The Role of microRNA-210 in the Pathogenesis and Diagnosis of Preeclampsia—A Systematic Review
Abstract
1. Introduction
1.1. Biogenesis and Regulation of miR-210
1.2. Mechanistic Role of miR-210 in Trophoblast Biology
1.3. miR-210 as a Biomarker in Preeclampsia
1.4. Therapeutic Potential of Targeting miR-210
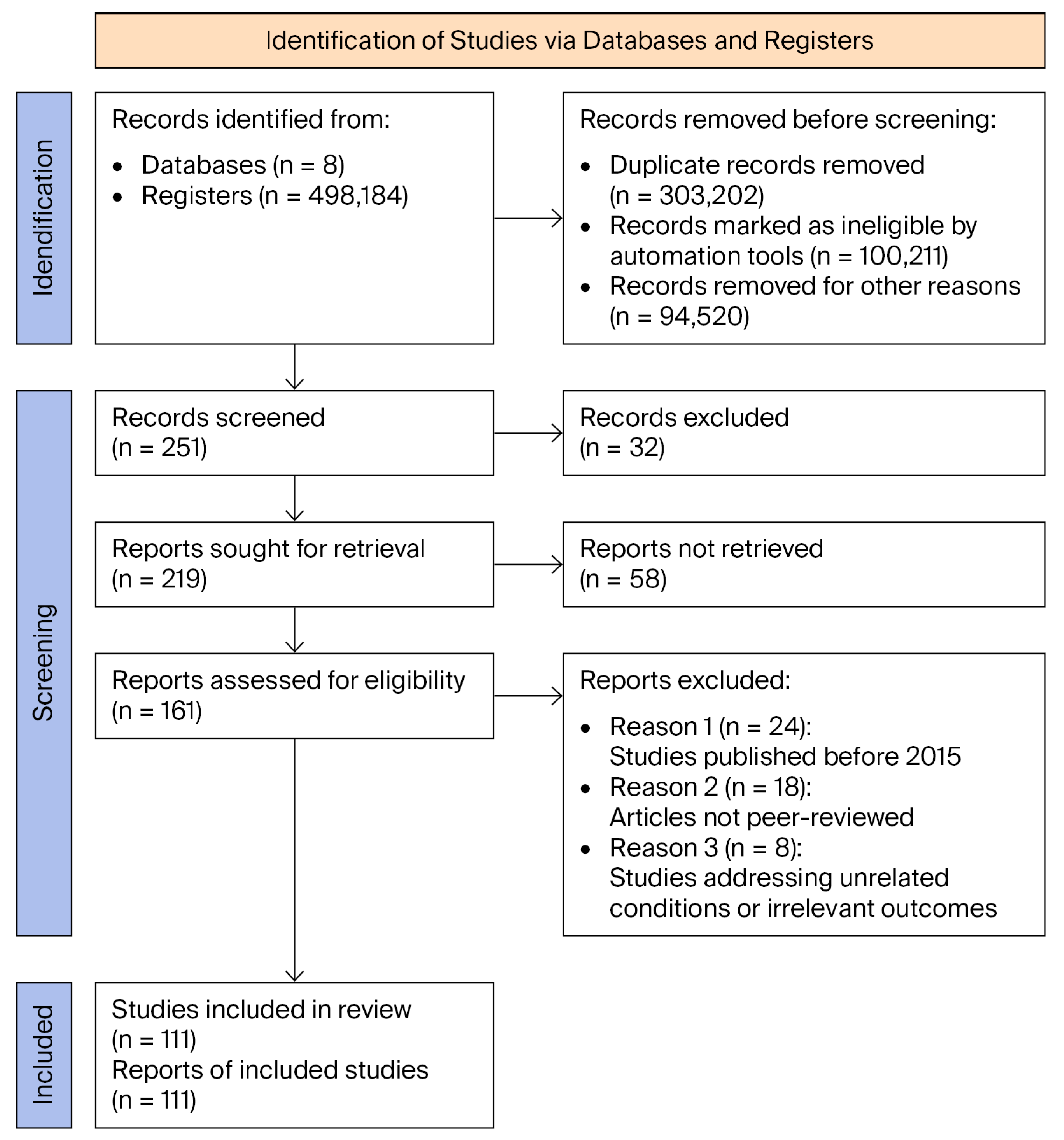
2. Materials and Methods
- The function of miR-210 in response to placental hypoxia, through HIF-1α-regulated mechanisms and its effects on mitochondria and angiogenesis;
- Its potential role as a diagnostic and prognostic biomarker, assessed through its levels in plasma, serum, and placental tissue;
- The importance of an interdisciplinary approach in defining a molecular profile of preeclampsia, by correlating data from genetics, epigenetics, obstetrics, and placental pathology.
2.1. Eligibility Criteria for Study Selection
2.1.1. Inclusion Criteria
- Selected articles needed to address preeclampsia from a molecular perspective, with a particular focus on the role of microRNA-210. Publications were included if they provided relevant insights into the following aspects:
- ○
- Dysregulation of miR-210 expression in placental tissue or maternal circulation in the context of preeclampsia.
- ○
- Molecular mechanisms influenced by miR-210, such as mitochondrial function, trophoblast invasion, angiogenesis, and oxidative stress.
- ○
- The potential of miR-210 as a non-invasive diagnostic biomarker for preeclampsia.
- ○
- Experimental therapeutic approaches targeting the inhibition of miR-210.
- Included studies comprised original research articles, systematic reviews, meta-analyses, and peer-reviewed publications from indexed journals, ensuring both scientific quality and relevance.
2.1.2. Exclusion Criteria
- Studies not adhering to PRISMA methodological standards, as compliance is essential for ensuring the rigour of the review process.
- Isolated case reports lacking generalisability or clear relevance to the molecular role of miR-210 in preeclampsia.
- Studies addressing unrelated pathologies or those in which miR-210 was not analysed in a placental context.
2.1.3. Selection Process
2.2. Assessment of Risk of Bias and Robustness of Evidence
3. Results
3.1. Overview of Selected Studies
3.2. Pathogenic Mechanisms Involved in Preeclampsia: The Role of miR-210
3.3. Diagnostic and Prognostic Value of miR-210 in Preeclampsia
3.4. Therapeutic Perspectives in Targeting miR-210
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokori, E.; Aderinto, N.; Olatunji, G.; Komolafe, R.; Babalola, E.A.; Isarinade, D.T.; Omoworare, O.T. Prevalence and Materno-Fetal Outcomes of Preeclampsia/Eclampsia among Pregnant Women in Nigeria: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2024, 29, 482. [Google Scholar] [CrossRef]
- Thakur, A.S.; Tayade, S.; Patel, D.; Gupta, A.; Batra, N.; Batra, N. Unraveling the Predictive Power: Placenta Growth Factor and Pregnancy-Associated Plasma Protein A in Pre-Eclampsia. Cureus 2024, 16, e51367. [Google Scholar] [CrossRef]
- Nobles, C.J.; Mendola, P.; Mumford, S.L.; Silver, R.M.; Kim, K.; Andriessen, V.C.; Schisterman, E.F. Preconception Blood Pressure and Its Change into Early Pregnancy: Early Risk Factors for Preeclampsia and Gestational Hypertension. Hypertension 2020, 76, 922–929. [Google Scholar] [CrossRef]
- Dines, V.; Suvakov, S.; Kattah, A.; Vermunt, J.; Narang, K.; Jayachandran, M.; Garovic, V.D. Preeclampsia and the Kidney: Pathophysiology and Clinical Implications. Compr. Physiol. 2023, 13, 4231–4267. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, Q.Y.; Jiang, Y.Z.; Zheng, J. Role of Oxygen in Fetoplacental Endothelial Responses: Hypoxia, Physiological Normoxia, or Hyperoxia? Am. J. Physiol. Cell Physiol. 2020, 318, C943–C953. [Google Scholar] [CrossRef]
- Putra, I.W.A. Molecular Development of Placenta and its Relationship with Preeclampsia and Fetal Growth Restriction. Eur. J. Med. Health Sci. 2022, 4, 38–42. [Google Scholar] [CrossRef]
- Das, S.K. Insight into the Etiology of Preeclampsia. Indian J. Biochem. Biophys. 2025, 62, 449–456. [Google Scholar] [CrossRef]
- Margioula-Siarkou, G.; Margioula-Siarkou, C.; Petousis, S.; Margaritis, K.; Vavoulidis, E.; Gullo, G.; Mavromatidis, G. The Role of Endoglin and Its Soluble Form in Pathogenesis of Preeclampsia. Mol. Cell. Biochem. 2022, 477, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef]
- Deng, F.; Lei, J.; Qiu, J.; Zhao, C.; Wang, X.; Li, M.; Gao, Q. DNA Methylation Landscape in Pregnancy-Induced Hypertension: Progress and Challenges. Reprod. Biol. Endocrinol. 2024, 22, 77. [Google Scholar] [CrossRef]
- Frazier, S.; McBride, M.W.; Mulvana, H.; Graham, D. From Animal Models to Patients: The Role of Placental microRNAs, miR-210, miR-126, and miR-148a/152 in Preeclampsia. Clin. Sci. 2020, 134, 1001–1025. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Kokori, E.; Sanker, V.; Yusuf, I.A.; Adefusi, T.O.; Awuah, W.A. miR-210 in Ischaemic Stroke: Biomarker Potential, Challenges and Future Perspectives. Eur. J. Med. Res. 2024, 29, 432. [Google Scholar] [CrossRef]
- Jaszczuk, I.; Koczkodaj, D.; Kondracka, A.; Kwaśniewska, A.; Winkler, I.; Filip, A. The Role of miRNA-210 in Pre-Eclampsia Development. Ann. Med. 2022, 54, 1350–1356. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Mammdoh, Y.M.; Omar, H.; Mohamed, O.A.; Abbas, A.M.; El-Din, L.T. Predictive Value of microRNA-210 in Preeclampsia. J. Curr. Med. Res. Pract. 2023, 8, 74–78. [Google Scholar] [CrossRef]
- Zaccagnini, G.; Greco, S.; Longo, M.; Maimone, B.; Voellenkle, C.; Fuschi, P.; Martelli, F. Hypoxia-Induced miR-210 Modulates the Inflammatory Response and Fibrosis upon Acute Ischemia. Cell Death Dis. 2021, 12, 435. [Google Scholar] [CrossRef]
- Linna-Kuosmanen, S.; Tomas Bosch, V.; Moreau, P.R.; Bouvy-Liivrand, M.; Niskanen, H.; Kansanen, E.; Kaikkonen, M.U. NRF2 Is a Key Regulator of Endothelial microRNA Expression under Proatherogenic Stimuli. Cardiovasc. Res. 2021, 117, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Liu, C.P.; Wang, H.W.; Xu, R.M. Structural Basis for Pri-miRNA Recognition by Drosha. Mol. Cell 2020, 78, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Vergani-Junior, C.A.; Tonon-da-Silva, G.; Inan, M.D.; Mori, M.A. DICER: Structure, Function, and Regulation. Biophys. Rev. 2021, 13, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Fan, P.; Ma, R.; Wang, Q.; He, L.; Niu, H.; Luo, Q. miR-210 Regulates Lung Adenocarcinoma by Targeting HIF-1α. Heliyon 2023, 9, e15261. [Google Scholar] [CrossRef]
- Silina, M.V.; Dzhalilova, D.S.; Makarova, O.V. Role of microRNAs in Regulation of Cellular Response to Hypoxia. Biochemistry 2023, 88, 741–757. [Google Scholar]
- Li, B.; Dasgupta, C.; Huang, L.; Meng, X.; Zhang, L. miRNA-210 Induces Microglial Activation and Regulates Microglia-Mediated Neuroinflammation in Neonatal Hypoxic-Ischemic Encephalopathy. Cell. Mol. Immunol. 2020, 17, 976–991. [Google Scholar] [CrossRef]
- Chen, Y.M.; He, X.Z.; Wang, S.M.; Xia, Y. δ-Opioid Receptors, microRNAs, and Neuroinflammation in Cerebral Ischemia/Hypoxia. Front. Immunol. 2020, 11, 421. [Google Scholar] [CrossRef]
- Ma, Q.; Dasgupta, C.; Shen, G.; Li, Y.; Zhang, L. MicroRNA-210 Downregulates TET2 and Contributes to Inflammatory Response in Neonatal Hypoxic-Ischemic Brain Injury. J. Neuroinflamm. 2021, 18, 6. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, G.; Lee, J.; Lee, Y.; Kim, J.H. Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming. Tissue Eng. Regen. Med. 2022, 19, 19–33. [Google Scholar] [CrossRef]
- Deng, M.; Tong, R.; Zhang, Z.; Wang, T.; Liang, C.; Zhou, X.; Hou, G. EFNA3 as a Predictor of Clinical Prognosis and Immune Checkpoint Therapy Efficacy in Patients with Lung Adenocarcinoma. Cancer Cell Int. 2021, 21, 535. [Google Scholar] [CrossRef]
- Arenas, G.A.; Lorca, R.A. Effects of Hypoxia on Uteroplacental and Fetoplacental Vascular Function during Pregnancy. Front. Physiol. 2024, 15, 1490154. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.G.; Zerbib, E.; Favier, A.; Dabi, Y.; Daraï, E. Value of Non-Coding RNA Expression in Biofluids to Identify Patients at Low Risk of Pathologies Associated with Pregnancy. Diagnostics 2024, 14, 729. [Google Scholar] [CrossRef] [PubMed]
- Eccher, A.; Girolami, I.; Lucenteforte, E.; Troncone, G.; Scarpa, A.; Pantanowitz, L. Diagnostic Mesothelioma Biomarkers in Effusion Cytology. Cancer Cytopathol. 2021, 129, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, J.; Yang, Q.; Liu, Y.; Jia, W.; Zhang, X.; Wang, Y.L. MicroRNA-210 Regulates Placental Adaptation to Maternal Hypoxic Stress during Pregnancy. Biol. Reprod. 2021, 104, 418–429. [Google Scholar] [CrossRef]
- Yagel, S.; Cohen, S.M.; Goldman-Wohl, D.; Beharier, O. Redefining Pre-Eclampsia as Type I or II: Implementing an Integrated Model of the Maternal–Cardiovascular–Placental–Fetal Array. Ultrasound Obstet. Gynecol. 2023, 61, 293–301. [Google Scholar] [CrossRef]
- Hemmatzadeh, M.; Shomali, N.; Yousefzadeh, Y.; Mohammadi, H.; Ghasemzadeh, A.; Yousefi, M. MicroRNAs: Small Molecules with a Large Impact on Pre-Eclampsia. J. Cell. Physiol. 2020, 235, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Rencelj, A.; Gvozdenovic, N.; Cemazar, M. MitomiRs: Their Roles in Mitochondria and Importance in Cancer Cell Metabolism. Radiol. Oncol. 2021, 55, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.; Gupta, N.; Sharma, V.; Sarkar, C.; Mishra, D.P.; Kulshreshtha, R. ALDH5A1/miR-210 Axis Plays a Key Role in Reprogramming Cellular Metabolism and Has a Significant Correlation with Glioblastoma Patient Survival. Cancer Cell Int. 2024, 24, 259. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Chen, Y.; Wu, C.; Cao, Z.; Xia, L.; Meng, J.; Wang, Z. MicroRNAs: Key Regulators of the Trophoblast Function in Pregnancy Disorders. J. Assist. Reprod. Genet. 2023, 40, 3–17. [Google Scholar] [CrossRef]
- Alexander, T.; Roman, I.; Elena, V.; Andrei, G. Publication-Based Analysis of miR-210 Dependent Biomarkers of Pre-Eclampsia. Biol. Commun. 2020, 65, 163–177. [Google Scholar]
- Hu, X.Q.; Dasgupta, C.; Song, R.; Romero, M.; Wilson, S.M.; Zhang, L. MicroRNA-210 Mediates Hypoxia-Induced Repression of Spontaneous Transient Outward Currents in Sheep Uterine Arteries during Gestation. Hypertension 2021, 77, 1412–1427. [Google Scholar] [CrossRef]
- Ghosh, S.; Thamotharan, S.; Fong, J.; Lei, M.Y.; Janzen, C.; Devaskar, S.U. Circulating Extracellular Vesicular microRNA Signatures in Early Gestation Show an Association with Subsequent Clinical Features of Pre-Eclampsia. Sci. Rep. 2024, 14, 16770. [Google Scholar] [CrossRef] [PubMed]
- Meghana, K.S.; Yaliwal, R.G.; Kadakol, G.S.; Bidri, S.R.; Kori, S.; Kadakol Sr, G.S.; Bidri, S. Evaluation of miRNA-210 as a Prognostic Biomarker for Pre-Eclampsia: A Case-Control Study. Cureus 2025, 17, e83453. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Zheng, Y.; Duan, M.; Qiu, Z.; Huang, C. CircRNA as an Achilles Heel of Cancer: Characterization, Biomarker and Therapeutic Modalities. J. Transl. Med. 2024, 22, 752. [Google Scholar] [CrossRef]
- Serati, A.; Novielli, C.; Anelli, G.M.; Mandalari, M.; Parisi, F.; Cetin, I.; Mandò, C. Characterization of Maternal Circulating microRNAs in Obese Pregnancies and Gestational Diabetes Mellitus. Antioxidants 2023, 12, 515. [Google Scholar] [CrossRef]
- Wang, H.; Luo, C.; Wu, X.; Zhang, J.; Xu, Z.; Liu, Y.; Xie, J. Circular RNA hsa_circ_0081343 Promotes Trophoblast Cell Migration and Invasion and Inhibits Trophoblast Apoptosis by Regulating miR-210-5p/DLX3 Axis. Reprod. Biol. Endocrinol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Zou, Z.; Forbes, K.; Harris, L.K.; Heazell, A.E. The Potential Role of the ESRRG Pathway in Placental Dysfunction. Reproduction 2021, 161, R45–R60. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, J.; Zhang, B.; Wang, W.; Liu, J.; Liang, C.; Shi, S. The Promising Role of Noncoding RNAs in Cancer-Associated Fibroblasts: An Overview of Current Status and Future Perspectives. J. Hematol. Oncol. 2020, 13, 154. [Google Scholar] [CrossRef]
- Estrada-Meza, C.; Torres-Copado, A.; Loreti González-Melgoza, L.; Ruiz-Manriquez, L.M.; De Donato, M.; Sharma, A.; Paul, S. Recent Insights into the microRNA and Long Non-Coding RNA-Mediated Regulation of Stem Cell Populations. 3 Biotech 2022, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Sayres, L.; Flockton, A.R.; Ji, S.; Rey Diaz, C.; Gumina, D.L.; Su, E.J. Angiogenic Function of Human Placental Endothelial Cells in Severe Fetal Growth Restriction Is Not Rescued by Individual Extracellular Matrix Proteins. Cells 2023, 12, 2339. [Google Scholar] [CrossRef]
- Kieran, N.W.; Suresh, R.; Dorion, M.F.; MacDonald, A.; Blain, M.; Wen, D.; Healy, L.M. MicroRNA-210 Regulates the Metabolic and Inflammatory Status of Primary Human Astrocytes. J. Neuroinflamm. 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. Abnormal microRNA expression profile at early stages of gestation in pregnancies destined to develop placenta previa. Front. Med. 2024, 11, 1469855. [Google Scholar] [CrossRef]
- Karadzov Orlic, N.; Joksić, I. Preeclampsia pathogenesis and prediction—Where are we now: The focus on the role of galectins and miRNAs. Hypertens. Pregnancy 2025, 44, 2470626. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349. [Google Scholar] [CrossRef]
- Gerede, A.; Stavros, S.; Danavasi, M.; Potiris, A.; Moustakli, E.; Machairiotis, N.; Zikopoulos, A.; Nikolettos, K.; Drakakis, P.; Nikolettos, N.; et al. MicroRNAs in Preeclampsia: Bridging Diagnosis and Treatment. J. Clin. Med. 2025, 14, 2003. [Google Scholar] [CrossRef] [PubMed]
- Giannubilo, S.R.; Cecati, M.; Marzioni, D.; Ciavattini, A. Circulating miRNAs and Preeclampsia: From Implantation to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1418. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, K.; Fijałkowska, A.; Issat, T.; Maciejewski, T.M. Insight into the Key Points of Preeclampsia Pathophysiology: Uterine Artery Remodeling and the Role of MicroRNAs. Int. J. Mol. Sci. 2021, 22, 3132. [Google Scholar] [CrossRef]
- Meruvu, S.; Ding, Z.; Choudhury, M. Mono-(2-ethylhexyl) phthalate induces trophoblast hypoxia and mitochondrial dysfunction through HIF-1α-miR-210-3p axis in HTR-8/SVneo cell line. Curr. Res. Toxicol. 2024, 7, 100188. [Google Scholar] [CrossRef]
- Jairajpuri, D.S.; Malalla, Z.H.; Sarray, S.; Mahmood, N. Analysis of differential expression of hypoxia-inducible microRNA-210 gene targets in mild and severe preeclamptic patients. Non-Coding RNA Res. 2021, 6, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bavelloni, A.; Ramazzotti, G.; Poli, A.; Piazzi, M.; Focaccia, E.; Blalock, W.; Faenza, I. MiRNA-210: A current overview. Anticancer Res. 2017, 37, 6511–6521. [Google Scholar]
- Bakirtzi, K.; Law, I.K.M.; Xue, X.; Iliopoulos, D.; Shah, Y.M.; Pothoulakis, C. Neurotensin promotes the development of colitis and intestinal angiogenesis via Hif-1α–miR-210 signaling. J. Immunol. 2016, 196, 4311–4321. [Google Scholar] [CrossRef]
- Huang, X. Regulation of the hypoxic response by non-coding RNAs. In Tumor Hypoxia; Springer: Cham, Switzerland, 2017; pp. 189–224. [Google Scholar]
- Troise, D.; Infante, B.; Mercuri, S.; Netti, G.S.; Ranieri, E.; Gesualdo, L.; Pontrelli, P. Hypoxic state of cells and immunosenescence: A focus on the role of the HIF signaling pathway. Biomedicines 2023, 11, 2163. [Google Scholar] [CrossRef]
- Srivani, G.; Imran, M.; Merchant, N.; Mandala, J.P.; Nagaraju, G.P. Role of succinate dehydrogenase in hepatocellular carcinoma. In Theranostics and Precision Medicine for the Management of Hepatocellular Carcinoma; Academic Press: Cambridge, MA, USA, 2022; pp. 167–180. [Google Scholar]
- Goyal, A.; Afzal, M.; Goyal, K.; Ballal, S.; Sharma, G.C.; Kavitha, V.; Ali, H. miR-210: A non-invasive biomarker for hypoxia-driven lung cancer diagnosis and therapy. Clin. Chim. Acta 2025, 571, 120215. [Google Scholar] [CrossRef]
- Kornacki, J.; Olejniczak, O.; Sibiak, R.; Gutaj, P.; Wender-Ożegowska, E. Pathophysiology of Pre-Eclampsia—Two Theories of the Development of the Disease. Int. J. Mol. Sci. 2024, 25, 307. [Google Scholar] [CrossRef]
- Bardan, C.R.; Ioniță, I.; Iordache, M.; Călămar-Popovici, D.; Todorescu, V.; Popescu, R.; Bernad, E.S. Epigenetic Biomarkers in Thrombophilia-Related Pregnancy Complications: Mechanisms, Diagnostic Potential, and Therapeutic Implications: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 13634. [Google Scholar] [CrossRef] [PubMed]
- Erbilen, E.A.; Varol, G.F.; Sut, N.; Turker, N.P.; Sayin, N.C. Over-Expression of MicroRNA-210 and MicroRNA-185-5p in Normotensive Late-Fetal Growth Restriction: Preliminary Cohort Study. Gynecol. Obstet. Reprod. Med. 2024, 30, 83–92. [Google Scholar] [CrossRef]
- Bigagli, E.; Spataro, E.; Pasquini, L.; Cinci, L.; D’Ambrosio, M.; De Blasi, C.; Luceri, C. Vaginal miR-210-3p as a potential biomarker for pregnancies complicated by early fetal growth restriction: A proof-of-concept case-control study. Placenta 2025, 163, 8–15. [Google Scholar] [CrossRef]
- Salimbayeva, D.; Kurmanova, A.; Nurmakova, A.; Smailov, M.; Kypshakbayeva, Z. Placental Biomarkers of Preeclampsia: Systematic Review. Bratisl. Med. J. 2025, 126, 1–14. [Google Scholar] [CrossRef]
- Biró, O.; Alasztics, B.; Molvarec, A.; Joó, J.; Nagy, B.; Rigó Jr, J. Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens. 2017, 10, 207–212. [Google Scholar] [CrossRef]
- Trongpisutsak, A.; Phupong, V. Prediction of preeclampsia using a combination of serum micro RNA-210 and uterine artery Doppler ultrasound. Sci. Prog. 2021, 104, 00368504211036856. [Google Scholar] [CrossRef] [PubMed]
- Nikuei, P.; Davoodian, N.; Tahamtan, I.; Keshtkar, A.A. Predictive value of miR-210 as a novel biomarker for pre-eclampsia: A systematic review protocol. BMJ Open 2016, 6, e011920. [Google Scholar] [CrossRef]
- Youssef, H.M.G.; Marei, E.S. Association of MicroRNA-210 and MicroRNA-155 with severity of preeclampsia. Pregnancy Hypertens. 2019, 17, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, E.; Kozyra-Pydyś, E.; Zajkowska, A.; Pankiewicz, K.; Szewczyk, G.; Maciejewski, T.; Fijałkowska, A. Exploring the Role of miRNA-101a in the Circulatory System’s Adaptive Mechanisms in Hypertensive Disorders of Pregnancy. Diagnostics 2025, 15, 535. [Google Scholar] [CrossRef]
- Oancea, M.; Mihu, D.; Braicu, C.; Isachesku, E.; Nati, I.-D.; Boitor-Borza, D.; Diculescu, D.M.; Strilciuc, S.; Pană, A. MicroRNAs in Preeclampsia: An Overview of Biomarkers and Potential Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 5607. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Hou, B.; Wang, J.; Chen, A.; Liu, S. Exploring the role of exosomal MicroRNAs as potential biomarkers in preeclampsia. Front. Immunol. 2024, 15, 1385950. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Hu, M.; Zheng, Y.; Xu, P.; Zhang, L.; Kilby, M.D. Upregulated LncZBTB39 in pre-eclampsia and its effects on trophoblast invasion and migration via antagonizing the inhibition of miR-210 on THSD7A expression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef]
- Cirkovic, A.; Stanisavljevic, D.; Milin-Lazovic, J.; Rajovic, N.; Pavlovic, V.; Milicevic, O.; Milic, N. Preeclamptic women have disrupted placental microRNA expression at the time of preeclampsia diagnosis: Meta-analysis. Front. Bioeng. Biotechnol. 2021, 9, 782845. [Google Scholar] [CrossRef]
- Kochhar, P.; Dwarkanath, P.; Ravikumar, G.; Thomas, A.; Crasta, J.; Thomas, T.; Mukhopadhyay, A. Placental expression of miR-21-5p, miR-210-3p and miR-141-3p: Relation to human fetoplacental growth. Eur. J. Clin. Nutr. 2022, 76, 730–738. [Google Scholar] [CrossRef]
- Awamleh, Z.; Han, V.K. Identification of miR-210-5p in human placentae from pregnancies complicated by preeclampsia and intrauterine growth restriction, and its potential role in the pregnancy complications. Pregnancy Hypertens. 2020, 19, 159–168. [Google Scholar] [CrossRef]
- Munaut, C.; Tebache, L.; Blacher, S.; Noël, A.; Nisolle, M.; Chantraine, F. Dysregulated circulating miRNAs in preeclampsia. Biomed. Rep. 2016, 5, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Song, H. Circ-0002814 participates in proliferation and migration through miR-210 and FUS/VEGF pathway of preeclampsia. J. Obstet. Gynaecol. Res. 2022, 48, 1698–1709. [Google Scholar] [CrossRef]
- Mora-Palazuelos, C.; Villegas-Mercado, C.E.; Avendaño-Félix, M.; Lizárraga-Verdugo, E.; Romero-Quintana, J.G.; López-Gutiérrez, J.; Bermúdez, M. The role of ncRNAs in the immune dysregulation of preeclampsia. Int. J. Mol. Sci. 2023, 24, 15215. [Google Scholar] [CrossRef]
- Popova, A.K.; Vashukova, E.S.; Illarionov, R.A.; Maltseva, A.R.; Pachuliia, O.V.; Postnikova, T.B.; Glotov, A.S. Extracellular Vesicles as Biomarkers of Pregnancy Complications. Int. J. Mol. Sci. 2024, 25, 11944. [Google Scholar] [CrossRef]
- Fasanaro, P.; Romani, S.; Voellenkle, C.; Maimone, B.; Capogrossi, M.C.; Martelli, F. ROD1 is a seedless target gene of hypoxia-induced miR-210. PLoS ONE 2012, 7, e44651. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, P.; Zhang, L. Fetal hypoxia impacts on proliferation and differentiation of Sca-1+ cardiac progenitor cells and maturation of cardiomyocytes: A role of microRNA-210. Genes 2020, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Portugal, C.; Montaño-Samaniego, M.; Guttman-Bazbaz, R.; Bravo-Estupiñan, D.M.; Ibáñez-Hernández, M. Therapeutic Applications of Poly-miRNAs and miRNA Sponges. Int. J. Mol. Sci. 2025, 26, 4535. [Google Scholar] [CrossRef]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating microRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Cheeran, E.A.; Salamah, J.; Enabulele, D.B.; Tahir, A.; Lee, B.K. Long non-coding RNAs: A summary of their roles in placenta development and pathology. Biol. Reprod. 2024, 110, 431–449. [Google Scholar] [CrossRef]
- Dai, W.; Guo, R.; Na, X.; Jiang, S.; Liang, J.; Guo, C.; Li, D. Hypoxia and the endometrium: An indispensable role for HIF-1α as therapeutic strategies. Redox Biol. 2024, 73, 103205. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed]
- Mouillet, J.F.; Chu, T.; Sadovsky, Y. Expression patterns of placental microRNAs. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 737–743. [Google Scholar] [CrossRef]
- Lycoudi, A.; Mavreli, D.; Mavrou, A.; Papantoniou, N.; Kolialexi, A. miRNAs in pregnancy-related complications. Expert Rev. Mol. Diagn. 2015, 15, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Surico, D.; Venkatesan, S.; Ola Pour, M.M.; Aquino, C.I.; Remorgida, V. Extracellular Vesicles and Pregnancy-Related Hypertensive Disorders: A Descriptive Review on the Possible Implications “From Bench to Bedside”. Biology 2025, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Muralimanoharan, S.; Maloyan, A.; Mele, J.; Guo, C.; Myatt, L.G.; Myatt, L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 2012, 33, 816–823. [Google Scholar] [CrossRef]
- Colleoni, F.; Padmanabhan, N.; Yung, H.W.; Watson, E.D.; Cetin, I.; Tissot van Patot, M.C.; Murray, A.J. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: A role for miRNA-210 and protein synthesis inhibition. PLoS ONE 2013, 8, e55194. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Favaro, R.R.; Markert, U.R. Placental miRNAs in feto-maternal communication mediated by extracellular vesicles. Placenta 2020, 102, 27–33. [Google Scholar] [CrossRef]
- Inno, R.; Kikas, T.; Lillepea, K.; Laan, M. Coordinated expressional landscape of the human placental miRNome and transcriptome. Front. Cell Dev. Biol. 2021, 9, 697947. [Google Scholar] [CrossRef]
- Harapan, H.; Andalas, M. The role of microRNAs in the proliferation, differentiation, invasion, and apoptosis of trophoblasts during the occurrence of preeclampsia—A systematic review. Tzu Chi Med. J. 2015, 27, 54–64. [Google Scholar] [CrossRef]
- Fu, G.; Brkić, J.; Hayder, H.; Peng, C. MicroRNAs in human placental development and pregnancy complications. Int. J. Mol. Sci. 2013, 14, 5519–5544. [Google Scholar] [CrossRef]
- Skalis, G.; Katsi, V.; Miliou, A.; Georgiopoulos, G.; Papazachou, O.; Vamvakou, G.; Makris, T. MicroRNAs in preeclampsia. Microrna 2019, 8, 28–35. [Google Scholar] [CrossRef]
- Chan, S.Y.; Loscalzo, J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle 2010, 9, 1072–1083. [Google Scholar] [CrossRef]
- Luo, R.; Wang, Y.; Xu, P.; Cao, G.; Zhao, Y.; Shao, X.; Wang, Y.L. Hypoxia-inducible miR-210 contributes to preeclampsia via targeting thrombospondin type I domain containing 7A. Sci. Rep. 2016, 6, 19588. [Google Scholar] [CrossRef]
- Anton, L.; DeVine, A.; Polyak, E.; Olarerin-George, A.; Brown, A.G.; Falk, M.J.; Elovitz, M.A. HIF-1α stabilization increases miR-210 eliciting first trimester extravillous trophoblast mitochondrial dysfunction. Front. Physiol. 2019, 10, 699. [Google Scholar] [CrossRef]
- Krawczynski, K.; Mishima, T.; Huang, X.; Sadovsky, Y. Intact feto-placental growth in microRNA-210 deficient mice. Placenta 2016, 47, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Gui, J.; Wu, X.; Wu, W. Downregulation of miR-424 in placenta is associated with severe preeclampsia. Pregnancy Hypertens. 2019, 17, 109–112. [Google Scholar] [CrossRef]
- Jelena, M.; Sopić, M.; Joksić, I.; Zmrzljak, U.P.; Karadžov-Orlić, N.; Egić, A.; Spasojević-Kalimanovska, V. Placenta-specific plasma miR518b is a potential biomarker for preeclampsia. Clin. Biochem. 2020, 79, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Atan, N.A.D.; Omidi-Ardali, H.; Tavirani, M.R. Assessment of correlation between miR-210 expression and pre-eclampsia risk: A meta-analysis. Rep. Biochem. Mol. Biol. 2018, 7, 94. [Google Scholar]
- Awoyemi, T.; Jiang, S.; Rahbar, M.; Logentherian, P.; Collett, G.; Zhang, W.; Vatish, M. MicroRNA analysis of medium/large placenta extracellular vesicles in normal and preeclampsia pregnancies. Front. Cardiovasc. Med. 2024, 11, 1371168. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Wei, M.; Chen, Y.; Yang, X.; Chen, L.; Xiao, X. MiR-210 and miR-155 as potential diagnostic markers for pre-eclampsia pregnancies. Medicine 2017, 96, e7515. [Google Scholar] [CrossRef]
- Nejad, R.M.A.; Saeidi, K.; Gharbi, S.; Salari, Z.; Saleh-Gohari, N. Quantification of circulating miR-517c-3p and miR-210-3p levels in preeclampsia. Pregnancy Hypertens. 2019, 16, 75–78. [Google Scholar] [CrossRef]
- Trăistaru, V.A.; Zamfirescu, V.; Burnei Rusu, A.; Bohîlțea, R.; Nastasia, Ș.; Vlădăreanu, R. Ultrasound markers in early pregnancy predictive for preeclampsia. In Proceedings of the 5th Romanian Congress of the Romanian Society of Ultrasound in Obstetrics and Gynecology, Târgu Mureș, Romania, 20–22 April 2017; pp. 593–597, ISBN 978-88-95922-881. [Google Scholar]
- Mitran, M.; Velicu, O.; Comandașu, D.-E.; Vlădăreanu, S. Diagnosis and prevention of preeclampsia–literature review. Ginecol. Ro 2017, 3, 10–12. [Google Scholar] [CrossRef]
- Yalcin, S.E.; Ozler, M.R.; Yalcin, Y.; Saglam, E.; Yilmaz, E.S.; Nerez, N. Association of Early Pregnancy Inflammatory Indices with Preterm Birth and Perinatal Outcomes in Pregnancies with Pregestational Diabetes. J. Clin. Med. 2025, 14, 6834. [Google Scholar] [CrossRef] [PubMed]
- Russu, M.; Stanculescu, R.; Nastasia, S.; Paun, M.; Mubarak, N.; Marin, J.A.; Lachanas, I. Pregnancy Outcomes Following Preconception, Early and Late Administration of Vaginal Micronized Progesterone for Recurrent Pregnancy Loss; GINECO RO: Bucharest, Romania, 2009; Volume 5, pp. 10–15. [Google Scholar]
| Aspect | Details | References |
|---|---|---|
| Primary Objective | miR-210 is a hypoxia-induced microRNA, upregulated in preeclampsia. Its primary role in placental molecular dysfunction lies in the inhibition of genes involved in mitochondrial metabolism, angiogenesis, and trophoblast invasion, thereby contributing to placental insufficiency and the pathogenesis of preeclampsia. | [52,53,54,55] |
| Trophoblast Dysfunction | miR-210 inhibits trophoblast invasion by suppressing the expression of genes essential for cellular motility (e.g., MNT, THSD7A), leading to shallow invasion and deficient placentation. | [56,57] |
| Impaired Angiogenesis | miR-210-mediated repression of EFNA3 reduces angiogenic signalling, limiting vascular development and spiral artery remodelling. | [58,59] |
| Mitochondrial Dysfunction | miR-210 suppresses key genes such as ISCU1/2 and SDHD, disrupting the electron transport chain and increasing oxidative stress. | [60,61] |
| HIF-1α Pathway Activation | Under hypoxic conditions, HIF-1α induces miR-210, which in turn exacerbates hypoxia by inhibiting mitochondrial function, forming a pathological self-perpetuating loop. | [62,63] |
| Cumulative Effects | Sustained miR-210 expression contributes to a pro-inflammatory placental environment, reduced uteroplacental perfusion, and systemic endothelial dysfunction. | [64,65] |
| Clinical Significance | Understanding these mechanisms offers insights for the development of biomarkers and targeted therapies, with potential for early diagnosis and prevention of preeclampsia. | [39,66] |
| Aspect | Diagnostic Biomarker | Prognostic Biomarker | References |
|---|---|---|---|
| Definition | A molecular indicator detectable in plasma or serum, reflecting the presence of preeclampsia. | An early marker indicating the risk of developing preeclampsia before clinical symptoms emerge. | [67,68] |
| Mechanism | Elevated levels of miR-210 in maternal circulation in response to placental hypoxia. | Overexpression of miR-210 correlates with early molecular disturbances in trophoblast function and angiogenesis. | [69,70] |
| Timing of Detection | Detectable during the second and third trimesters, at symptomatic stages. | May be identified as early as the second trimester, prior to clinical onset. | [71,72] |
| Stability | Remarkably stable in plasma, resistant to RNase degradation, ideal for non-invasive testing. | Stable under varied storage conditions, suitable for prenatal screening. | [73] |
| Detection Tools | RT-qPCR, microarray, RNA sequencing. | Standardised techniques compatible with routine testing. | [74,75] |
| Clinical Benefits | Complements traditional markers (blood pressure, proteinuria) by providing molecular insight. | Enables early intervention and monitoring of high-risk pregnancies. | [76,77] |
| Limitations | Absence of universally accepted reference thresholds, inter-study variability. | Circulating miRNA profiles may be influenced by comorbidities. | [78,79] |
| Practical Relevance | Useful in differential diagnosis and confirmation of preeclampsia. | Aids in risk stratification and the individualisation of prenatal care. | [80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cretu, O.E.; Dirlau, A.A.; Neacsu, A.V.; Nenciu, A.E.; Ceausu, I. The Role of microRNA-210 in the Pathogenesis and Diagnosis of Preeclampsia—A Systematic Review. J. Clin. Med. 2025, 14, 7593. https://doi.org/10.3390/jcm14217593
Cretu OE, Dirlau AA, Neacsu AV, Nenciu AE, Ceausu I. The Role of microRNA-210 in the Pathogenesis and Diagnosis of Preeclampsia—A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7593. https://doi.org/10.3390/jcm14217593
Chicago/Turabian StyleCretu, Oana Eliza, Alina Alexandra Dirlau, Adrian Valeriu Neacsu, Adina Elena Nenciu, and Iuliana Ceausu. 2025. "The Role of microRNA-210 in the Pathogenesis and Diagnosis of Preeclampsia—A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7593. https://doi.org/10.3390/jcm14217593
APA StyleCretu, O. E., Dirlau, A. A., Neacsu, A. V., Nenciu, A. E., & Ceausu, I. (2025). The Role of microRNA-210 in the Pathogenesis and Diagnosis of Preeclampsia—A Systematic Review. Journal of Clinical Medicine, 14(21), 7593. https://doi.org/10.3390/jcm14217593








