Relationship Between Liver Steatosis, Pancreas Steatosis, Metabolic Comorbidities, and Subclinical Vascular Markers in Children with Obesity: An Imaging-Based Study
Abstract
1. Introduction
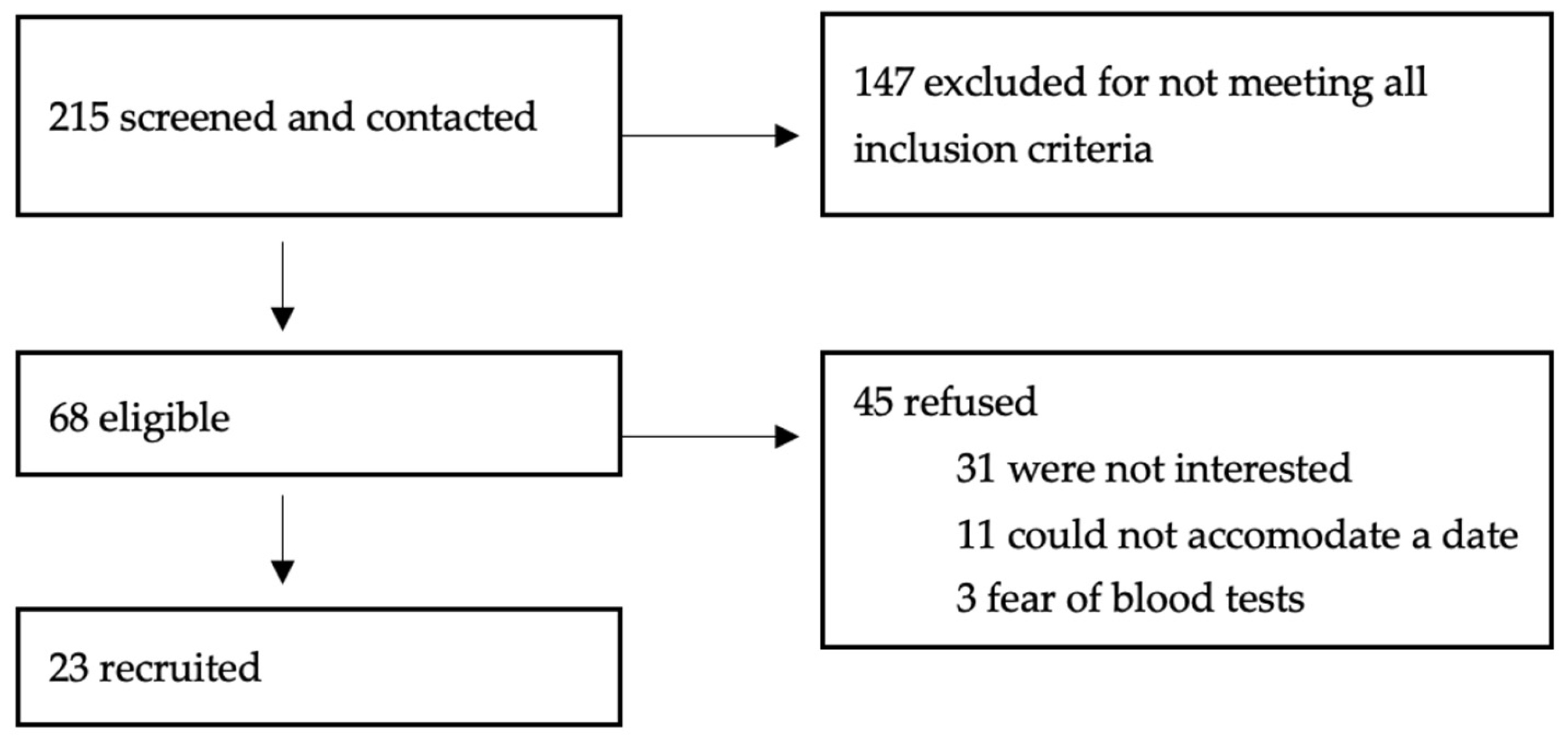
2. Materials and Methods
2.1. Description
2.2. Protocol
2.3. Image Acquisition
2.3.1. Hepatic and Pancreatic Measurements
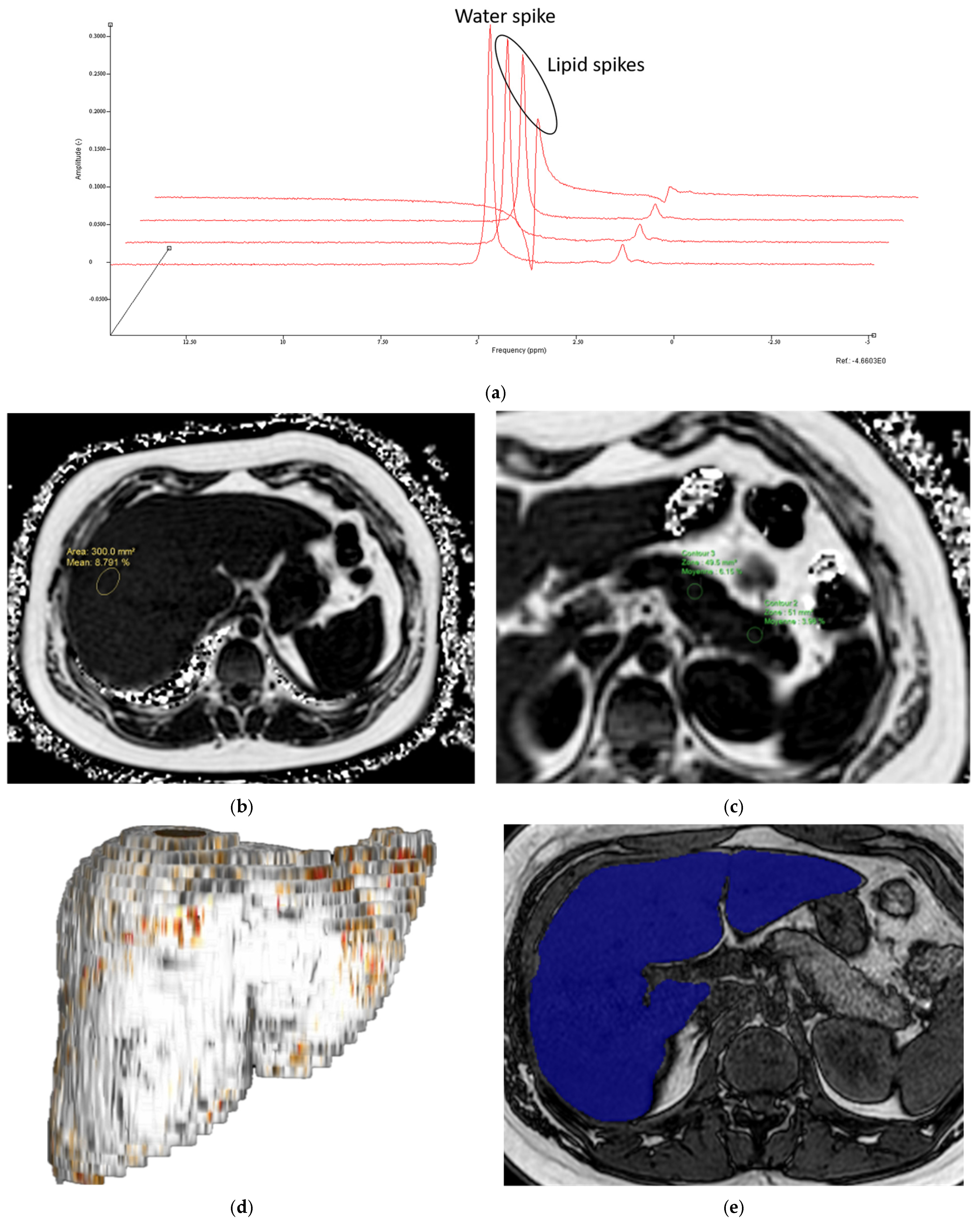
- MR Spectroscopy
- MRI-PDFF
- Liver and pancreas volumes
2.3.2. Subclinical Vascular Markers on Imaging
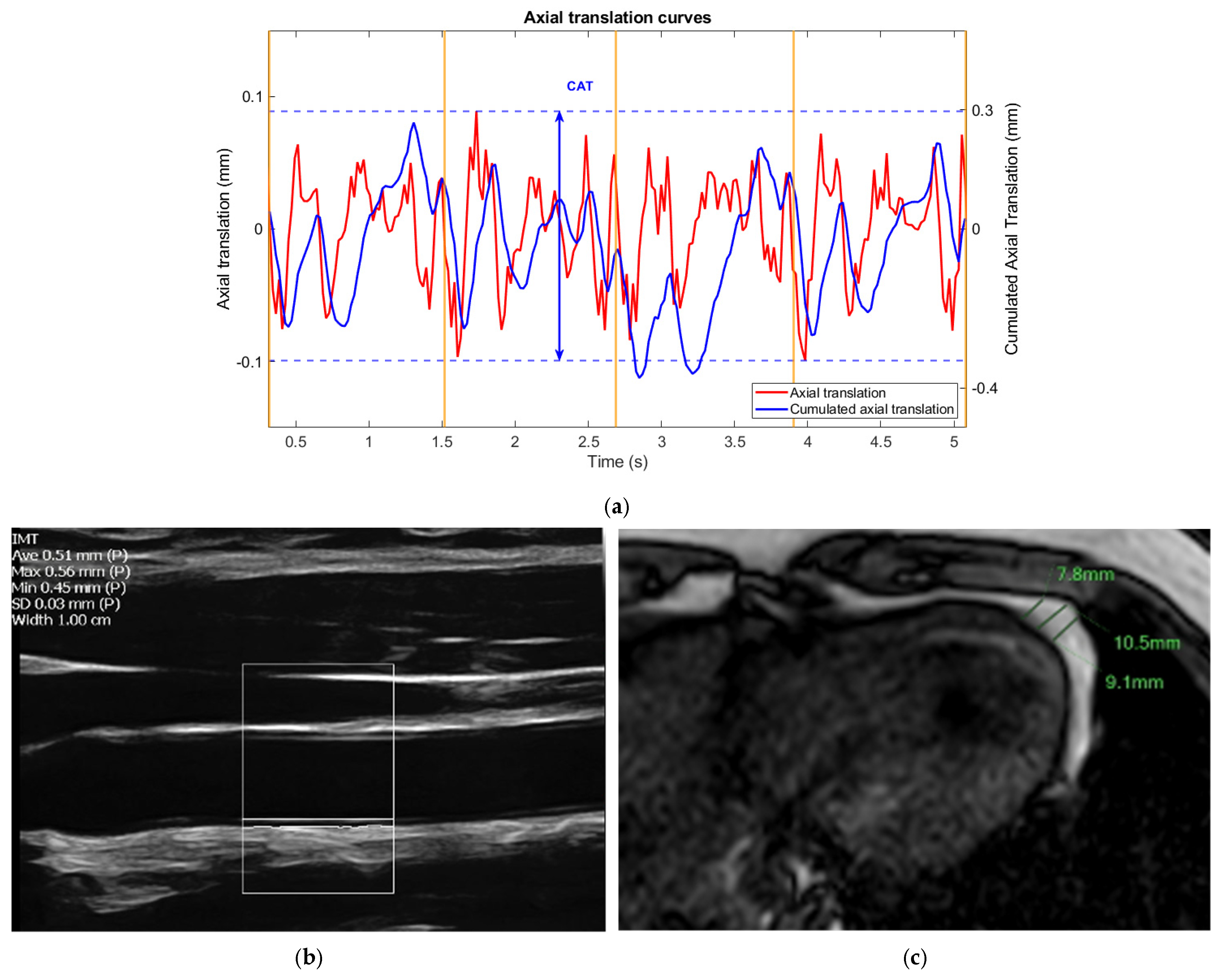
- Carotid ultrasound
- Non-Invasive Vascular Elastography (NIVE)
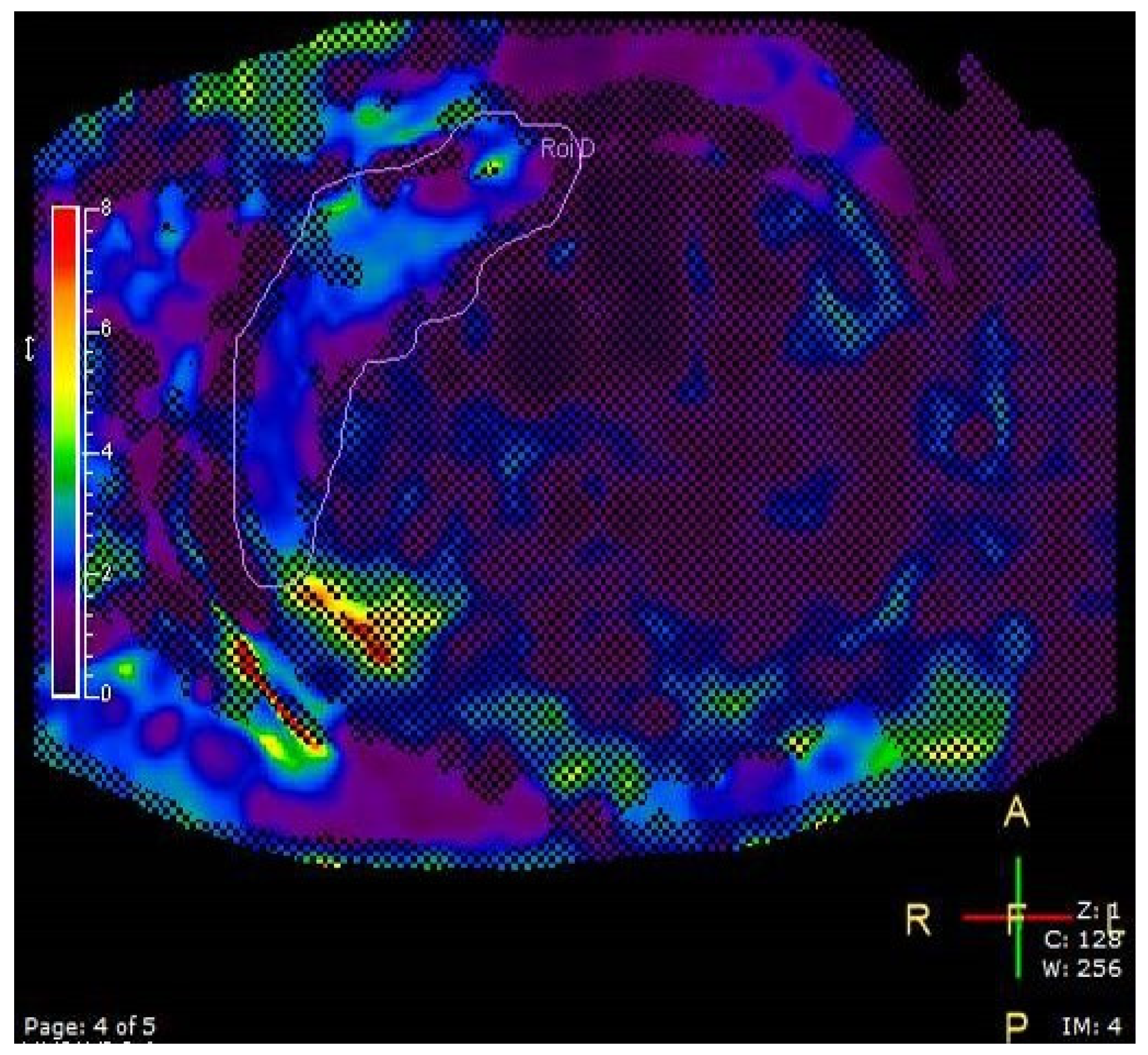
- Measurement of pericardial fat thickness
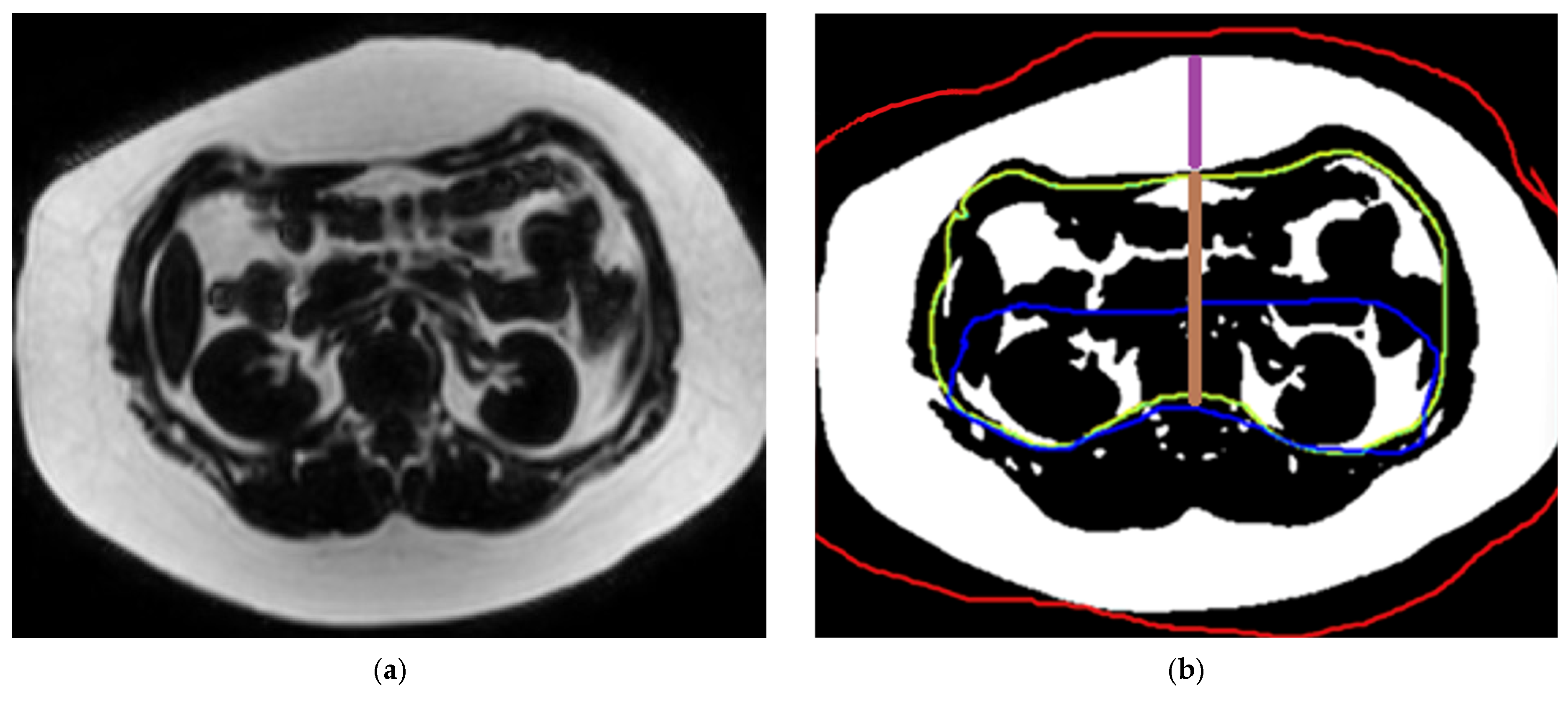
2.3.3. Measurement of Abdominal Fat Compartment Surface Area and Thickness
2.3.4. Liver Elastography
2.4. Statistics
3. Results
3.1. Anthropometric
3.2. Correlation Between Imaging Modalities and Reproducibility
3.3. Liver Steatosis
3.4. Elastography
3.5. Pancreas MRI-PDFF
3.6. Measurement of Abdominal Fat Surface Area and Thickness
4. Discussion
4.1. Reproducibility of Imaging Measurements
4.2. Exploring Associations Between Subclinical Vascular Markers and MASLD
4.3. Exploring Associations Between Subclinical Vascular Markers and Pancreas Measurements
4.4. Exploring Associations Between Subclinical Vascular Markers and Abdominal Fat Compartments
4.5. Insulin Resistance
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | metabolic dysfunction-associated steatosic liver disease |
| NIVE | non-invasive vascular elastography |
| IMT | intima-media thickness |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| MRI-PDFF | magnetic resonance imaging proton density fat fraction |
| VAT | visceral adipose tissue |
References
- Stroes, A.S.R.; Vos, M.; Benninga, M.A.; Koot, B.G.P. Pediatric MASLD: Current understanding and practical approach. Eur. J. Pediatr. 2025, 184, 29. [Google Scholar] [CrossRef]
- Tzifi, F.; Fretzayas, A.; Chrousos, G.; Kanaka-Gantenbein, C. Non-alcoholic fatty liver infiltration in children: An underdiagnosed evolving disease. Hormones 2019, 18, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Nobili, V.; Anania, C.; Verdecchia, P.; Chiesa, C. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J. Gastroenterol. 2011, 17, 3082–3091. [Google Scholar] [PubMed] [PubMed Central]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024, 30, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Berenson, G.S. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease: The Bogalusa Heart Study. Am. J. Cardiol. 2002, 90, 3L–7L. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Du, S.; Zhang, Q.; Xiao, J.; Dong, Q.; Xin, Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: A meta-analysis. Eur. Radiol. 2019, 29, 3564–3573. [Google Scholar] [CrossRef]
- Kemp, J.M.; Ghosh, A.; Dillman, J.R.; Krishnasarma, R.; Manhard, M.K.; Tipirneni-Sajja, A.; Shrestha, U.; Trout, A.T.; Morin, C.E. Practical approach to quantitative liver and pancreas MRI in children. Pediatr. Radiol. 2025, 55, 36–57. [Google Scholar] [CrossRef]
- Madan, S.A.; John, F.; Pyrsopoulos, N.; Pitchumoni, C.S. Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1237–1248. [Google Scholar] [CrossRef]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: Meta-analysis of 119 clinical trials involving 100,667 patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Cote, A.T.; Phillips, A.A.; Harris, K.C.; Sandor, G.G.; Panagiotopoulos, C.; Devlin, A.M. Obesity and arterial stiffness in children: Systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1038–1044. [Google Scholar] [CrossRef]
- El Jalbout, R.; Cloutier, G.; Roy-Cardinal, M.H.; Henderson, M.; Levy, E.; Lapierre, C.; Soulez, G.; Dubois, J. The value of non-invasive vascular elastography (NIVE) in detecting early vascular changes in overweight and obese children. Eur. Radiol. 2019, 29, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- Roy Cardinal, M.H.; Heusinkveld, M.H.G.; Qin, Z.; Lopata, R.G.P.; Naim, C.; Soulez, G.; Cloutier, G. Carotid artery plaque vulnerability assessment using noninvasive ultrasound elastography: Validation with MRI. Am. J. Roentgenol. 2017, 209, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Alchourron, E.; Dubois, J.; Cloutier, G.; Stein, N.; Farhat, Z.; Roy-Cardinal, M.H.; Moretti, J.B.; Lapierre, C.; El Jalbout, R. Non-invasive vascular elastography as a one-step imaging technique to evaluate early vascular changes in children compared to B-mode-based intima-media thickness technique: A validation study using inter- and intra-rater reliability. Can. Assoc. Radiol. J. 2022, 74, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Anderson, A.; Ding, J.; Budoff, M.; Rider, O.; Petersen, S.E.; Jensen, M.K.; Koch, M.; Allison, M.; Kawel-Boehm, N.; et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging 2017, 10, 1016–1027. [Google Scholar] [CrossRef]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef]
- Krishnan, A.; Sharma, H.; Yuan, D.; Trollope, A.F.; Chilton, L. The role of epicardial adipose tissue in the development of atrial fibrillation, coronary artery disease and chronic heart failure in the context of obesity and type 2 diabetes mellitus: A narrative review. J. Cardiovasc. Dev. Dis. 2022, 9, 217. [Google Scholar] [CrossRef]
- Sicari, R.; Sironi, A.M.; Petz, R.; Frassi, F.; Chubuchny, V.; De Marchi, D.; Positano, V.; Lombardi, M.; Picano, E.; Gastaldelli, A. Pericardial rather than epicardial fat is a cardiometabolic risk marker: An MRI vs echo study. J. Am. Soc. Echocardiogr. 2011, 24, 1156–1162. [Google Scholar] [CrossRef]
- Yun, C.H.; Jhuang, J.R.; Tsou, M.T. Pericardial fat, thoracic peri-aortic adipose tissue, and systemic inflammatory marker in nonalcoholic fatty liver and abdominal obesity phenotype. Sci. Rep. 2022, 12, 1958. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Pisano, G.; Consonni, D.; Tiraboschi, S.; Baragetti, A.; Bertelli, C.; Norata, G.D.; Dongiovanni, P.; Valenti, L.; Grigore, L.; et al. Epicardial adipose tissue (EAT) thickness is associated with cardiovascular and liver damage in nonalcoholic fatty liver disease. PLoS ONE 2016, 11, e0162473. [Google Scholar] [CrossRef]
- Swauger, S.E.; Fashho, K.; Hornung, L.N.; Elder, D.A.; Thapaliya, S.; Anton, C.G.; Trout, A.T.; Abu-El-Haija, M. Association of pancreatic fat on imaging with pediatric metabolic co-morbidities. Pediatr. Radiol. 2023, 53, 2030–2039. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, J.L.; Li, M.L.; Zhou, J.; Sun, X.L. The association between pancreas steatosis and metabolic syndrome: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3142. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Alexakis, K.; Mavrikaki, V.; Mikhailidis, D.P. Nonalcoholic Fatty Pancreas Disease: Role in Metabolic Syndrome, “Prediabetes,” Diabetes, and Atherosclerosis. Dig. Dis. Sci. 2021, 67, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Zhou, J.; Chen, X.; Sun, Y.; Mao, Z.; Chai, K. Prevalence and factors associated with nonalcoholic fatty pancreas disease and its severity in China. Medicine 2018, 97, e11293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Petrov, M.S. Relationship of fat in the pancreas with cardiovascular disease. Obes. Rev. 2025, 26, e13914. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Marketou, M.E.; Buechler, N.S.; Fragkiadakis, K.; Plevritaki, A.; Zervakis, S.; Maragkoudakis, S.; Tsiavos, A.; Simantirakis, E.; Kochiadakis, G. Visceral fat and cardiometabolic future in children and adolescents: A critical update. Pediatr. Res. 2023, 94, 1639–1647. [Google Scholar] [CrossRef]
- Gast, K.B.; den Heijer, M.; Smit, J.W.; Widya, R.L.; Lamb, H.J.; de Roos, A.; Jukema, J.W.; Rosendaal, F.R.; de Mutsert, R.; NEO study group. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis 2015, 241, 547–554. [Google Scholar] [CrossRef]
- Hung, C.S.; Lee, J.K.; Yang, C.Y.; Hsieh, H.R.; Ma, W.Y.; Lin, M.S.; Liu, P.H.; Shih, S.R.; Liou, J.M.; Chuang, L.M.; et al. Measurement of visceral fat: Should we include retroperitoneal fat? PLoS ONE 2014, 9, e112355. [Google Scholar] [CrossRef]
- Tanaka, M.; Okada, H.; Hashimoto, Y.; Kumagai, M.; Nishimura, H.; Fukui, M. Intraperitoneal, but not retroperitoneal, visceral adipose tissue is associated with diabetes mellitus: A cross-sectional, retrospective pilot analysis. Diabetol. Metab. Syndr. 2020, 12, 103. [Google Scholar] [CrossRef]
- Moretti, J.B.; Drouin, A.; Truong, C.; Youn, E.; Cloutier, A.; Alvarez, F.; Paganelli, M.; Grzywacz, K.; Jantchou, P.; Dubois, J.; et al. Effects of polyphenol supplementation on hepatic steatosis, intima-media thickness and non-invasive vascular elastography in obese adolescents: A pilot study protocol. BMJ Open 2024, 14, e074882. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011): An update on behalf of the Advisory Board of the 3rd, 4th and 5th Watching the Risk Symposia. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Demircelik, M.B.; Yilmaz, O.C.; Gurel, O.M.; Selcoki, Y.; Atar, I.A.; Bozkurt, A.; Akin, K.; Eryonucu, B. Epicardial adipose tissue and pericoronary fat thickness measured with 64-multidetector computed tomography: Potential predictors of the severity of coronary artery disease. Clinics 2014, 69, 388–392. [Google Scholar] [CrossRef]
- Sadouni, M.; Boldeanu, I.; Durand, M.; Juneau, D.; Blais, S.; Tremblay, C.; Chartrand-Lefebvre, C. Quantification of epicardial fat using non-contrast cardiac CT in an HIV population: Reproducibility and association with other body fat indices. Eur. J. Radiol. Open 2021, 8, 100317. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Hunte, D.E.; Mouzaki, M.; Xanthakos, S.A.; Su, W.; Zhang, B.; Dillman, J.R. Relationship between abdominal fat stores and liver fat, pancreatic fat, and metabolic comorbidities in a pediatric population with non-alcoholic fatty liver disease. Abdom. Radiol. 2019, 44, 3107–3114. [Google Scholar] [CrossRef]
- Trout, A.T.; Serai, S.; Mahley, A.D.; Wang, H.; Zhang, Y.; Zhang, B.; Dillman, J.R. Liver stiffness measurements with MR elastography: Agreement and repeatability across imaging systems, field strengths, and pulse sequences. Radiology 2016, 281, 657–984. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- CHU Sainte Justine. Available online: https://www.chusj.org/CORPO/files/7f/7fdf0f0f-4c86-40f1-afd0-75f34c4814b6.pdf (accessed on 11 April 2025).
- Trout, A.T.; Anupindi, S.A.; Gee, M.S.; Khanna, G.; Xanthakos, S.A.; Serai, S.D.; Baikpour, M.; Calle-Toro, J.S.; Ozturk, A.; Zhang, B.; et al. Normal liver stiffness measured with MR elastography in children. Radiology 2020, 297, 495–742. [Google Scholar] [CrossRef]
- Sawh, M.C.; Newton, K.P.; Goyal, N.P.; Angeles, J.E.; Harlow, K.; Bross, C.; Schlein, A.N.; Hooker, J.C.; Sy, E.Z.; Glaser, K.J.; et al. Normal range for MR elastography measured liver stiffness in children without liver disease. J. Magn. Reson. Imaging 2020, 51, 647–963. [Google Scholar] [CrossRef]
- Demircioğlu, F.; Koçyiğit, A.; Arslan, N.; Cakmakçi, H.; Hizli, S.; Sedat, A.T. Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 68–75. [Google Scholar] [CrossRef]
- Pacifico, L.; Cantisani, V.; Ricci, P.; Osborn, J.F.; Schiavo, E.; Anania, C.; Ferrara, E.; Dvisic, G.; Chiesa, C. Nonalcoholic Fatty Liver Disease and Carotid Atherosclerosis in Children. Pediatr. Res. 2008, 63, 423–427. [Google Scholar] [CrossRef][Green Version]
- Alp, H.; Karaarslan, S.; Selver Eklioğlu, B.; Atabek, M.E.; Altın, H.; Baysal, T. Association between nonalcoholic fatty liver disease and cardiovascular risk in obese children and adolescents. Can. J. Cardiol. 2013, 29, 1118–1125. [Google Scholar] [CrossRef]
- Koot, B.G.; de Groot, E.; van der Baan-Slootweg, O.H.; Bohte, A.E.; Nederveen, A.J.; Jansen, P.L.; Stoker, J.; Benninga, M.A. Nonalcoholic fatty liver disease and cardiovascular risk in children with obesity. Obesity 2015, 23, 1239–1243. [Google Scholar] [CrossRef]
- Monasso, G.S.; Santos, S.; Silva, C.C.V.; Geurtsen, M.L.; Oei, E.; Gaillard, R.; Felix, J.F.; Jaddoe, V.W.V. Body fat, pericardial fat, liver fat and arterial health at age 10 years. Pediatr. Obes. 2022, 17, e12926. [Google Scholar] [CrossRef]
- Pacifico, L.; Di Martino, M.; Anania, C.; Andreoli, G.M.; Bezzi, M.; Catalano, C.; Chiesa, C. Pancreatic fat and β-cell function in overweight/obese children with nonalcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 4688–4695. [Google Scholar] [CrossRef]
| MR Elastography | PDFF | MRS | FEE-BH Dual | |
|---|---|---|---|---|
| Sequence | MR Elastography-4SL, Fast field echo (FFE) | mDixon- Quant-BH FFE | Steam-mTE Echo | Ax dual-FFE-BH |
| FOV (mm) | 400 | 400 | - | 340 |
| Matrix | 272 × 64 | 144 × 116 | - | 208 × 128 |
| Number of slices | 4 | 70 | - | 28 |
| Slice thickness (mm) | 10 | 6 | Spectroscopic volume of interest 25 mm3 | 6 |
| TE (msec) | 20 | 1.19 | 12 | 2.3 |
| TR (msec) | 50 | 6.7 | 3000 | 105 |
| Slice orientation | Transverse | Transverse | Transverse | Transverse |
| Gap (mm) | 1 | 0 | - | 1.2 |
| Flip angle (degree) | 20 | 5 | 90 | 75 |
| Time (s) | 5 × 13 | 1 × 14 | 14 (3 sequences) | 2 × 7 |
| Parallel imaging | 2 (SENSE) | - | - | 2 (SENSE) |
| Breath hold | Yes (after expiration) | Yes | No | Yes |
| Variables | Mean ± Standard Deviation |
|---|---|
| Sex, No. (%) | |
| Males | 18 (78.2%) |
| Females | 5 (21.8%) |
| Age (years) | 14.8 ± 1.7 |
| Origin, No. (%) | |
| Caucasian | 12 (52.2%) |
| Hispanic | 5 (21.8%) |
| First Nation | 3 (13%) |
| Other | 3 (13%) |
| Tanner Stage, No. (%) | |
| 1 | 1 (4.4%) |
| 2 | 2 (8.7%) |
| 3 | 7 (30.4%) |
| 4 | 7(30.4%) |
| 5 | 6 (26.1%) |
| Weight (kg) | 108.0 ± 24.3 |
| Weight percentile | 96.1 ± 2.4 |
| Height (cm) | 173.8 ± 8.0 |
| Height percentile | 79.8 ± 21.9 |
| BMI (kg/m2) | 35.5 ± 6.7 |
| BMI percentile | 98.6 ± 2.3 |
| Liver steatosis PDFF | 20.84 ± 11.4 |
| Systolic blood pressure (mm Hg) | 124 ± 20 |
| Waist circumference (cm) | 112.4 ± 17.9 |
| Glucose * (mmol/L) | 5.2 ± 0.5 |
| Triglycerides * (mmol/L) | 1.5 ± 0.7 |
| Insulin * (pmol/L) | 195.0 ± 98.8 |
| ICC Inter-Operator | 95% CI | ICC Intra-Operator | 95% CI | |
|---|---|---|---|---|
| Liver volume | 0.996 | 0.984–0.999 | 0.988 | 0.949–0.997 |
| Liver PDFF | 0.998 | 0.992–0.999 | 0.995 | 0.982–0.998 |
| Pancreas volume | 0.995 | 0.982–0.999 | 0.995 | 0.981–0.999 |
| Pancreas PDFF (average) | 0.987 | 0.957–0.996 | 0.971 | 0.902–0.992 |
| Pancreas PDFF (tail) | 0.970 | 0.898–0.991 | 0.934 | 0.793–0.980 |
| Pancreas PDFF (body) | 0.984 | 0.945–0.995 | 0.961 | 0.873–0.988 |
| Pancreas PDFF (head) | 0.953 | 0.845–0.986 | 0.926 | 0.763–0.978 |
| PFT | 0.970 | 0.884–0.992 | 0.985 | 0.943–0.996 |
| Peri-coronary fat | 0.865 | 0.549–0.965 | 0.878 | 0.588–0.968 |
| Peri-apical fat | 0.961 | 0.852–0.990 | 0.807 | 0.383–0.949 |
| IMT | IMT/ Diameter | CAT | CAS | CAS/CAT | PFT | Peri Coronary Fat | Peri Apical Fat | HOMA-IR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Liver volume | ρ [95% CI] | 0.024 [−0.423–0.462] | −0.038 [−0.473–0.412] | 0.20 [−0.278–0.600] | −0.20 [−0.598–0.281] | −0.33 [−0.684–0.141] | 0.19 [−0.273–0.586] | 0.17 [−0.291–0.572] | 0.28 [−0.187–0.643] | 0.28 [−0.225–0.671] |
| p-value | 0.92 | 0.87 | 0.39 | 0.40 | 0.15 | 0.40 | 0.45 | 0.22 | 0.25 | |
| Liver PDFF | ρ [95% CI] | −0.012 [−0.453–0.433] | 0.039 [−0.411–0.474] | −0.29 [−0.656–0.190] | −0.051 [−0.493–0.412] | 0.27 [−0.213–0.642] | −0.20 [−0.591–0.265] | −0.16 [−0.566–0.301] | −0.20 [−0.590–0.268] | 0.55 * [0.115–0.809] |
| p-value | 0.96 | 0.87 | 0.22 | 0.83 | 0.26 | 0.38 | 0.48 | 0.39 | 0.015 | |
| MRS | ρ [95% CI] | 0.033 [−0.405–0.459] | 0.025 [−0.412–0.453] | −0.35 [−0.687–0.109] | −0.075 [−0.501–0.380] | 0.32 [−0.144–0.668] | 0.036 [−0.413–0.472] | −0.042 [−0.476–0.409] | −0.064 [−0.492–0.390] | 0.66 * [0.283–0.862] |
| p-value | 0.88 | 0.91 | 0.12 | 0.75 | 0.16 | 0.88 | 0.86 | 0.78 | 0.002 | |
| Pancreas volume | ρ [95% CI] | 0.085 [−0.371–0.509] | 0.057 [−0.396–0.488] | −0.13 [−0.553–0.342] | −0.38 [−0.711–0.090] | −0.12 [−0.544–0.353] | 0.49 * [0.055–0.764] | 0.33 [−0.131–0.675] | 0.49 * [0.063–0.768] | 0.65 * [0.251–0.861] |
| p-value | 0.71 | 0.81 | 0.58 | 0.099 | 0.61 | 0.026 | 0.14 | 0.023 | 0.003 | |
| Pancreas PDFF (average) | ρ [95% CI] | −0.18 [−0.577–0.286] | −0.013 [−0.453–0.432] | −0.10 [−0.530–0.370] | −0.23 [−0.619–0.250] | −0.081 [−0.516–0.387] | 0.32 [−0.141–0.669] | −0.04 [−0.474–0.410] | 0.67 * [0.325–0.859] | 0.53 * [0.071–0.805] |
| p-value | 0.44 | 0.96 | 0.67 | 0.33 | 0.73 | 0.15 | 0.86 | <0.001 | 0.023 | |
| Pancreas PDFF (tail) | ρ [95% CI] | −0.13 [−0.543–0.330] | −0.012 [−0.452–0.433] | −0.13 [−0.549–0.347] | −0.16 [−0.570–0.320] | −0.030 [−0.477–0.430] | 0.46 * [0.015–0.747] | 0.088 [−0.369–0.511] | 0.73 * [0.429–0.887] | 0.70 * [0.331–0.882] |
| p-value | 0.57 | 0.96 | 0.60 | 0.51 | 0.90 | 0.038 | 0.70 | <0.001 | 0.001 | |
| Pancreas PDFF (body) | ρ [95% CI] | −0.23 [−0.611–0.236] | −0.11 [−0.525–0.352] | −0.17 [−0.576–0.312] | −0.34 [−0.688–0.135] | −0.071 [−0.508–0.396] | 0.31 [−0.159–0.659] | 0.015 [−0.431–0.455] | 0.55 * [0.143–0.799] | 0.62 * 0.202–0.847] |
| p-value | 0.31 | 0.64 | 0.49 | 0.14 | 0.77 | 0.18 | 0.95 | 0.010 | 0.006 [ | |
| Pancreas PDFF (head) | ρ [95% CI] | −0.089 [−0.512–0.368] | 0.079 [−0.377–0.504] | −0.19 [−0.595–0.285] | −0.20 [−0.598–0.281] | 0.023 [−0.436–0.471] | 0.23 [−0.240–0.609] | −0.059 [−0.489–0.394] | 0.64 * [0.280–0.845] | 0.35 [−0.152–0.711] |
| p-value | 0.70 | 0.73 | 0.41 | 0.40 | 0.93 | 0.32 | 0.80 | 0.002 | 0.15 | |
| Total fat area | ρ [95% CI] | 0.003 [−0.451–0.456] | −0.008 [−0.460–0.448] | −0.17 [−0.587–0.324] | −0.24 [−0.636–0.252] | −0.03 [−0.489–0.442] | 0.43 [−0.026–0.741] | 0.25 [−0.230–0.632] | 0.45 * [−0.003–0.751] | 0.57 * [0.102–0.828] |
| p-value | 0.99 | 0.98 | 0.50 | 0.32 | 0.90 | 0.056 | 0.29 | 0.046 | 0.018 | |
| Visceral fat area (VAT) | ρ [95% CI] | −0.099 [−0.529–0.372] | −0.12 [−0.541–0.357] | −0.58 * [−0.823–−0.155] | −0.37 [−0.714–0.113] | 0.29 [−0.204–0.665] | 0.26 [−0.223–0.637] | 0.032 [−0.428–0.479] | 0.57 * [0.155–0.813] | 0.54 * [0.071–0.818] |
| p-value | 0.68 | 0.63 | 0.009 | 0.12 | 0.23 | 0.27 | 0.90 | 0.009 | 0.024 | |
| Retroperitoneal fat area | ρ [95% CI] | 0.37 [−0.098–0.707] | 0.35 [−0.128–0.691] | −0.32 [−0.681–0.176] | −0.17 [−0.586–0.326] | 0.21 [−0.286–0.614] | 0.36 [−0.108–0.702] | 0.23 [−0.246–0.622] | 0.53 * [0.099–0.793] | 0.16 [−0.359–0.606] |
| p-value | 0.11 | 0.14 | 0.19 | 0.50 | 0.40 | 0.12 | 0.32 | 0.016 | 0.54 | |
| Intraperitoneal fat area | ρ [95% CI] | −0.26 [−0.637–0.223] | −0.26 [−0.636–0.224] | −0.57 * [−0.818–−0.140] | −0.46 * [−0.763–0.005] | 0.20 [−0.291–0.610] | 0.22 [−0.257–0.614] | −0.031 [−0.478–0.429] | 0.53 * [0.102–0.793] | 0.62 * [0.176–0.850] |
| p-value | 0.27 | 0.28 | 0.011 | 0.047 | 0.41 | 0.35 | 0.90 | 0.016 | 0.009 | |
| Subcutaneous area (SAT) | ρ [95% CI] | 0.062 [−0.403–0.501] | 0.029 [−0.431–0.476] | 0.00 [−0.466–0.466] | −0.14 [−0.571–0.345] | −0.12 [−0.553–0.368] | 0.46 * [0.006–0.755] | 0.32 [−0.153–0.678] | 0.34 [−0.133–0.688] | 0.45 [−0.056–0.771] |
| p-value | 0.80 | 0.91 | 1.0 | 0.56 | 0.63 | 0.042 | 0.17 | 0.14 | 0.071 | |
| Thickness VAT | ρ [95% CI] | −0.12 [−0.544–0.353] | 0.12 [−0.357–0.541] | −0.19 [−0.602–0.303] | −0.29 [−0.665–0.204] | −0.056 [−0.508–0.420] | 0.22 [−0.259–0.613] | 0.063 [−0.402–0.503] | 0.36 [−0.108–0.701] | 0.63 * [0.203–0.858] |
| p-value | 0.62 | 0.63 | 0.44 | 0.23 | 0.82 | 0.35 | 0.79 | 0.12 | 0.006 | |
| Thickness SAT | ρ [95% CI] | −0.018 [−0.468–0.439] | −0.079 [−0.514–0.389] | 0.038 [−0.436–0.495] | −0.18 [−0.599–0.308] | −0.13 [−0.563–0.356] | 0.096 [−0.374–0.526] | 0.24 [−0.243–0.623] | 0.009 [−0.447–0.461] | −0.017 [−0.505–0.479] |
| p-value | 0.94 | 0.74 | 0.88 | 0.45 | 0.59 | 0.69 | 0.32 | 0.97 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Ghomari, K.; Voia, A.; Moretti, J.-B.; Cloutier, A.; Cloutier, G.; El Jalbout, R. Relationship Between Liver Steatosis, Pancreas Steatosis, Metabolic Comorbidities, and Subclinical Vascular Markers in Children with Obesity: An Imaging-Based Study. J. Clin. Med. 2025, 14, 7048. https://doi.org/10.3390/jcm14197048
El Ghomari K, Voia A, Moretti J-B, Cloutier A, Cloutier G, El Jalbout R. Relationship Between Liver Steatosis, Pancreas Steatosis, Metabolic Comorbidities, and Subclinical Vascular Markers in Children with Obesity: An Imaging-Based Study. Journal of Clinical Medicine. 2025; 14(19):7048. https://doi.org/10.3390/jcm14197048
Chicago/Turabian StyleEl Ghomari, Kenza, Anna Voia, Jean-Baptiste Moretti, Anik Cloutier, Guy Cloutier, and Ramy El Jalbout. 2025. "Relationship Between Liver Steatosis, Pancreas Steatosis, Metabolic Comorbidities, and Subclinical Vascular Markers in Children with Obesity: An Imaging-Based Study" Journal of Clinical Medicine 14, no. 19: 7048. https://doi.org/10.3390/jcm14197048
APA StyleEl Ghomari, K., Voia, A., Moretti, J.-B., Cloutier, A., Cloutier, G., & El Jalbout, R. (2025). Relationship Between Liver Steatosis, Pancreas Steatosis, Metabolic Comorbidities, and Subclinical Vascular Markers in Children with Obesity: An Imaging-Based Study. Journal of Clinical Medicine, 14(19), 7048. https://doi.org/10.3390/jcm14197048






