Anterior Cervical Meningocele: Systematic Review of the Literature and Illustrative Case
Abstract
1. Introduction
2. Materials and Methods
2.1. Illustrative Case
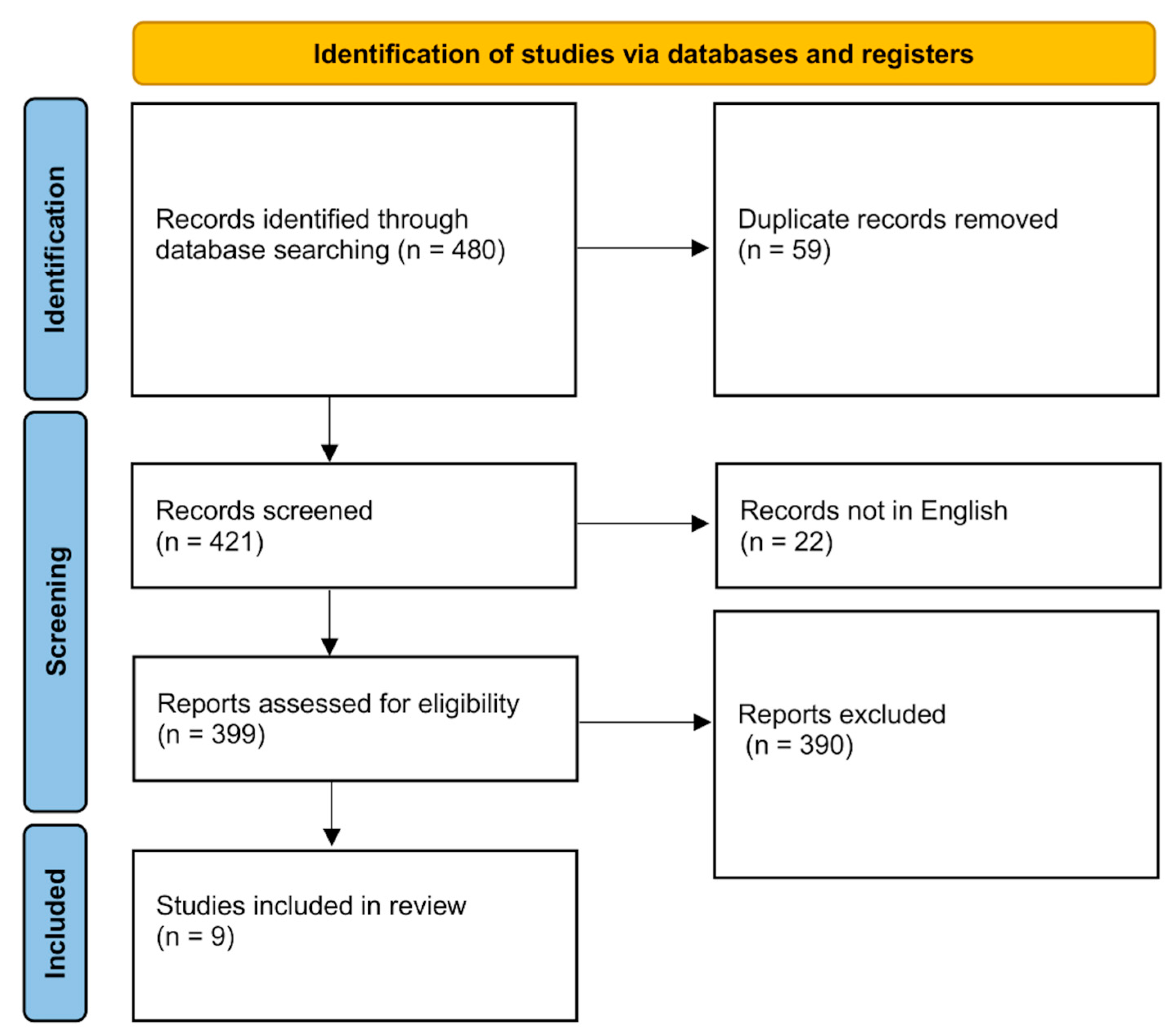
2.2. Systematic Review of the Literature
2.3. Quality Assessment
3. Results
3.1. Illustrative Case
3.2. Systematic Review of the Literature
- A newborn female presented with an anterolateral cervical meningocele located in the posterior lateral triangle, causing torticollis and mass effect. Surgical excision was performed; however, the postoperative course was complicated by a cerebrospinal fluid fistula and subsequent meningitis [6].
- A 51-year-old woman presented with motor deficit and paresthesia in the right upper limb, accompanied by pain triggered by neck movements. Cervical X-ray revealed multiple meningoceles. Physical examination identified multiple café-au-lait spots. Details regarding subsequent treatment and follow-up were not available [16].
- A 40-year-old man with NF1 presented with neck pain accompanied by dysphagia. The pain radiated to the ipsilateral shoulder and was exacerbated by cough. Physical examination revealed soft tissue swelling in the neck and left upper limb weakness. Computed tomography myelography demonstrated multiple contrast-filled meningoceles in the prevertebral spaces. A conservative follow-up strategy was adopted, while neurosurgical intervention was not deemed necessary [1].
- A 55-year-old woman with NF1 presented with worsening cervicothoracic pain of longstanding duration. Radiographs showed spinal canal enlargement with posterior scalloping at T6 and widened intervertebral foramina of C4–T1, predominantly on the right side. An MRI revealed multiple large anterior cervical meningoceles communicating with the dural sac through wide right-sided openings from C3–4 to the cervicothoracic junction, with minor dilations on the left. The cervical spinal cord was preserved without displacement. A myelogram and CT confirmed the findings. In the absence of neurological deficits, a conservative approach was adopted [7].
- A 59-year-old woman with NF1 presented with progressive neck swelling, dysphagia, voice changes, and cervical pain. Physical examination revealed a 10 × 5 cm soft right-sided neck mass, enlarging on cough, multiple café-au-lait macules, cutaneous neurofibromas, and marked cervicothoracic kyphosis but no neurological deficits. CT and MRI showed a large fluid-filled anterior cervical mass, initially indistinguishable from cystic neurofibroma. A myelo-CT demonstrated thecal communication at the C3/4–C4/5 level, as confirmed by technetium-99 scintigraphy, consistent with an anterior cervical meningocele [17].
- A 44-year-old man with NF1 presented with neck pain radiating to the left hand. Examination revealed cutaneous neurofibromas, mild weakness of the intrinsic hand muscles, and sensory loss in the C6–C8 dermatomes. Cervical radiographs demonstrated widening of the left C6–7 foramen, while CT myelography and MRI identified left-sided meningoceles at the C5–6 and C6–7 level, together with a suspected neuroma at C5–6. Due to persistent painful dysesthesia, the patient underwent a left C5 laminectomy, which revealed a neuroma arising from the posterior division of the C5 root and extending into the C5–6 foramen. The tumor was completely excised, and although the meningocele neck could not be repaired, it was wrapped with muscle and hemostatic material. The patient was discharged pain-free within one week, although sensory deficit in the C6–C8 dermatomes persisted. Histopathology confirmed neuroma. At the 6-month follow-up, an MRI showed stable meningoceles. Since the patient declined further intervention, no additional surgery was performed [8].
- A 49-year-old man with NF1 presented with a 10-year history of globus sensation and progressive dysphagia, accompanied more recently by dysphonia, inspiratory stridor, and obstructive sleep apnea (OSAS). ENT evaluation revealed a large submucosal oropharyngeal–hypopharyngeal mass obstructing visualization of the larynx, along with polysomnographic evidence of severe OSAS. An MRI demonstrated three anterior cervical meningoceles (C3–6), the largest protruding through eroded foramina at C3–4 and C4–5, displacing the larynx, esophagus, and trachea. CT confirmed osseous dysplasia and foraminal erosion (C2–C5). Given the airway compromise, a tracheotomy was first performed, followed by a right anterolateral cervical approach. Two large meningoceles with surrounding neurofibromatous tissue were dissected, ligated, and excised, while a smaller intervertebral meningocele at C5–6 was left in place. The postoperative course was complicated by a cerebrospinal fluid leak, which required reoperation and lumbar drainage. The patient had an excellent recovery, with the complete resolution of dysphagia, airway obstruction, and OSAS and normal findings on follow-up laryngoscopy, videofluoroscopy, and polysomnography [9].
- A 45-year-old woman with congenital fusion of C5–C7 presented after a fall and was investigated with an MRI, which demonstrated a cortical defect of the left C6–C7 through which an anterior cervical meningocele extended into the prevertebral space down to T1, as well as an associated syringomyelia from C4/5 to C7/T1. The cranio-cervical junction and the remainder of the spine and brain were normal, and laboratory studies were unremarkable. Neurological examination revealed no objective deficits, although the patient reported subjective upper limb weakness. There were no cutaneous or musculoskeletal abnormalities. She was conservatively managed with advice to avoid extreme cervical movements. At the 6-month follow-up, both her symptoms and MRI findings remained stable. She continues outpatient surveillance with serial imaging [10].
- A 19-year-old woman with NF1 presented with long-standing torticollis, progressive bilateral visual loss over four months, and cerebellar symptoms for three months. Examination revealed early papilledema, positive bilateral cerebellar signs, and multiple café-au-lait macules and freckles on the trunk without evidence of symptoms attributable to the ACM. MRI of the brain demonstrated thickened optic nerves and a left cerebellar pilocytic astrocytoma with obstructive hydrocephalus, while cervical imaging revealed anterior herniation of the meninges into the retropharyngeal space at C2–C4, absent right lateral masses of C3–C4, hypoplastic right C2 pars and pedicle, and fusion of the C2–C3 posterior elements. Dynamic radiographs confirmed abnormal mobility at C3–C4 with atlanto-axial dislocation. The patient underwent suboccipital craniectomy with tumor excision, stabilization with bilateral lateral mass screws (C1, C3, and C4), and temporary prophylactic lumbar drainage. Postoperatively, she demonstrated complete tumor resection with the correction of torticollis. The patient underwent chemotherapy for optic glioma. At the 18-month follow-up, an MRI showed stable ACM size, although the patient remained asymptomatic [18].
3.3. Summary of Patients’ Outcome
- Surgical approach:
- Clinical outcome complicated by cerebrospinal fluid leakage and subsequent meningitis [6].
- Clinical outcome characterized solely by a persistent sensory deficit in the C6–C8 dermatomes [8].
- The clinical course was initially complicated by a cerebrospinal fluid leak requiring reoperation and lumbar drainage. At the six-month follow-up, the patient showed excellent recovery with no residual neurological deficits [9].
- The neurological status of the patient remained stable and was asymptomatic at final follow-up [18].
- Conservative approach:
- Follow-up not available [16];
- Follow-up not available [1];
- Follow-up not available [7];
- Follow-up not available [17];
- The neurological status of the patient remained stable and was asymptomatic at final follow-up [10];
- Our illustrative case: The neurological status of the patient remained stable and was asymptomatic at final follow-up.
3.4. Quality Assessment
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM | anterior cervical meningocele |
| NF1 | neurofibromatosis type 1 |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| N/A | not available |
| KFS | Klippel–Feil syndrome |
| CSF | cerebrospinal fluid. |
Appendix A
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | |
|---|---|---|---|---|---|---|---|---|
| Shore et al. (1982) [6] | Y | Y | Y | Y | Y | Y | Y | Y |
| O’Neil et al. (1983) [16] | Y | Y | Y | Y | N/A | N/A | N/A | Y |
| Kaiser et al. (1986) [1] | Y | Y | Y | Y | Y | N/A | N/A | Y |
| So et al. (1989) [7] | Y | Y | Y | N | Y | N/A | N/A | Y |
| Freund et al. (1992) [17] | Y | Y | Y | Y | N/A | N/A | N/A | Y |
| Göçer et al. (1999) [8] | Y | Y | Y | Y | Y | Y | Y | Y |
| Kos et al. (2009) [9] | Y | Y | Y | Y | Y | Y | Y | Y |
| Gallagher et al. (2015) [10] | Y | Y | Y | Y | Y | Y | Y | Y |
| Kumar et al. (2021) [18] | Y | Y | Y | Y | Y | Y | Y | Y |
| LEGEND | ||||||||
| Q1: Question 1, were patients’ demographic characteristics clearly described? | ||||||||
| Q2: Question 2, was the patient’s history clearly described and presented as a timeline? | ||||||||
| Q3: Question 3, was the current clinical condition of the patient on presentation clearly described? | ||||||||
| Q4: Question 4, were diagnostic tests or assessment methods and the results clearly described? | ||||||||
| Q5: Question 5, was the intervention(s) or treatment procedure(s) clearly described? | ||||||||
| Q6: Question 6, was the post-intervention clinical condition clearly described? | ||||||||
| Q7: Question 7, were adverse events (harms) or unanticipated events identified and described? | ||||||||
| Q8: Question 8, does the case report provide takeaway lessons? | ||||||||
| Y: yes; N: no; unclear; and N/A: not applicable. | ||||||||
References
- Kaiser, M.C.; De Slegte, R.G.; Crezée, F.C.; Valk, J. Anterior cervical meningoceles in neurofibromatosis. Am. J. Neuroradiol. 1986, 7, 1105. [Google Scholar]
- Jaffray, D.; O’Brein, J.P. A true anterior thoracic meningocele associated with a congenital kyphoscoliosis. J. Pediatr. Orthop. 1985, 5, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Laurence, K.M.; Tew, B.J. Natural history of spina bifida cystica and cranium bifidum cysticum: Major central nervous system malformations in South Wales. Part IV. Arch. Dis. Child. 1971, 46, 127–138. [Google Scholar] [CrossRef]
- Walton, M.; Bass, J.; Soucy, P. Tethered cord with anorectal malformation, sacral anomalies and presacral masses: An underrecognized association. Eur. J. Pediatr. Surg. 1995, 5, 59–62. [Google Scholar] [CrossRef]
- Cheng, C.; Tao, B.; Bai, S.; Gao, G.; Li, S.; Shang, A. Anterior Sacral Meningocele: A New Classification and Treatment Using the Dorsal Transsacral Approach. Spine 2020, 45, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.M.; Chun, R.W.; Strother, C.M. Lateral cervical meningocele. Clin. Pediatr. 1982, 21, 430–433. [Google Scholar] [CrossRef]
- So, C.B.; Li, D.K. Anterolateral cervical meningocele in association with neurofibromatosis: MR and CT studies. J. Comput. Assist. Tomogr. 1989, 13, 692–695. [Google Scholar] [CrossRef]
- Göçer, A.I.; Tuna, M.; Gezercan, Y.; Boyar, B.; Bağdatoğlu, H. Multiple anterolateral cervical meningoceles associated with neurofibromatosis. Neurosurg. Rev. 1999, 22, 124–126. [Google Scholar] [CrossRef]
- Kos, M.P.; Peerdeman, S.M.; David, E.F.; Mahieu, H.F. Multiple cervical anterior meningoceles in a patient with neurofibromatosis type 1 cause dysphagia and dyspnea. Otolaryngol.–Head Neck Surg. 2009, 140, 612–613. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Chavredakis, E.; Carter, D.; Bhojak, M.; Jenkinson, M.D.; Clark, S.R. Cervical vertebral fusion with anterior meningocele. Neuroradiol. J. 2015, 28, 205–208. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Gutmann, D.H. Neurofibromatosis type 1: A multidisciplinary approach to care. Lancet Neurol. 2014, 13, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.R.; Dormans, J.P.; Kusumi, K. Klippel-Feil syndrome: Clinical features and current understanding of etiology. Clin. Orthop. Relat. Res. 2004, 424, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Guille, J.T.; Miller, A.; Bowen, J.R.; Forlin, E.; Caro, P.A. The natural history of Klippel-Feil syndrome: Clinical, roentgenographic, and magnetic resonance imaging findings at adulthood. J. Pediatr. Orthop. 1995, 15, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Settle, S.H., Jr.; Rountree, R.B.; Sinha, A.; Thacker, A.; Higgins, K.; Kingsley, D.M. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev. Biol. 2003, 254, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Neill, P.; Whatmore, W.J.; Booth, A. Spinal Meningoceles in Association with Neurofibromatosis. Neurosurgery 1983, 13, 82–84. [Google Scholar] [CrossRef]
- Freund, B.; Timon, C. Cervical meningocele presenting as a neck mass in a patient with neurofibromatosis 1. J. Laryngol. Otol. 1992, 106, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mehrotra, A.; Verma, P.K.; Das, K.K.; Jaiswal, A.K.; Behari, S. Anterior cervical meningocele with craniovertebral junction instability—A case report and literature review. J. Craniovertebr. Junction Spine 2021, 12, 440–444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pessoa, B.L.; Lima, Y.; Orsini, M. True Cervicothoracic Meningocele: A Rare and Benign Condition. Neurol. Int. 2015, 7, 6079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohta, A.; Das, S.; Jindal, R. Anterior sacral meningocele presenting as constipation. J. Pediatr. Neurosci. 2011, 6, 40–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beyazal, M. An asymptomatic large anterior sacral meningocele in a patient with a history of gestation: A case report with radiological findings. Case Rep. Radiol. 2013, 2013, 842620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, F.M.; Burke, B.L. Anterior sacral meningocele. A presentation of three cases. JAMA 1977, 237, 39–42. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | N | Onset Symptoms | Age (yo) | Sex | Genetic Syndrome | Airway Comp. | Dysphagia | Level | Management | Follow-Up | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shore et al. (1982) [6] | 1 | Neck pain | Newborn | F | None | No | No | C2-C3 | Cyst excision duraplasty | 6 months | CSF leak and meningitis |
| O’Neil et al. (1983) [16] | 1 | Paresthesia | 51 | F | NF1 | No | No | C4-C5 | Conservative | N/A | N/A |
| Kaiser et al. (1986) [1] | 1 | Neck pain | 40 | M | NF1 | No | Yes | C3 | Conservative | N/A | N/A |
| So et al. (1989) [7] | 1 | Low backache | 55 | F | NF1 | No | No | C3-T2 | Conservative | N/A | N/A |
| Freund et al. (1992) [17] | 1 | Hypoesthesia C6-C8 and neck pain | 59 | F | NF1 | Yes | Yes | C3-C5 | Conservative | N/A | N/A |
| Göçer et al. (1999) [8] | 1 | Neck pain | 44 | M | NF1 | No | No | C5-C7 | Cyst excision duraplasty | 6 months | None |
| Kos et al. (2009) [9] | 1 | Asymptomatic | 49 | F | NF1 | Yes | Yes | C3-C6 | Cyst excision duraplasty | 6 months | CSF leak |
| Gallagher et al. (2015) [10] | 1 | Neck pain | 45 | F | KFS | No | No | C6-C7 | Conservative | 6 months | None |
| Kumar et al. (2021) [18] | 1 | Neck pain Headache | 19 | F | NF1 | No | No | C2-C4 | Posterior arthrodesis (C1-C4) | 18 months | None |
| Present case | 1 | Asymptomatic | 62 | M | None | No | No | C3-C5 | Conservative | 6 months | None |
| Variables | n = 10 |
|---|---|
| Demographic variables | |
| Age (years) | 47 (41–54) |
| Females [n (%)] | 7 (70) |
| Symptoms [n (%)] | |
| Headache | 1 (10) |
| Neck pain | 6 (60) |
| Paresthesia/Hypoesthesia | 2 (20) |
| Compressive signs | 3 (30) |
| Genetic syndrome [n (%)] | |
| NF1 | 7 (70) |
| KFS | 1 (10) |
| Treatment [n (%)] | |
| Conservative | 4 (40) |
| Excision | 3 (30) |
| Rod fixation | 1 (10) |
| Hemilaminectomy | 1 (10) |
| Not available | 1 (10) |
| Clinical outcomes [n (%)] | |
| Asymptomatic | 4 (40) |
| CSF leak | 2 (20) |
| Meningitis | 1 (10) |
| Not available | 4 (40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, E.; Meola, A.; Scali, I.; Manganotti, P.; Tacconi, L. Anterior Cervical Meningocele: Systematic Review of the Literature and Illustrative Case. J. Clin. Med. 2025, 14, 7530. https://doi.org/10.3390/jcm14217530
Ricci E, Meola A, Scali I, Manganotti P, Tacconi L. Anterior Cervical Meningocele: Systematic Review of the Literature and Illustrative Case. Journal of Clinical Medicine. 2025; 14(21):7530. https://doi.org/10.3390/jcm14217530
Chicago/Turabian StyleRicci, Edoardo, Antonio Meola, Ilario Scali, Paolo Manganotti, and Leonello Tacconi. 2025. "Anterior Cervical Meningocele: Systematic Review of the Literature and Illustrative Case" Journal of Clinical Medicine 14, no. 21: 7530. https://doi.org/10.3390/jcm14217530
APA StyleRicci, E., Meola, A., Scali, I., Manganotti, P., & Tacconi, L. (2025). Anterior Cervical Meningocele: Systematic Review of the Literature and Illustrative Case. Journal of Clinical Medicine, 14(21), 7530. https://doi.org/10.3390/jcm14217530






