Magnetic Resonance Imaging-Based Assessment of Bone Marrow Fat and T2 Relaxation in Adolescents with Obesity and Liver Steatosis: A Feasibility Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
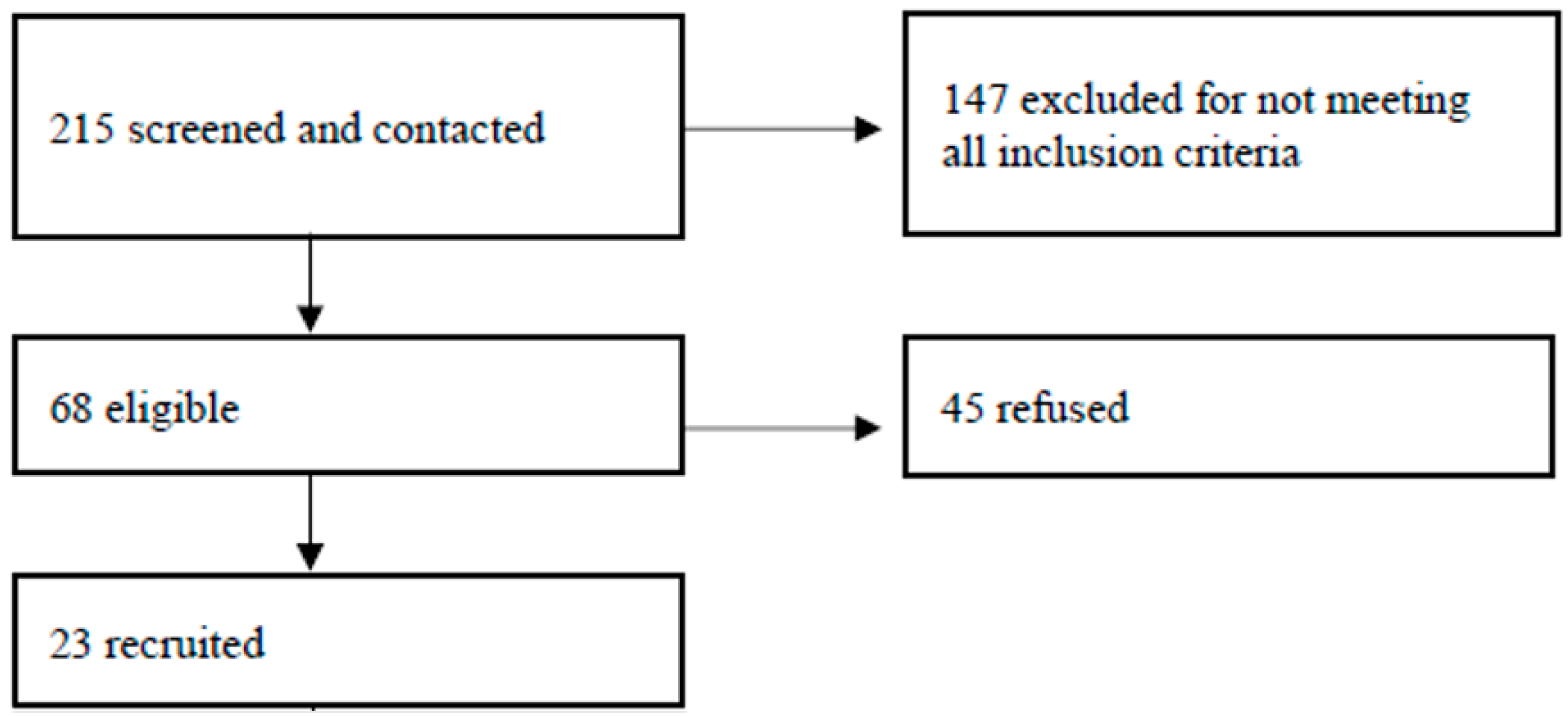
2.2. Participants and Eligibility Criteria
2.3. Data Collection and Anthropometrics
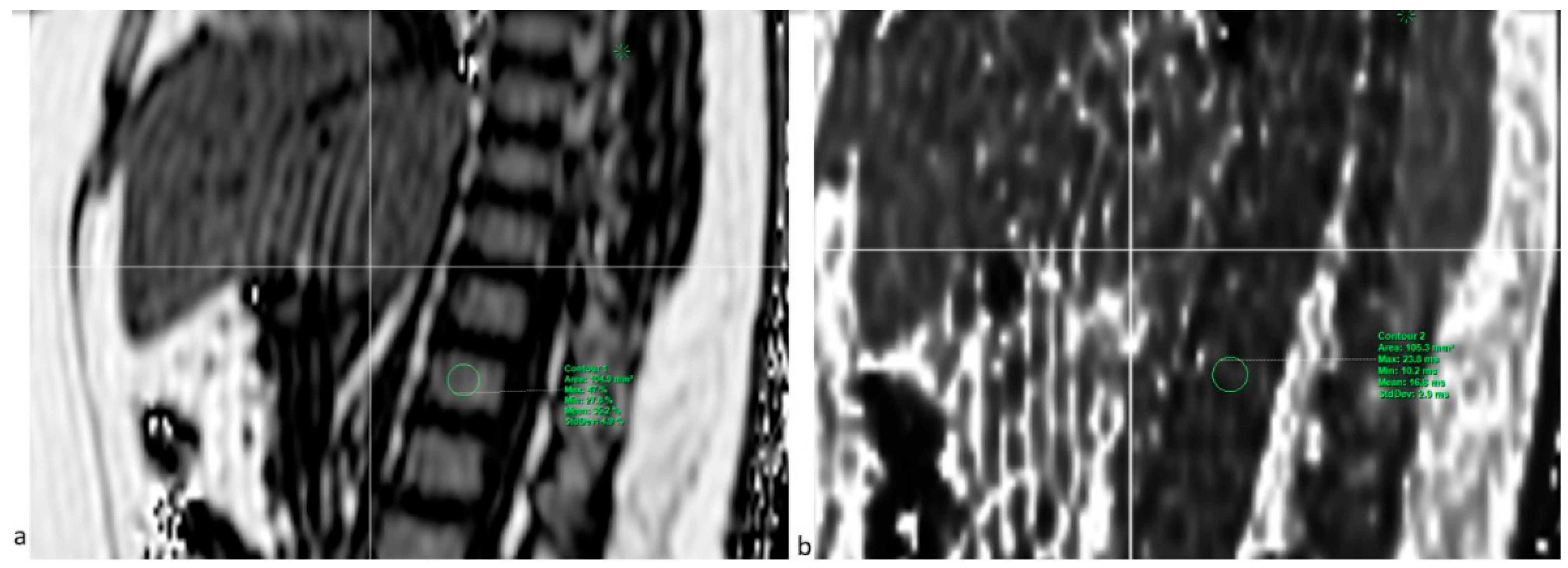
2.4. Imaging Procedures
2.4.1. PDFF Sequence
2.4.2. Liver Elastography
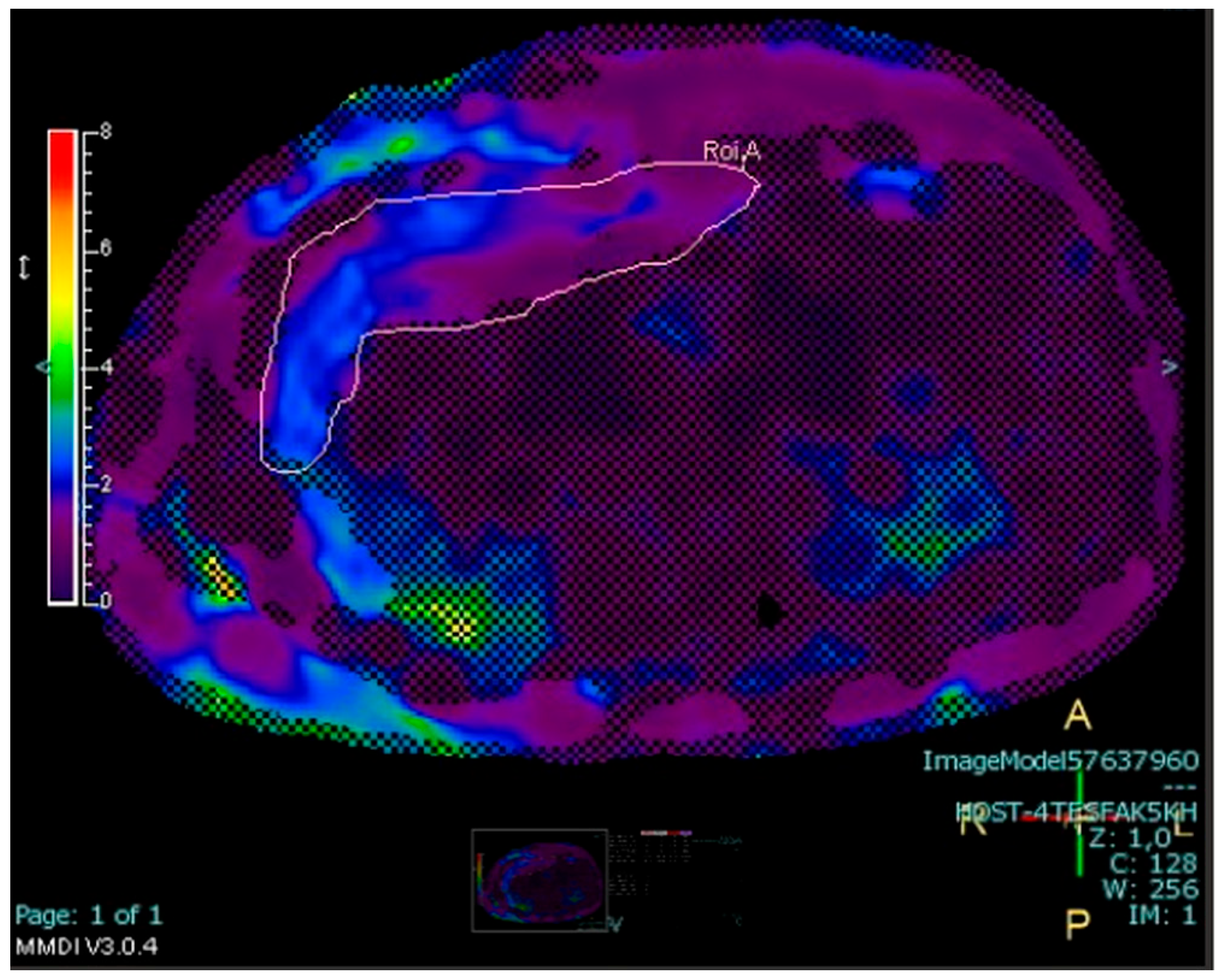
2.4.3. Measurement of Abdominal Fat Stores
2.5. DXA Examination
2.6. Statistical Analysis
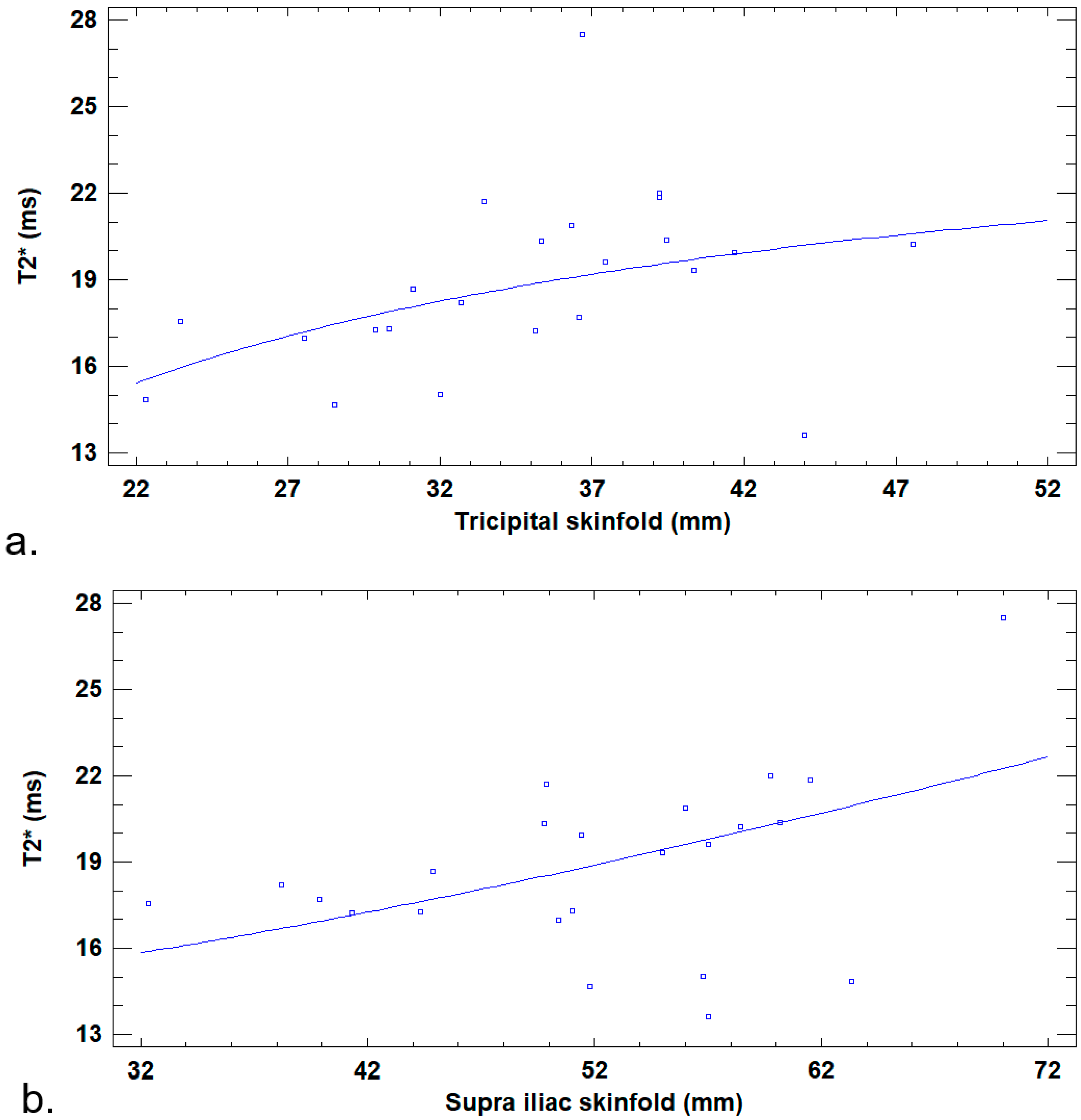
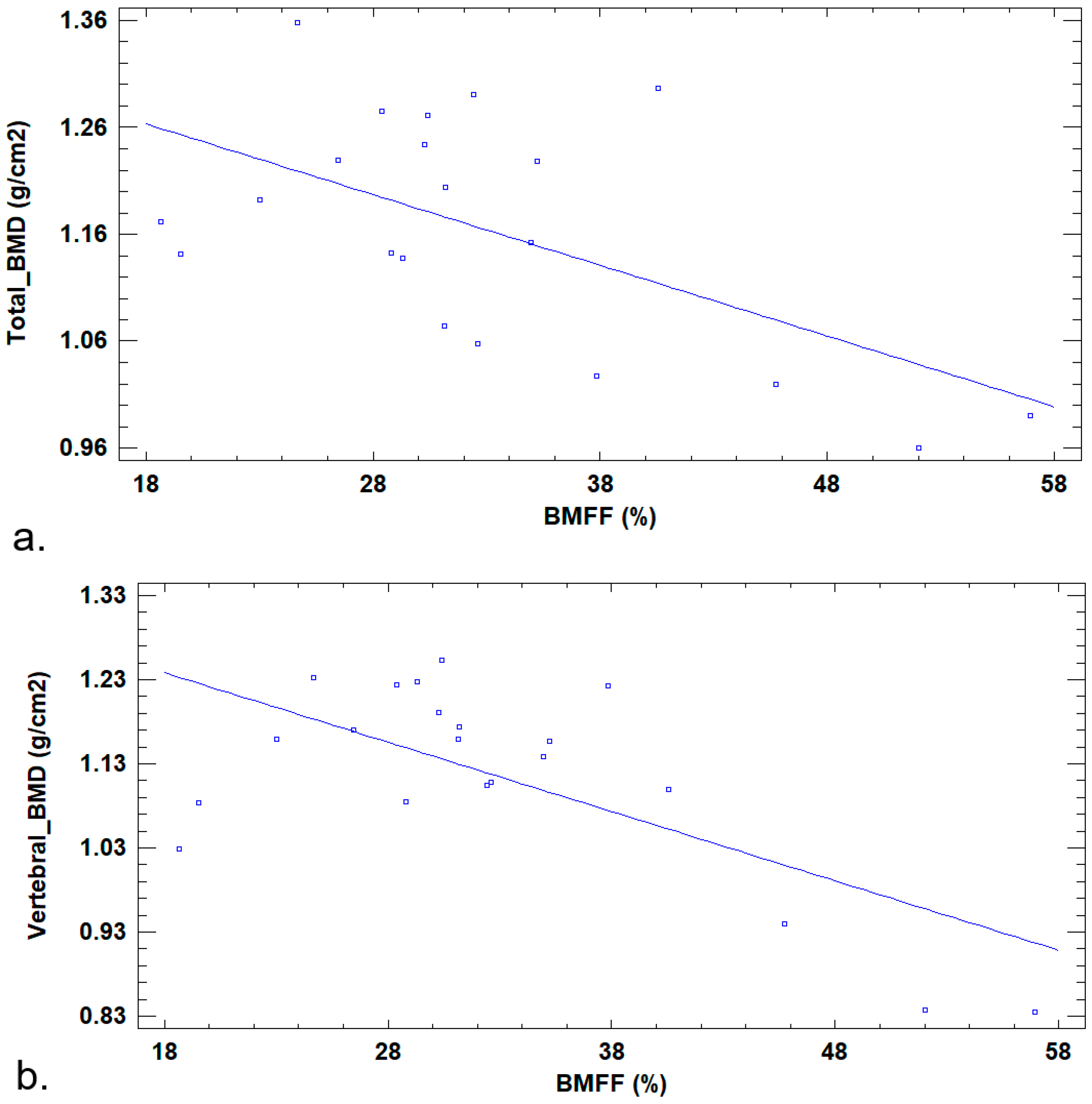
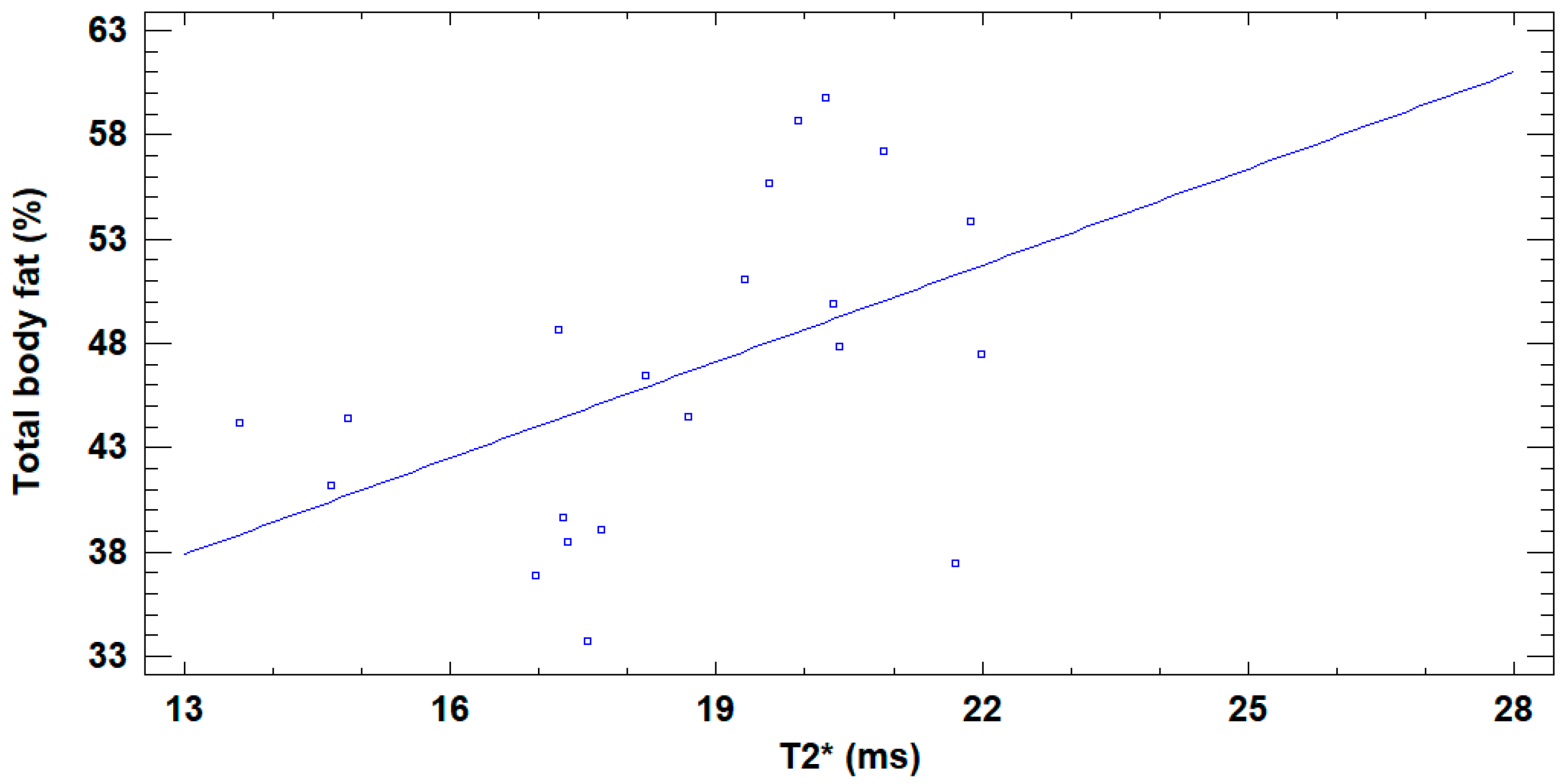
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| BMFF | Bone marrow fat fraction |
| BMD | Bone mineral density |
| DXA | Dual-energy X-ray absorptiometry |
| BMI | Body mass index |
| MRI | Magnetic resonance imaging |
| PDFF | Proton density fat fraction |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ROI | Region of interest |
| VAT | Visceral fat |
| ICC | Correlation coefficient |
References
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. (accessed on 15 August 2025).
- Biro, S.; Barber, D.; Williamson, T.; Morkem, R.; Khan, S.; Janssen, I. Prevalence of toddler, child and adolescent overweight and obesity derived from primary care electronic medical records: An observational study. Can. Med. Assoc. Open Access J. 2016, 4, E538–E544. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.T.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [PubMed]
- Mooli, R.G.R.; Ramakrishnan, S.K. Liver Steatosis is a Driving Factor of Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1267–1270. [Google Scholar] [CrossRef]
- Vachliotis, I.D.; Anastasilakis, A.D.; Goulas, A.; Goulis, D.G.; Polyzos, S.A. Nonalcoholic fatty liver disease and osteoporosis: A potential association with therapeutic implications. Diabetes Obes. Metab. 2022, 24, 1702–1720. [Google Scholar] [CrossRef]
- Labayen, I.; Cadenas-Sánchez, C.; Idoate, F.; Medrano, M.; Tobalina, I.; Villanueva, A.; Rodríguez-Vigil, B.; Álvarez de Eulate, N.; Osés, M.; Cabeza, R. Liver Fat, Bone Marrow Adipose Tissue, and Bone Mineral Density in Children with Overweight. J. Clin. Endocrinol. Metab. 2023, 109, e253–e258. [Google Scholar] [CrossRef]
- Tencerova, M.; Figeac, F.; Ditzel, N.; Taipaleenmäki, H.; Nielsen, T.K.; Kassem, M. High-Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J. Bone Min. Res. 2018, 33, 1154–1165. [Google Scholar] [CrossRef]
- Yu, E.W.; Greenblatt, L.; Eajazi, A.; Torriani, M.; Bredella, M.A. Marrow adipose tissue composition in adults with morbid obesity. Bone 2017, 97, 38–42. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784.e6. [Google Scholar] [CrossRef]
- Morris, D.M.; Wang, C.; Papanastasiou, G.; Gray, C.D.; Xu, W.; Sjöström, S.; Badr, S.; Paccou, J.; Semple, S.I.; MacGillivray, T.; et al. A novel deep learning method for large-scale analysis of bone marrow adiposity using UK Biobank Dixon MRI data. Comput. Struct. Biotechnol. J. 2024, 24, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Koebnick, C.; Smith, N.; Adams, A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin. Orthop. Relat. Res. 2013, 471, 1199–1207. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ahn, J.; Kim, H.K.; Kim, J.H. Obese children experience more extremity fractures than nonobese children and are significantly more likely to die from traumatic injuries. Acta Paediatr. 2016, 105, 1152–1157. [Google Scholar] [CrossRef]
- Wasserman, H.; O’Donnell, J.M.; Gordon, C.M. Use of dual energy X-ray absorptiometry in pediatric patients. Bone 2017, 104, 84–90. [Google Scholar] [CrossRef]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012, 27, 119–124. [Google Scholar] [CrossRef]
- Fintini, D.; Cianfarani, S.; Cofini, M.; Andreoletti, A.; Ubertini, G.M.; Cappa, M.; Manco, M. The Bones of Children with Obesity. Front. Endocrinol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Hilton, C.; Sabaratnam, R.; Drakesmith, H.; Karpe, F. Iron, glucose and fat metabolism and obesity: An intertwined relationship. Int. J. Obes. 2023, 47, 554–563. [Google Scholar] [CrossRef]
- Bouglé, D.; Brouard, J. Iron in child obesity. Relationships with inflammation and metabolic risk factors. Nutrients 2013, 5, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.; Kwack, K.-S.; Yun, J.S. CT and MR for bone mineral density and trabecular bone score assessment in osteoporosis evaluation. Sci. Rep. 2023, 13, 16574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Z.; Wang, C.; Cheng, X.; Wang, L.; Duanmu, Y.; Zhang, C.; Veronese, N.; Guglielmi, G. Reliability of measuring the fat content of the lumbar vertebral marrow and paraspinal muscles using MRI mDIXON-Quant sequence. Diagn. Interv. Radiol. 2018, 24, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Tang, G.; Hua, T.; Tu, Y.; Ji, R.; Zhu, J. mDIXON-Quant technique diagnostic accuracy for assessing bone mineral density in male adult population. BMC Musculoskelet. Disord. 2023, 24, 125. [Google Scholar] [CrossRef]
- Chang, R.; Ma, X.; Jiang, Y.; Huang, D.; Chen, X.; Zhang, M.; Hao, D. Percentage fat fraction in magnetic resonance imaging: Upgrading the osteoporosis-detecting parameter. BMC Med. Imaging 2020, 20, 30. [Google Scholar] [CrossRef]
- Moretti, J.-B.; Drouin, A.; Truong, C.; Youn, E.; Cloutier, A.; Alvarez, F.; Paganelli, M.; Grzywacz, K.; Jantchou, P.; Dubois, J.; et al. Effects of polyphenol supplementation on hepatic steatosis, intima-media thickness and non-invasive vascular elastography in obese adolescents: A pilot study protocol. BMJ Open 2024, 14, e074882. [Google Scholar] [CrossRef]
- Trout, A.T.; Serai, S.; Mahley, A.D.; Wang, H.; Zhang, Y.; Zhang, B.; Dillman, J.R. Liver Stiffness Measurements with MR Elastography: Agreement and Repeatability across Imaging Systems, Field Strengths, and Pulse Sequences. Radiology 2016, 281, 793–804. [Google Scholar] [CrossRef]
- Trout, A.T.; Hunte, D.E.; Mouzaki, M.; Xanthakos, S.A.; Su, W.; Zhang, B.; Dillman, J.R. Relationship between abdominal fat stores and liver fat, pancreatic fat, and metabolic comorbidities in a pediatric population with non-alcoholic fatty liver disease. Abdom. Radiol 2019, 44, 3107–3114. [Google Scholar] [CrossRef]
- Sawh, M.C.; Newton, K.P.; Goyal, N.P.; Angeles, J.E.; Harlow, K.; Bross, C.; Schlein, A.N.; Hooker, J.C.; Sy, E.Z.; Glaser, K.J.; et al. Normal range for MR elastography measured liver stiffness in children without liver disease. J. Magn. Reson. Imaging 2020, 51, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Normal Liver Stiffness Measured with MR Elastography in Children—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32960728/ (accessed on 11 March 2025).
- Forte, Y.S.; Renovato-Martins, M.; Barja-Fidalgo, C. Cellular and Molecular Mechanisms Associating Obesity to Bone Loss. Cells 2023, 12, 521. [Google Scholar] [CrossRef]
- Farella, I.; Chiarito, M.; Vitale, R.; D’Amato, G.; Faienza, M.F. The “Burden” of Childhood Obesity on Bone Health: A Look at Prevention and Treatment. Nutrients 2025, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef]
- da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity 2016, 24, 2522–2532. [Google Scholar] [CrossRef]
- Justesen, J.; Stenderup, K.; Ebbesen, E.N.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bandirali, M.; Di Leo, G.; Papini, G.D.E.; Messina, C.; Sconfienza, L.M.; Ulivieri, F.M.; Sardanelli, F. A new diagnostic score to detect osteoporosis in patients undergoing lumbar spine MRI. Eur. Radiol. 2015, 25, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Löffler, M.T.; Kronthaler, S.; Böhm, C.; Dieckmeyer, M.; Ruschke, S.; Kirschke, J.S.; Carballido-Gamio, J.; Karampinos, D.C.; Krug, R.; et al. MRI-Based Quantitative Osteoporosis Imaging at the Spine and Femur. J. Magn. Reson. Imaging 2021, 54, 12–35. [Google Scholar] [CrossRef] [PubMed]

| MR Elastography | PDFF | |
|---|---|---|
| Sequence | MR Elastography-4SL FFE | mDixonQuant-BH FFE |
| FOV (mm) | 400 | 400 |
| Matrix | 272 × 64 | 144 × 116 |
| Number of slices | 4 | 70 |
| Slice thickness (mm) | 10 | 6 |
| TE (msec) | 20 | 1.22 |
| TR (msec) | 50 | 6.7 |
| Slice orientation | Transverse | Transverse |
| Gap (mm) | 1 | 0 |
| Flip angle (degree) | 20 | 5 |
| Time (seconds) | 5 × 13 | 1 × 14 |
| Parallel imaging | 2 (SENSE) | |
| Inversion time | No | No |
| Breath hold | Yes (after expiration) | Yes |
| Variables | Mean (Standard Deviation) |
|---|---|
| Age (years) | 14.8 (interval 12–17) |
| BMI (kg/m2) | 35.5 (±6.7) |
| Hepatic steatosis (%) | 20.3 (±11.2) |
| Hepatic elastography on MRI (kPa) | 2.2 (±0.5) |
| Total bone mineral density (g/cm2) | 1.5 (±0.1) |
| Vertebral mineral density (g/cm2) | 1.4 (± 0.1) |
| Total body fat (%) | 45.3 (±7.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letissier, C.; El Ghomari, K.; Gervais, S.; Ahmarani, L.; El Jalbout, R. Magnetic Resonance Imaging-Based Assessment of Bone Marrow Fat and T2 Relaxation in Adolescents with Obesity and Liver Steatosis: A Feasibility Pilot Study. J. Clin. Med. 2025, 14, 7594. https://doi.org/10.3390/jcm14217594
Letissier C, El Ghomari K, Gervais S, Ahmarani L, El Jalbout R. Magnetic Resonance Imaging-Based Assessment of Bone Marrow Fat and T2 Relaxation in Adolescents with Obesity and Liver Steatosis: A Feasibility Pilot Study. Journal of Clinical Medicine. 2025; 14(21):7594. https://doi.org/10.3390/jcm14217594
Chicago/Turabian StyleLetissier, Camille, Kenza El Ghomari, Sylvie Gervais, Léna Ahmarani, and Ramy El Jalbout. 2025. "Magnetic Resonance Imaging-Based Assessment of Bone Marrow Fat and T2 Relaxation in Adolescents with Obesity and Liver Steatosis: A Feasibility Pilot Study" Journal of Clinical Medicine 14, no. 21: 7594. https://doi.org/10.3390/jcm14217594
APA StyleLetissier, C., El Ghomari, K., Gervais, S., Ahmarani, L., & El Jalbout, R. (2025). Magnetic Resonance Imaging-Based Assessment of Bone Marrow Fat and T2 Relaxation in Adolescents with Obesity and Liver Steatosis: A Feasibility Pilot Study. Journal of Clinical Medicine, 14(21), 7594. https://doi.org/10.3390/jcm14217594






