Modified Hofmann Articulated Spacer in the Treatment of Peri-Prosthetic Joint Infection of the Knee—Surgical Technique and Early Clinical Evaluation
Abstract
1. Introduction
2. Materials and Methods
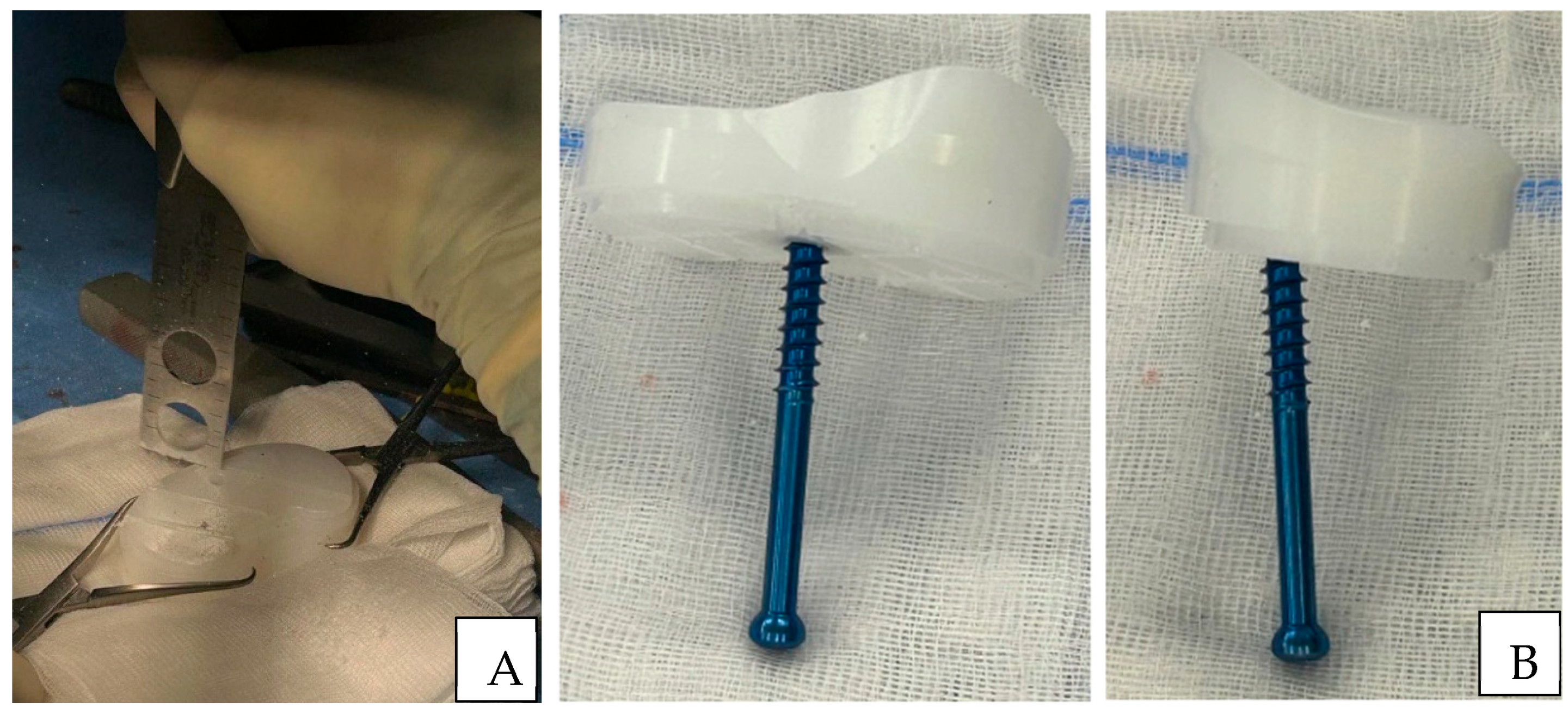
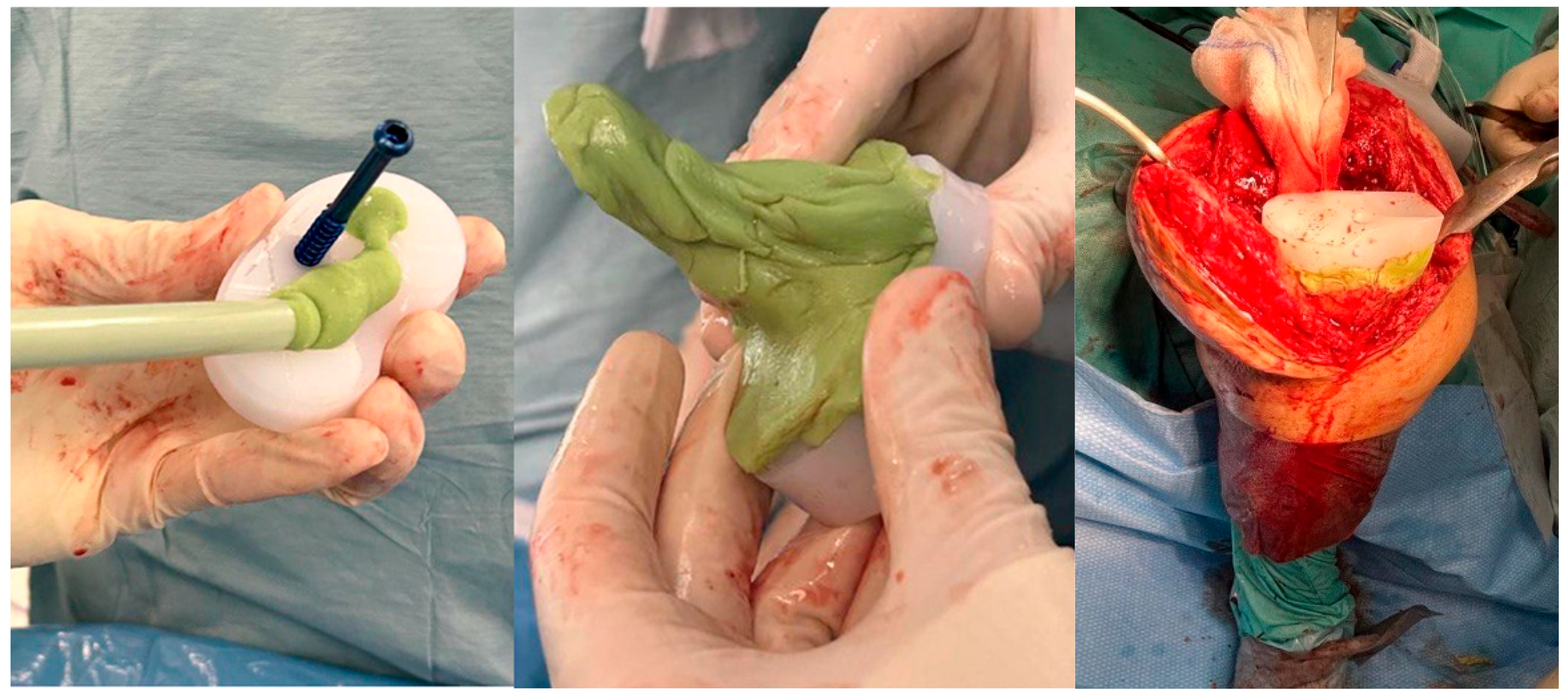
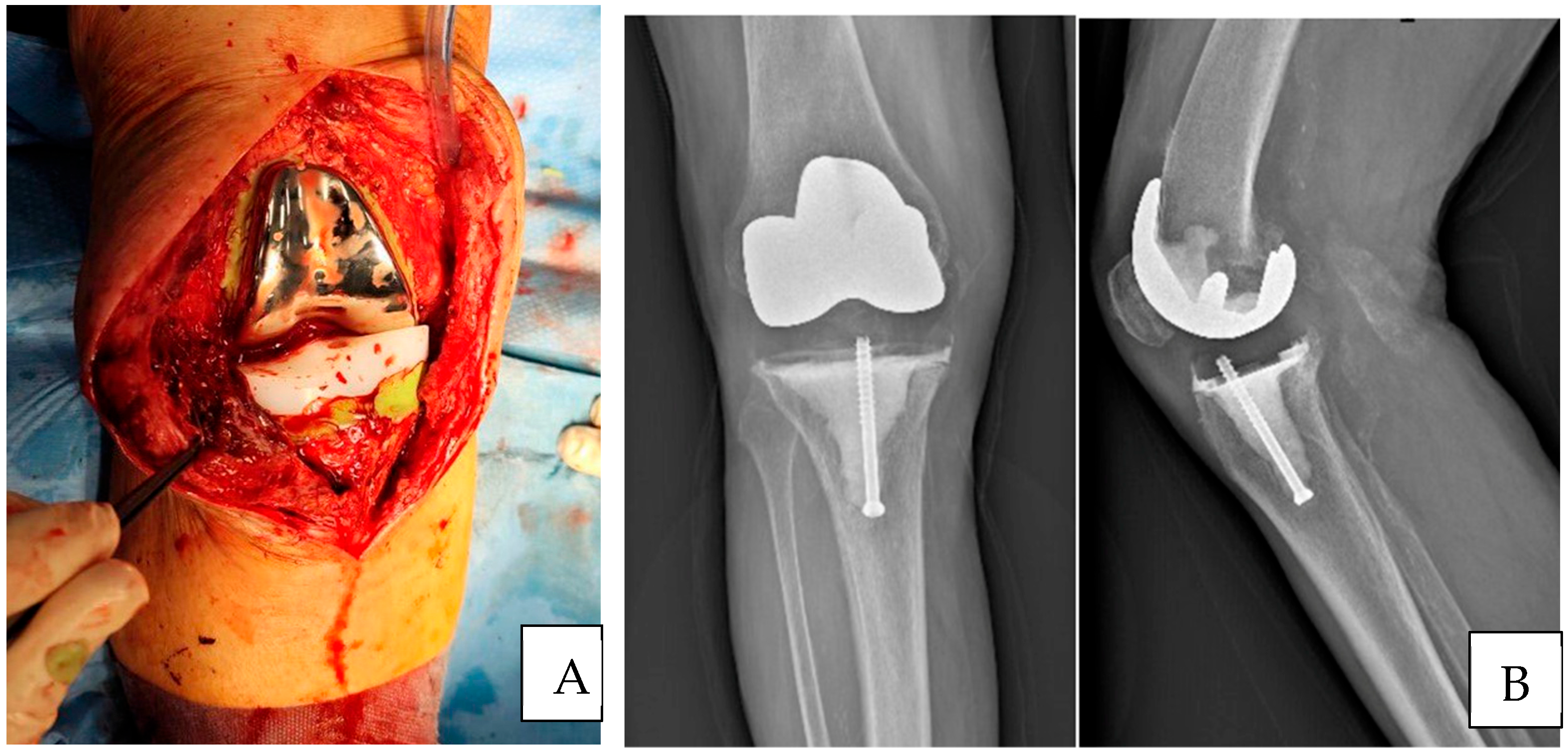
Surgical Technique
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| mHAS | Modified Hofmann Articulated Spacer |
| PROMs | Patient-Reported Outcome Measures |
| KSS | Knee Society Score |
| OKS | Oxford Knee Score |
| TKA | Total knee arthroplasty |
| PJI | Periprosthetic Joint Infection |
| DAIR | Debridement, Antibiotic and Retention of the Implant |
| DAPRI | Debridement, Antibiotic Pearls and Retention of Implant |
| AORI | Anderson Orthopedic Research Institute |
| CR | Cruciate Retaining |
| MSSA | Methicillin-Sensitive Staphylococcus Aureus |
| MRSE | Methicillin-Resistant Staphylococcus Epidermidis |
| EQ-5D-5L VAS | EuroQol-5 Dimension-5 Level Visual Analogue Scale |
| CCK | constrained condylar knee |
References
- Varacallo, M.A.; Luo, T.D.; Mabrouk, A.; Johanson, N.A. Total Knee Arthroplasty Techniques. In StatPearls; Updated 6 May 2024; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499896/ (accessed on 1 March 2025).
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int. J. Artif. Organs 2012, 35, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, G.; Bolduc, M.E.; Atkins, B.L.; Kendrick, B.J.L.; McLardy-Smith, P.; Murray, D.W.; Gundle, R.; Taylor, A.H. Functional outcome of debridement, antibiotics and implant retention in periprosthetic joint infection involving the hip: A case-control study. Bone Jt. J. 2017, 99-B, 614–622. [Google Scholar] [CrossRef]
- Kocaoğlu, H.; Hennes, F.; Abdelaziz, H.; Neufeld, M.E.; Gehrke, T.; Citak, M. Survival analysis of one-stage exchange of infected unicompartmental knee arthroplasty: A single-center study with minimum 3 years follow-up. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gililland, J.M.; Carlson, V.R.; Fehring, K.; Springer, B.D.; Griffin, W.L.; Anderson, L.A. Balanced, Stemmed, and Augmented Articulating Total Knee Spacer Technique. Arthroplast. Today 2020, 6, 981–986. [Google Scholar] [CrossRef] [PubMed]
- De Mauro, D.; Festa, E.; Di Gennaro, D.; Ascione, T.; Coletta, G.; Mariconda, M.; Balato, G. Augmented Articulating Spacers in Infected Total Knee Arthroplasty: Surgical Technique. Healthcare 2024, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K. Therapeutic Use of Antibiotic-loaded Bone Cement in the Treatment of Hip and Knee Joint Infections. J. Bone Jt. Infect. 2017, 2, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.A.; Kane, K.R.; Tkach, T.K.; Plaster, R.L.; Camargo, M.P. Treatment of infected total knee arthroplasty using an articulating spacer. Clin. Orthop. Relat. Res. 1995, 321, 45–54. [Google Scholar] [CrossRef]
- Roof, M.A.; Baylor, J.L.; Bernstein, J.A.; Antonelli, B.J.; Kugelman, D.N.; Egol, A.J.; Melnic, C.M.; Chen, A.F.; Long, W.J.; Aggarwal, V.K.; et al. Comparing the Efficacy of Articulating Spacer Constructs for Knee Periprosthetic Joint Infection Eradication: All-Cement vs. Real-Component Spacers. J. Arthroplast. 2021, 36, S320–S327. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Mahmoud, Y.; Forte, S.A.; Novack, T.A.; Nace, J. One and a Half-Stage Revision with Prosthetic Articulating Spacer for Definitive Management of Knee Periprosthetic Joint Infection. Arthroplast. Today 2023, 19, 100993. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, P.; Su, S.; Hedlund, H.; Suh, G.; Maloney, W.J.; Goodman, S.B.; Huddleston, J.I., 3rd. Treatment of Periprosthetic Knee Infection with a Two-stage Protocol Using Static Spacers. Clin. Orthop. Relat. Res. 2016, 474, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Engh, G.A.; Ammeen, D.J. Bone loss with revision total knee arthroplasty: Defect classification and alternatives for reconstruction. Instr. Course Lect. 1999, 48, 167–175. [Google Scholar] [PubMed]
- Matthews, P.C.; Berendt, A.R.; McNally, M.A.; Byren, I. Diagnosis and management of prosthetic joint infection. BMJ 2009, 338, b1773. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D.; INFORM Team. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Fehring, K.A.; Ollivier, M.; Mabry, T.M.; Hanssen, A.D.; Abdel, M.P. Repeat two-stage exchange arthroplasty for prosthetic hip re-infection. Bone Jt. J. 2018, 100-B, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Belay, E.S.; Wixted, C.M.; Kim, B.I.; Wellman, S.S.; Jiranek, W.A.; Bolognesi, M.P.; Seyler, T.M. A Permanent Articulating Spacer Versus Two-Stage Exchange for Chronic Periprosthetic Joint Infection: A Propensity Score-Matched Study. J. Arthroplast. 2023, 38, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Khnanisho, M.; Horne, C.; Deckey, D.G.; Tarabichi, S.; Seyler, T.M.; Bingham, J.S. 1.5-Stage Revision for the Treatment of Periprosthetic Joint Infection: A Systematic Review. J. Arthroplast. 2025, 40, 1945–1951.e2. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Hwang, S.H.; Baek, H.J.; Park, C.H. Aseptic survival of the 1.5-stage exchange arthroplasty for periprosthetic joint infection was acceptable when using an autoclaved femoral component and a new polyethylene insert. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Freiberg, A.A.; Malchau, H.; Rubash, H.E.; Kwon, Y.M. The fate of unplanned retention of prosthetic articulating spacers for infected total hip and total knee arthroplasty. J. Arthroplast. 2014, 29, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Regis, D.; Sandri, A.; Magnan, B.; Bartolozzi, P. Six-year follow-up of a preformed spacer for the management of chronically infected total hip arthroplasty. Arch. Orthop. Trauma Surg. 2010, 130, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean Value |
|---|---|
| Total number of patients | 9 |
| Sex (M/F) | 5/4 |
| Age (years) | 74 (65–83) |
| Surgical time (minutes) | 128.5 (SD: 30.2) |
| Follow-up (months) | 8.12 |
| Bacterial Pathogens | % |
|---|---|
| Methicillin-Sensitive Staphylococcus aureus (MSSA) | 56% |
| Streptococcus agalactiae | 11% |
| Streptococcus anginosus pathogen | 11% |
| Corynebacterium acnes | 11% |
| Methicillin-Resistant Staphylococcus epidermidis (MRSE) | 11% |
| Outcomes | Mean Value |
|---|---|
| Knee range of motion | 95° (80–110°) |
| Knee Society Score (KSS) | 71.9 (53.7–90) |
| Oxford Knee Score (OKS) | 30.8 (22–39) |
| Patient | Bacterial Pathogens | Antibiotic | Duration of Antibiotic Therapy | Type of Ambulation | Final Decision |
|---|---|---|---|---|---|
| 1 | S. agalactiae | Amoxicillin OP Cefazolin IV | 12 weeks | Walking without crutches | Maintained Hofmann spacer |
| 2 | S. anginosus | Ceftriaxone IVAmoxicillin OP | 6 weeks | Walking with crutches | Maintained Hofmann spacer |
| 3 | MRSE | Ceftriaxone+ Teicoplanin IV, Dalbavancin IV | 3 weeks | Walking without crutches | Implantation of CCK prosthesis (2-stage) |
| 4 | MSSA | Cefazolin IV Dalbavancin IV | 3 weeks | Walking with crutches | Knee arthrodesis |
| 5 | MSSA | Cefazolin IV Dalbavancin IV | 3 weeks | Walking without crutches | Implantation of CCK prosthesis (2-stage) |
| 6 | MSSA | Ceftriaxone IV Doxiciclin OP | 8 weeks | Walking without crutches | Maintained Hofmann spacer |
| 7 | MSSA | Ceftriaxone IV Doxiciclin OP | 8 weeks | Walking with crutches | Maintained Hofmann spacer |
| 8 | MSSA | Cefazolin IV Dalbavancin IV | 3 weeks | Walking without crutches | Implantation of CCK prosthesis (2-stage) |
| 9 | C. acnes | Vancomicin IV Amoxicillin+ Rifampicin OP | 6 weeks | Walking without crutches | Implantation of CCK prosthesis (2-stage) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risitano, S.; Sanfilippo, S.; Limone, B.; Artiaco, S.; Faggiani, M.; Capella, M.; Massè, A. Modified Hofmann Articulated Spacer in the Treatment of Peri-Prosthetic Joint Infection of the Knee—Surgical Technique and Early Clinical Evaluation. J. Clin. Med. 2025, 14, 7605. https://doi.org/10.3390/jcm14217605
Risitano S, Sanfilippo S, Limone B, Artiaco S, Faggiani M, Capella M, Massè A. Modified Hofmann Articulated Spacer in the Treatment of Peri-Prosthetic Joint Infection of the Knee—Surgical Technique and Early Clinical Evaluation. Journal of Clinical Medicine. 2025; 14(21):7605. https://doi.org/10.3390/jcm14217605
Chicago/Turabian StyleRisitano, Salvatore, Simone Sanfilippo, Beatrice Limone, Stefano Artiaco, Marianna Faggiani, Marcello Capella, and Alessandro Massè. 2025. "Modified Hofmann Articulated Spacer in the Treatment of Peri-Prosthetic Joint Infection of the Knee—Surgical Technique and Early Clinical Evaluation" Journal of Clinical Medicine 14, no. 21: 7605. https://doi.org/10.3390/jcm14217605
APA StyleRisitano, S., Sanfilippo, S., Limone, B., Artiaco, S., Faggiani, M., Capella, M., & Massè, A. (2025). Modified Hofmann Articulated Spacer in the Treatment of Peri-Prosthetic Joint Infection of the Knee—Surgical Technique and Early Clinical Evaluation. Journal of Clinical Medicine, 14(21), 7605. https://doi.org/10.3390/jcm14217605






