Long-Term Clinical Outcomes of Left Atrial Appendage Closure in Patients with Left Atrial Appendage Thrombus
Abstract
1. Introduction
2. Methods
2.1. Study Design
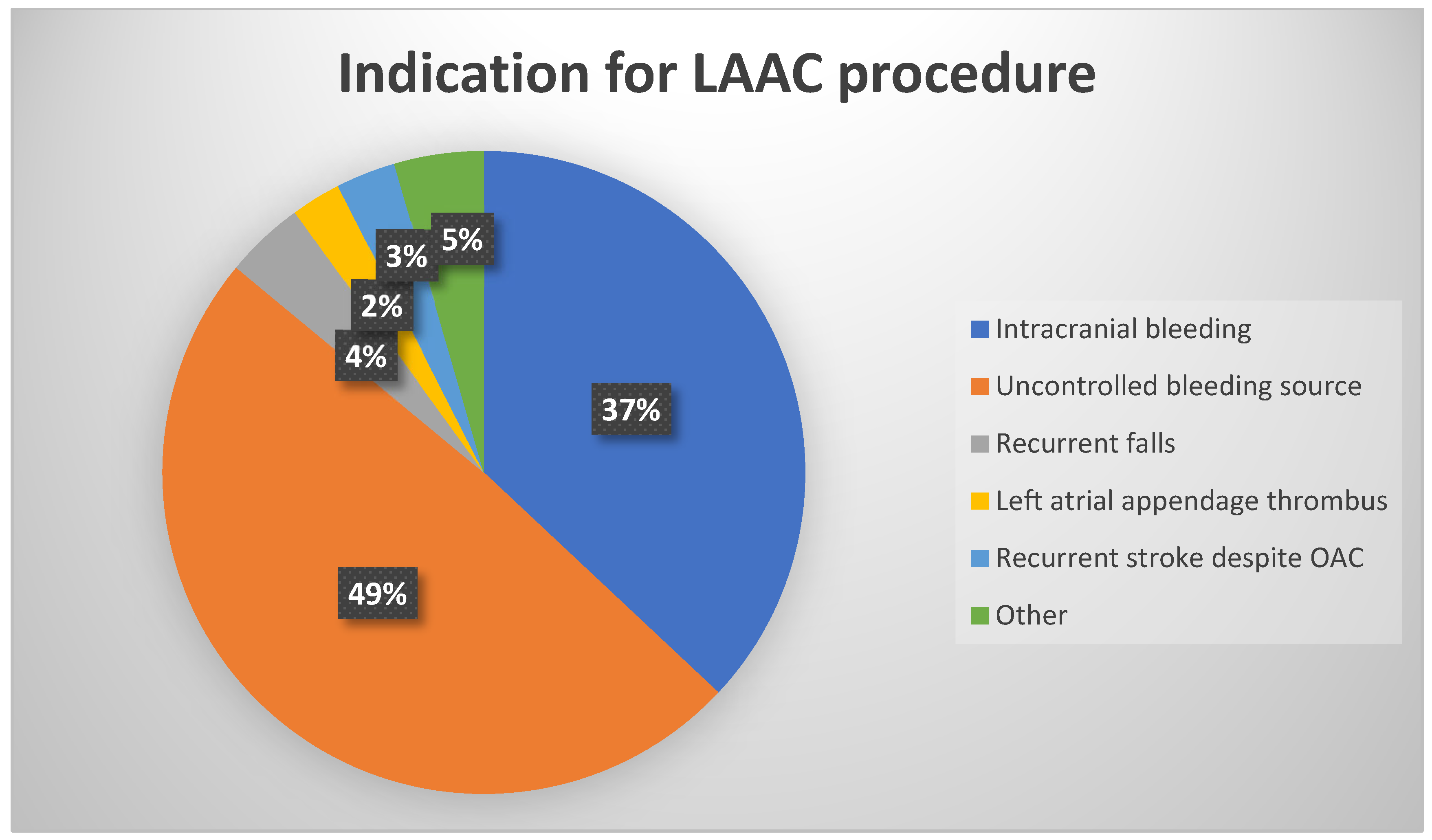
2.2. Thrombus Identification
2.3. Left Atrial Appendage Closure Procedure
- Avoidance of contrast injection into the LAA prior to device deployment.
- Maintenance of the delivery sheath outside the LAA ostium before deployment.
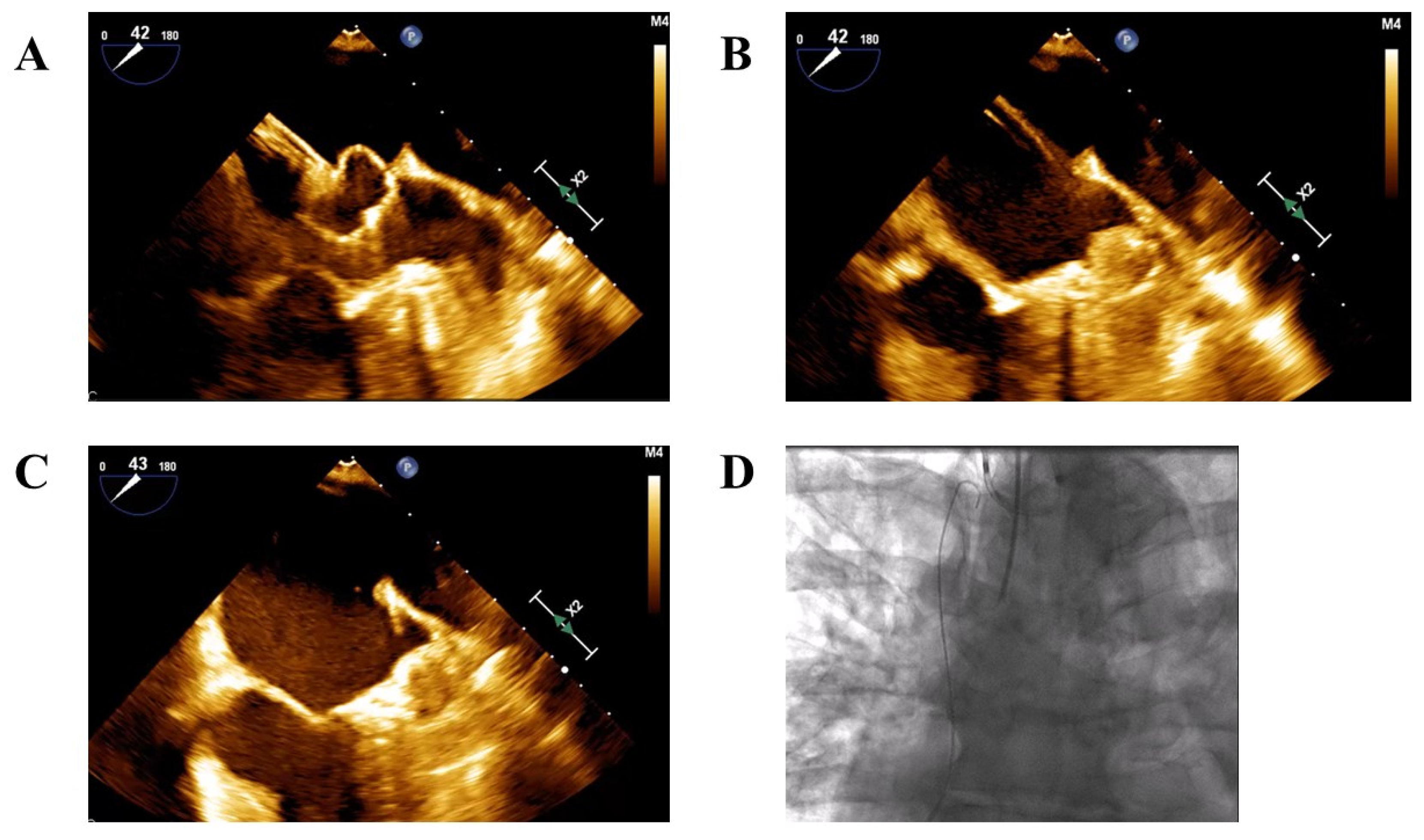
- Cautious advancement of the device and sheath into the LAA only after partial deployment of the device—either the lobe in Amplatzer devices or the ball-shaped structure in Watchman devices to reach the intended landing zone (Figure 1A–C).
2.4. Follow-Up and Endpoints
3. Statistical Analysis
4. Results
5. Discussion
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lip, G.Y.H.; Proietti, M.; Potpara, T.; Mansour, M.; Savelieva, I.; Tse, H.F.; Goette, A.; Camm, A.J.; Blomstrom-Lundqvist, C.; Gupta, D.; et al. Atrial fibrillation and stroke prevention: 25 years of research at EP Europace journal. Europace 2023, 25, euad226. [Google Scholar] [CrossRef]
- Kolominsky-Rabas, P.L.; Sarti, C.; Heuschmann, P.U.; Graf, C.; Siemonsen, S.; Neundoerfer, B.; Katalinic, A.; Lang, E.; Gassmann, K.G.; von Stockert, T.R. A prospective community-based study of stroke in Germany—The Erlangen Stroke Project (ESPro): Incidence and case fatality at 1, 3, and 12 months. Stroke 1998, 29, 2501–2506. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in non-valvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Potpara, T.S.; Polovina, M.M.; Licina, M.M.; Stojanovic, R.M.; Prostran, M.S.; Lip, G.Y. Novel oral anticoagulants for stroke prevention in atrial fibrillation: Focus on apixaban. Adv. Ther. 2012, 29, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Quinlan, D.J.; Eikelboom, J.W. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation 2012, 126, 2428–2432. [Google Scholar] [CrossRef]
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Sharma, S.P.; Cheng, J.; Turagam, M.K.; Gopinathannair, R.; Horton, R.; Lam, Y.Y.; Tarantini, G.; D’Amico, G.; Freixa Rofastes, X.; Lange, M.; et al. Feasibility of Left Atrial Appendage Occlusion in Left Atrial Appendage Thrombus: A Systematic Review. Clin. Electrophysiol. 2020, 6, 414–424. [Google Scholar] [CrossRef]
- Jalal, Z.; Iriart, X.; Dinet, M.L.; Selly, J.B.; Tafer, N.; Renou, P.; Sibon, I.; Thambo, J.B. Extending percutaneous left atrial appendage closure indications using the AMPLATZER™ Cardiac Plug device in patients with persistent left atrial appendage thrombus: The thrombus trapping technique. Arch. Cardiovasc. Dis. 2016, 109, 659–666. [Google Scholar] [CrossRef]
- Marroquin, L.; Tirado-Conte, G.; Pracoń, R.; Streb, W.; Gutierrez, H.; Boccuzzi, G.; Arzamendi-Aizpurua, D.; Cruz-González, I.; Ruiz-Nodar, J.M.; Kim, J.S.; et al. Management and outcomes of patients with left atrial appendage thrombus prior to percutaneous closure. Heart 2022, 108, 1098–1106. [Google Scholar] [CrossRef]
- Boccuzzi, G.G.; Montabone, A.; D’Ascenzo, F.; Colombo, F.; Ugo, F.; Muraglia, S.; De Backer, O.; Nombela-Franco, L.; Meincke, F.; Mazzone, P. Cerebral protection in left atrial appendage closure in the presence of appendage thrombosis. Catheter. Cardiovasc. Interv. 2021, 97, 511–515. [Google Scholar] [CrossRef]
- Sebag, F.A.; Garot, P.; Galea, R.; De Backer, O.; Lepillier, A.; De Meesteer, A.; Hildick-Smith, D.; Armero, S.; Moubarak, G.; Ducrocq, G.; et al. Left atrial appendage closure for thrombus trapping: The international, multicentre TRAPEUR registry. EuroIntervention 2022, 18, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; D’Amico, G.; Latib, A.; Montorfano, M.; Mazzone, P.; Fassini, G.; Maltagliati, A.; Ronco, F.; Saccà, S.; Cruz-Gonzalez, I.; et al. Percutaneous left atrial appendage occlusion in patients with atrial fibrillation and left appendage thrombus: Feasibility, safety and clinical efficacy. EuroIntervention 2018, 13, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Windecker, S.; Thaler, D.; Søndergaard, L.; Carroll, J.; Gold, M.R.; Guo, H.; Brunner, K.J.; Hermiller, J.B.; Diener, H.C.; et al. Rationale and design for AMPLATZER Amulet Left Atrial Appendage Occluder IDE randomized controlled trial (Amulet IDE Trial). Am. Heart J. 2019, 211, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017, 14, 1302–1308. [Google Scholar] [CrossRef]
- Landmesser, U.; Schmidt, B.; Nielsen-Kudsk, J.E.; Lam, S.C.C.; Park, J.W.; Tarantini, G.; Cruz-Gonzalez, I.; Geist, V.; Della Bella, P.; Colombo, A.; et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: Periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention 2017, 13, 867–876. [Google Scholar] [CrossRef]
- Kupó, P.; Pap, R.; Sághy, L.; Tényi, D.; Bálint, A.; Debreceni, D.; Basu-Ray, I.; Komócsi, A. Ultrasound guidance for femoral venous access in electrophysiology procedures-systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2020, 59, 407–414. [Google Scholar] [CrossRef]
- Ledwoch, J.; Sievert, K.; Boersma, L.V.A.; Bergmann, M.W.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; et al. Initial and long-term antithrombotic therapy after left atrial appendage closure with the WATCHMAN. Europace 2020, 22, 1036–1043. [Google Scholar] [CrossRef]
- Continisio, S.; Montonati, C.; Angelini, F.; Bocchino, P.P.; Carbonaro, C.; Giacobbe, F.; Dusi, V.; De Filippo, O.; Ielasi, A.; Giannino, G.; et al. Single versus dual antiplatelet therapy following percutaneous left atrial appendage closure-A systematic review and meta-analysis. Eur. J. Clin. Investig. 2024, 54, e14209. [Google Scholar] [CrossRef]

| Overall N = 403 | No LA Thrombus N = 379 | LA Thrombus N = 24 | p-Value | |
|---|---|---|---|---|
| Age, Years | 76 [71–82] | 76 [71–82] | 80 [75–85] | 0.054 |
| Sex, Male | 260 (65) | 242 | 18 | 0.268 |
| Hypertension, mmHg | 343 (85) | 324 (86) | 19 (79) | 0.379 |
| Active smoker | 39 (10) | 38 (10) | 1 (4) | 0.605 |
| AF pattern * | 0.109 | |||
| Paroxysmal | 237 (59) | 227 (60) | 10 (44) | |
| Persistent | 62 (15) | 59 (16) | 3 (13) | |
| Permanent | 100 (25) | 90 (24) | 10 (43) | |
| CHA2DS2VA | 4 [3–5] | 4 [3–5] | 5 [3–6] | 0.446 |
| CHF | 110 (27) | 102 (27) | 8 (33) | 0.494 |
| Diabetes mellitus | 194 (48) | 183 (48) | 11 (46) | 0.816 |
| Dyslipidemia | 273 (68) | 258 (68) | 15 (63) | 0.571 |
| CKD | 129 (32) | 124 (33) | 5 (21) | 0.226 |
| Carotid artery disease | 33 (8) | 32 (8) | 1 (4) | 0.709 |
| PVD | 69 (17) | 65 (17) | 4 (17) | 1 |
| Ischemic heart disease | 176 (44) | 166 (44) | 10 (42) | 0.838 |
| Permanent pacemaker | 74 (18) | 70 (19) | 4 (17) | 1 |
| Cognitive impairment | 44 (11) | 40 (11) | 4 (17) | 0.32 |
| Malignancy | 38 (9) | 35 (9) | 3 (13) | 0.756 |
| Previous stroke | 139 (35) | 128 (34) | 11 (46) | 0.228 |
| Previous hemorrhagic stroke | 88 (22) | 85 (22) | 3 (13) | 0.254 |
| Previous intracranial bleeding | 129 (32) | 124 (33) | 5 (21) | 0.226 |
| Previous GI bleeding | 162 (40) | 154 (41) | 8 (33) | 0.479 |
| Other bleeding source | 78 (19) | 71 (19) | 7 (29) | 0.283 |
| Recurrent falls | 37 (9) | 36 (10) | 1 (4) | 0.713 |
| BMI, kg/m2 | 27 [25–30] | 27 [25–30] | 26 [24–29] | 0.426 |
| Hemoglobin (g\dL) | 12 ± 1.9 | 11.9 ± 1.9 | 12.2 ± 1.6 | 0.457 |
| Platelets (×103/µL) | 184 [147–233] | 183 [146–231] | 205 [170–264] | 0.115 |
| Creatinine (mg\dL) | 1.11 [0.88–1.41] | 1.12 [0.89–1.41] | 0.97 [0.77–1.35] | 0.179 |
| eGFR (mL/min/1.73 m2) | 63 [45–80] | 61 [45–80] | 72 [48–90] | 0.144 |
| Overall N = 403 | No LA Thrombus N = 379 | LA Thrombus N = 24 | p Value | |
|---|---|---|---|---|
| LAA device size, mm | 27 [24–28] | 27 [24–28] | 27 [25–30] | 0.248 |
| LVEF, % | 60 [50–60] | 60 [50–60] | 55 [35–60] | 0.080 |
| LVEDD, cm | 4.7 [4.4–5.2] | 4.7 [4.4–5.2] | 4.85 [4.4–5.5] | 0.374 |
| LVESD, cm | 3.1 [2.7–3.6] | 3.1 [2.7–3.6] | 3.1 [2.8–4.1] | 0.503 |
| LA diameter, cm | 4.5 ± 0.7 | 4.5 ± 0.7 | 4.8 ± 0.6 | 0.027 |
| LA area, cm | 26 ± 6.4 | 26 ± 6.4 | 28.9 ± 6.8 | 0.054 |
| LAA morphology * | 0.267 | |||
| Chicken wing | 129 (33) | 122 (33) | 7 (32) | |
| Cactus | 27 (7) | 27 (8) | 0 | |
| Cauliflower | 57 (15) | 56 (15) | 1 (5) | |
| Windsock | 61 (16) | 55 (15) | 6 (27) | |
| Other | 115 (29) | 107 (29) | 8 (36) | |
| Device type | 0.003 | |||
| AGA | 23 (6) | 23 (6) | 0 | |
| Amulet | 204 (50) | 195 (51) | 9 (38) | |
| Watchman | 56 (14) | 56 (15) | 0 | |
| Watchman Flex | 120 (30) | 105 (28) | 15 (62) |
| No LA Thrombus N = 379 | LA Thrombus N = 24 | p-Value | |
|---|---|---|---|
| Death | 3 (0.8) | 1 (4.2) | 0.219 |
| Stroke | 1 (0.3) | 0 | 1 |
| Peripheral embolism | 0 | 0 | NA |
| Pericardial effusion | 2 (0.5) | 0 | 1 |
| Tamponade | 3 (0.8) | 0 | 1 |
| Minor bleeding (BARC 1–2) | 9 (2.4) | 1 (4.2) | 0.463 |
| Major bleeding (BARC 3–5) | 7 (1.8) | 1 (4.2) | 0.391 |
| Vascular complications | 8 (2.1) | 3 (12.5) | 0.022 |
| Device embolization | 4 (1.1) | 0 | 1 |
| Group | Procedure Indication | Antiplatelets/Anticoagulants Regimen at Discharge | Bleeding Event |
|---|---|---|---|
| No LA thrombus | GI bleeding | DAPT | Gastrointestinal |
| No LA thrombus | Intracranial bleeding | SAPT | Vascular injury |
| No LA thrombus | GI bleeding | DAPT | Gastrointestinal |
| No LA thrombus | GI bleeding | SAPT | Upper airway |
| No LA thrombus | GI bleeding and thrombocytopenia | SAPT | Gastrointestinal |
| No LA thrombus | GI bleeding | DAPT | Gastrointestinal |
| No LA thrombus | GI bleeding | DAPT | Gastrointestinal |
| LA thrombus | Recurrent stroke | Warfarin and SAPT | Gastrointestinal |
| No LA Thrombus N = 379 | LA Thrombus N = 24 | p-Value | |
|---|---|---|---|
| Antiplatelet therapy | 0.011 | ||
| Single antiplatelet | 143 (38) | 12 (50) | |
| Dual antiplatelet | 218 (58) | 8 (33) | |
| No antiplatelet | 18 (4) | 8 (17) | |
| Anticoagulation | 0.003 | ||
| Warfarin | 10 (3) | 1 (4) | |
| LMWH | 10 (3) | 3 (12) | |
| DOAC | 18 (4) | 4 (17) | |
| Bleeding events | 31 (8.2) | 2 (8.3) | 1 |
| Covariate | HR | 95% CI for HR | p-Value |
|---|---|---|---|
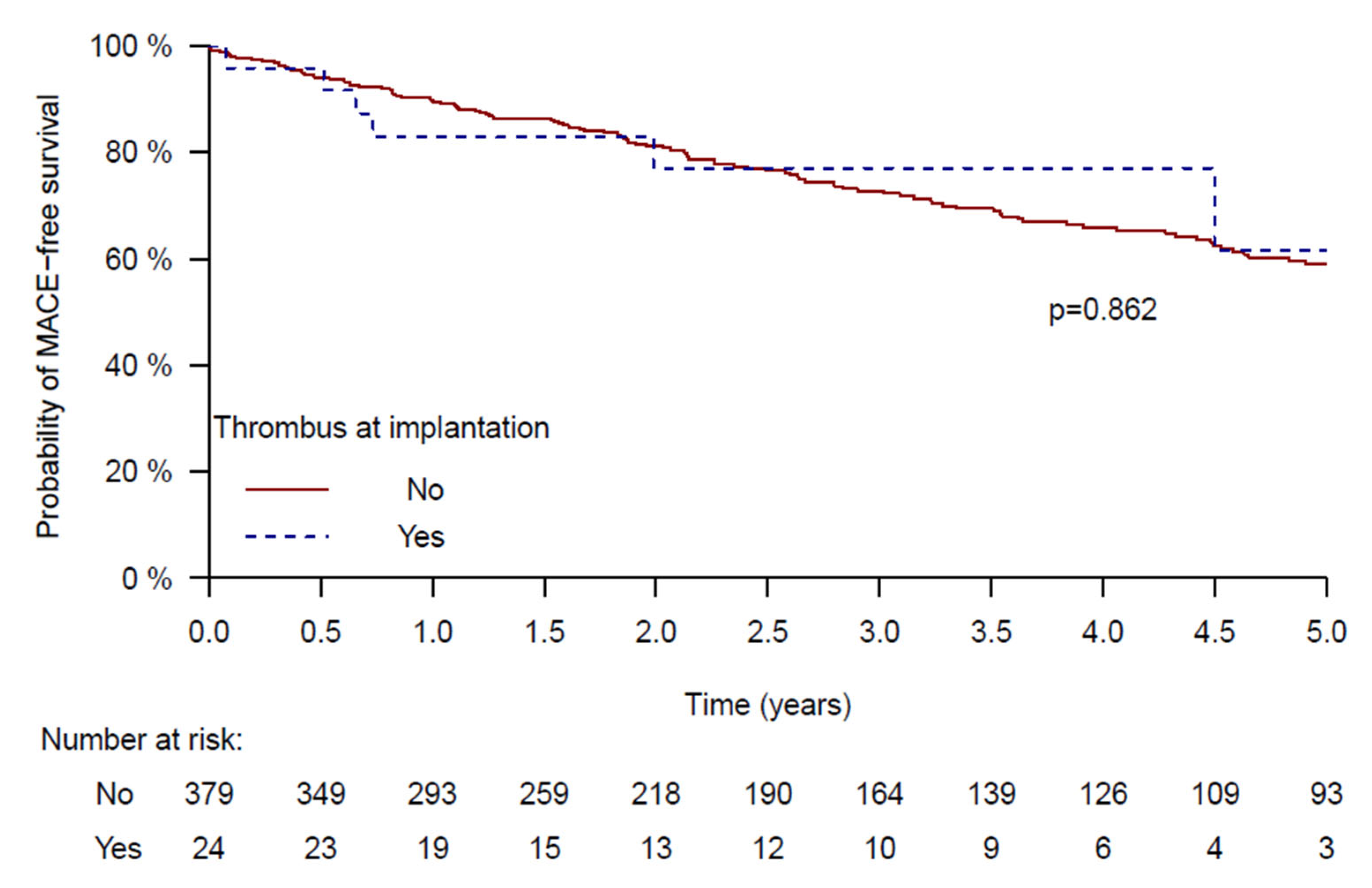
| LAA thrombus | 0.79 | 0.27–2.27 | 0.658 |
| Sex, Female | 0.75 | 0.68–1.73 | 0.747 |
| Age, Years | 1.03 | 1.00–1.07 | 0.039 |
| Congestive heart failure | 1.63 | 1.03–2.6 | 0.039 |
| Nursing assistant | 1.87 | 1.15–3.07 | 0.012 |
| eGFR | 0.988 | 0.978–0.999 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katz, M.; Nahmias Oz, R.; Massalha, E.; Sabag, A.; Nof, E.; Barbash, I.; Fefer, P.; Guetta, V.; Beinart, R. Long-Term Clinical Outcomes of Left Atrial Appendage Closure in Patients with Left Atrial Appendage Thrombus. J. Clin. Med. 2025, 14, 7589. https://doi.org/10.3390/jcm14217589
Katz M, Nahmias Oz R, Massalha E, Sabag A, Nof E, Barbash I, Fefer P, Guetta V, Beinart R. Long-Term Clinical Outcomes of Left Atrial Appendage Closure in Patients with Left Atrial Appendage Thrombus. Journal of Clinical Medicine. 2025; 14(21):7589. https://doi.org/10.3390/jcm14217589
Chicago/Turabian StyleKatz, Moshe, Rotem Nahmias Oz, Eias Massalha, Avi Sabag, Eyal Nof, Israel Barbash, Paul Fefer, Victor Guetta, and Roy Beinart. 2025. "Long-Term Clinical Outcomes of Left Atrial Appendage Closure in Patients with Left Atrial Appendage Thrombus" Journal of Clinical Medicine 14, no. 21: 7589. https://doi.org/10.3390/jcm14217589
APA StyleKatz, M., Nahmias Oz, R., Massalha, E., Sabag, A., Nof, E., Barbash, I., Fefer, P., Guetta, V., & Beinart, R. (2025). Long-Term Clinical Outcomes of Left Atrial Appendage Closure in Patients with Left Atrial Appendage Thrombus. Journal of Clinical Medicine, 14(21), 7589. https://doi.org/10.3390/jcm14217589






