoon/evening naps are not recommended [76] | Relaxation techniques (stretching, diaphragmatic breathing), warm and dim lighting, high tryptophan meal 2–3 h before bedtime [135], cool and quiet sleeping environment | Foam rolling (10–20 min) [137], active recovery (15–30 min), massage and compression after competition (2–4 h), cold therapies with mixed evidence (10–15 min), psychological skills (mindfulness, relaxation, imagery)—3–4×/week, 10–20 min), high performers applying wider range, coaches and peers as main sources, sauna (post-exercise/passive heat): short, repeated passive heating sessions after training (post-exercise sauna)—~20–30 min after the session, 2–3×/week for several weeks, mental techniques (imagery, mindfulness, breathing, relaxation): sessions ~15–30 min, 1–3×/week; shorter (5–10 min) breathing techniques before competition, cold-water immersion: 5–15 min at ~10–15 °C [138]. |
| Strength (e.g., weightlifting, bodybuilding, crossfit) | 8–9 h per night (up to 9–10 h during high-volume periods) | Short naps of 20–40 min, especially on days with high training load; avoid long naps >60 min and late evening naps [76] | Maintaining consistent schedules, creating a cool dark environment—environmental adjustments (16–21 °C room, limiting caffeine and screens pre-bed timing consistency (fixed bed/wake to align with circadian peaks), pre-sleep wind-down (1–2 h dim lights, no screens/caffeine post-3 PM, light reading), blackout curtains, white noise) [82] | 20 min of foam rolling applied immediately, then at 24 h and 48 h post-exercise [139], post-training cold-water Immersion (10 °C for 10 min) within 24–48 h, sauna (post-exercise/passive heat) ~20–30 min post-session, 2–3×/week, mental techniques (imagery, mindfulness, breathing, relaxation): sessions ~15–30 min, 1–3×/week; short 5–10 min visualization/breathing routines before heavy lifts or contests [141]. |
| Precision/Technical (e.g., archery, shooting, golf, gymnastics) | 8–9 h per night | Short naps of 20–30 min, preferably early afternoon (13:00–15:00); long or late naps are not recommended [76] | Fixed bed/wake schedules, avoiding shifts >1 h; wake with natural light, 1–2 h wind-down with relaxation (reading, warm bath, mindfulness); avoid stress-inducing content, no caffeine post-noon, alcohol/nicotine, high-intensity exercise 1–2 h pre-bed, or fluids close to bed, screens off 1–2 h, environment adjustments: cool (16–21 °C), dark (blackouts), quiet (earplugs/white noise) room; bed for sleep/intimacy only [82,140]. | Mental Training and visualization: 10–15 min daily sessions pre/post-training, mindfulness or breathing exercises (5–10 min), foam rolling: 5–10 min on key stabilizers post-session, sauna: 10–20 min infrared post-training, 2–3×/week, compression garments/cryotherapy for acute recovery (high evidence per reviews), core exercises for baseline stability [142,143] |
| Combat/Fighting (e.g., boxing, MMA, judo, wrestling) | 8–9 h per night (up to 9–10 h during intense preparation) | Short naps of 20–30 min, preferably early afternoon (13:00–15:00); long or late naps are not recommended [76] | Maintaining consistent bed and wake times, avoiding blue light from screens 2 h before bed to preserve melatonin, limiting caffeine after lunch, and creating a cool (60–70 °F), dark, quiet environment to enhance sleep quality and mitigate fatigue from high-impact training [82,144] | Foam rolling before training (90–120 s total per muscle group — e.g., 3 × 30–40 s per muscle group) — moderate pressure; avoid extreme pain [145], cold-water immersion immediately after very intense training sessions (10–15 °C, 5–15 min. immersion; alternatively 10–12 °C for shorter durations) [146], contrast baths/hydrotherapy after exercise or between intense training days via alternating cold/hot immersions (e.g., 1–3 min cold, 1–3 min hot) repeated to a total of 6–12 min; in practice, 6–12 min [147], sauna (traditional/infrared) after endurance sessions or as a heat-adaptation intervention during the preparatory phase or after training, post-exercise sauna: ~20–30 min sessions; in one protocol ~28 ± 2 min at 101–108 °C (dry, low humidity) 3×/week for 3 weeks, an alternative protocol (~20 min at ~43 °C) [148], mental techniques (relaxation, imagery, breathing, mental toughness)—applied systematically in psychological training (short sessions 2×/week) and as a pre-fight routine/ritual (brief breathing techniques, progressive relaxation before sleep/after a fight): sessions ~15–30 min, 1–3×/week in the training cycle; shorter (5–10 min), breathing exercises 3–10 min; progressive relaxation before sleep 10–20 min [149] |
| Team Sports (e.g., soccer, basketball, volleyball, hockey) | 8–10 h per night | Short or compensatory naps of 20–90 min, especially on match days and during travel; avoid late evening naps [63] | Relaxation routine, light exposure management, use of eye masks and earplugs during travel, cool and quiet sleeping environment [63] | Sleep and naps mitigate jet lag effects, support concentration, reaction time, and coordination; travel strategies: gradual sleep adjustment, daylight exposure, caffeine/alcohol avoidance, compensatory naps; insufficient sleep increases risk of injury and impairs cognitive and physical performance [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, F.; Bartkowiak-Wieczorek, J.; Matecka, M.; Jenczylik, K.; Brzezińska, K.; Gajniak, P.; Marchwiak, S.; Kaczmarek, K.; Nowak, M.; Kmiecik, M.; et al. Sleep and Athletic Performance: A Multidimensional Review of Physiological and Molecular Mechanisms. J. Clin. Med. 2025, 14, 7606. https://doi.org/10.3390/jcm14217606
Kaczmarek F, Bartkowiak-Wieczorek J, Matecka M, Jenczylik K, Brzezińska K, Gajniak P, Marchwiak S, Kaczmarek K, Nowak M, Kmiecik M, et al. Sleep and Athletic Performance: A Multidimensional Review of Physiological and Molecular Mechanisms. Journal of Clinical Medicine. 2025; 14(21):7606. https://doi.org/10.3390/jcm14217606
Chicago/Turabian StyleKaczmarek, Franciszek, Joanna Bartkowiak-Wieczorek, Monika Matecka, Karolina Jenczylik, Kinga Brzezińska, Paulina Gajniak, Sonia Marchwiak, Katarzyna Kaczmarek, Michał Nowak, Michał Kmiecik, and et al. 2025. "Sleep and Athletic Performance: A Multidimensional Review of Physiological and Molecular Mechanisms" Journal of Clinical Medicine 14, no. 21: 7606. https://doi.org/10.3390/jcm14217606
APA StyleKaczmarek, F., Bartkowiak-Wieczorek, J., Matecka, M., Jenczylik, K., Brzezińska, K., Gajniak, P., Marchwiak, S., Kaczmarek, K., Nowak, M., Kmiecik, M., Stężycka, J., Krupa, K. K., & Mądry, E. (2025). Sleep and Athletic Performance: A Multidimensional Review of Physiological and Molecular Mechanisms. Journal of Clinical Medicine, 14(21), 7606. https://doi.org/10.3390/jcm14217606









