Nationwide Trends in Hospitalizations for Sudden Cardiac Arrest Before and During the COVID Outbreak
Abstract
1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Population
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
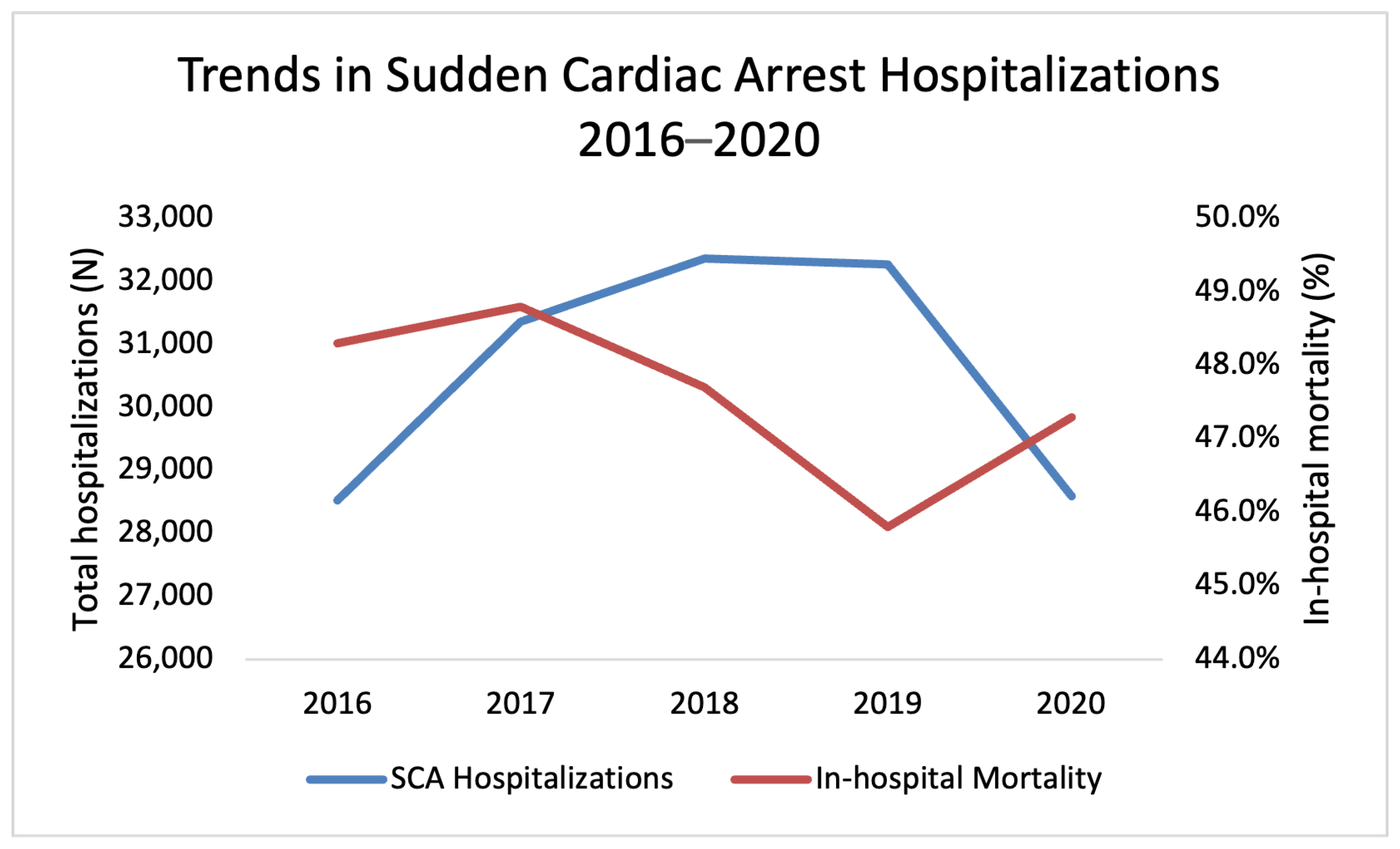
3.1. Trends in SCA Hospitalizations 2016–2020
3.2. Outcomes of SCA Hospitalizations 2016–2020
3.3. Comparison of Comorbidities for Survivors and Non-Survivors
3.4. Multivariable Regression Analysis for Associations with Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCA | sudden cardiac arrest |
| OHCA | out-of-hospital cardiac arrest |
| VF | ventricular fibrillation |
| VT | ventricular tachycardia |
| ICD | implantable cardioverter-defibrillator |
| CHF | congestive heart failure |
| CPR | cardiopulmonary resuscitation |
| AED | automated external defibrillator |
| ROSC | return of spontaneous circulation |
| ACE | angiotensin-converting enzyme |
Appendix A
| Condition | ICD-10 CM Codes |
|---|---|
| SCD | I46.2, I46.8, I46.9 |
| VT | I49.01, I49.02 |
| VF | I47.20, I47.21, I47.29 |
| Hypertension | I10, I11.0, I11.9, I12.0, I12.9, I13.0, I13.10, I13.11, I13.2 I15.0, I15.1, I15.2, I15.8, I15.9, I16.0, I16.1, I16.9 |
| Congestive Heart Failure | I50.1, I50.20, I50.22, I50.30, I50.32, I50.40, I50.42, I50.810, I50.812, I50.814, I50.82, I50.83, I50.84, I50.89, I50.9 |
| History of Ischemic Heart Disease | I25.2, Z95.5, Z95.1, Z95.8 |
| Diabetes Mellitus | E08.42, E08.649, E08.65, E08.9, E09.22, E09.649, E09.65, E09.8, E09.9, E10.0x, E10.1x, E10.6x, E10.8x, E10.9x, E11.0x, E11.1x, E11.6x, E11.8x, E11.9x, E13.0x, E13.1x, E13.6x, E13.8x, E13.9x |
| Renal Failure | I12.0, I12.9, I13.0, I13.10, I13.11, I13.2, N17.0, N17.1, N17.2, N17.8, N17.9, N18.1, N18.2, N18.3, N18.30, N18.31, N18.32, N18.4, N18.5, N18.6, N18.9, N19, Z49.01, Z49.02, Z49.31, Z49.32, Z94.0, Z99.2 |
| COVID-19 | U07.1, J12.82 |
| Acute Coronary Syndrome | I20.0, I20.1, I21.01, I21.02, I21.09, I21.11, I21.19 I21.21, I21.29, I21.3, I21.4, I21.9, I21.A1, I21.A9, I21.B, I22.0, I22.1, I22.2, I22.8, I22.9, I23.0, I24.0, I24.8, I24.9 |
| Peripheral Vascular Disease | I70.0, I70.1, I70.2x, I70.3x, I70.4x, I70.5x, I70.6x, I70.7x, I70.8x, I70.9x, I73.1, I73.8, I73.9, I771, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 |
| Cardiac Pacemaker | Z95.0 |
| Implantable Cardiac Defibrillator | Z95.810 |
| Pacemaker implantation | 0JH634Z, 0JH635Z, 0JH636Z, 0JH637Z, 0JH63MZ, 0JH604Z, 0JH605Z, 0JH606Z, 0JH607Z |
| ICD insertion | 0JH608Z, 0JH609Z, 0JH638Z, 0JH639Z, 0JH808Z, 0JH809Z, 0JH838Z, 0JH839Z, 0JH60FZ, 0JH63FZ, 02H43KZ, 02H60KZ, 02H63KZ, 02H64KZ, 02H70KZ, 02H73KZ, 02H74KZ, 02HK0KZ, 02HK3KZ, 02HK4KZ, 02HL0KZ, 02HL3KZ, 02HL4KZ |
| Catheter ablation | 025K0ZZ, 025K3ZZ, 025K4ZZ, 025L0ZZ, 025L3ZZ, 025L4ZZ, 025M0ZZ, 025M3ZZ, 025M4ZZ, 025D0ZZ, 025D3ZZ, 025D4ZZ |
| Coronary catheterization | 4A023N6, 4A023N7, 4A023N8 |
| Condition | ICD-10 CM Codes for Deyo-CCI Score | Score |
|---|---|---|
| Myocardial infarction | I21.x, I22.x, I25.2 | 1 |
| Congestive heart failure | I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5–I42.9, I43.x, I50.x, P29.0 | 1 |
| Peripheral vascular disease | I70.x, I71.x, I73.1, I73.8, I73.9, I77.1, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 | 1 |
| Cerebrovascular disease | G45.x, G46.x, H34.0, I60.x–I69.x | 1 |
| Dementia | F00.x–F03.x, F05.1, G30.x, G31.1 | 1 |
| Chronic pulmonary disease | I27.8, I27.9, J40.x–J47.x, J60.x–J67.x, J68.4, J70.1, J70.3 | 1 |
| Rheumatologic disease | M05.x, M06.x, M31.5, M32.x–M34.x, M35.1, M35.3, M36.0 | 1 |
| Peptic ulcer disease | K25.x–K28.x | 1 |
| Mild liver disease | B18.x, K70.0–K70.3, K70.9, K71.3–K71.5, K71.7, K73.x, K74.x, K76.0, K76.2–K76.4, K76.8, K76.9, Z94.4 | 1 |
| Diabetes | E10.0, E10.l, E10.6, E10.8, E10.9, E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9 | 1 |
| Diabetes with chronic complications | E10.2–E10.5, E10.7, E11.2–E11.5, E11.7, E12.2–E12.5, E12.7, E13.2–E13.5, E13.7, E14.2–E14.5, E14.7 | 2 |
| Hemiplegia or paraplegia | G04.1, G11.4, G80.1, G80.2, G81.x, G82.x, G83.0–G83.4, G83.9 | 2 |
| Renal disease | I12.0, I13.1, N03.2–N03.7, N05.2–N05.7, N18.x, N19.x, N25.0, Z49.0–Z49.2, Z94.0, Z99.2 | 2 |
| Any malignancy including leukemia and lymphoma | C00.x–C26.x, C30.x–C34.x, C37.x–C41.x, C43.x, C45.x–C58.x, C60.x–C76.x, C81.x–C85.x, C88.x, C90.x–C97.x | 2 |
| Moderate or severe liver disease | I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K72.1, K72.9, K76.5, K76.6, K76.7 | 3 |
| Metastatic solid tumor | C77.x–C80.x | 6 |
| Acquired immunodeficiency syndrome (AIDS) | B20.x–B22.x, B24.x | 6 |
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Yow, A.G.; Rajasurya, V.; Sharma, S. Sudden Cardiac Death. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Patel, A.; Jernigan, D.B. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak—United States, December 31, 2019–February 4, 2020. Am. J. Transplant. 2020, 20, 889–895. [Google Scholar] [CrossRef]
- Kole, C.; Stefanou, Ε.; Karvelas, N.; Schizas, D.; Toutouzas, K.P. Acute and Post-Acute COVID-19 Cardiovascular Complications: A Comprehensive Review. Cardiovasc. Drugs Ther. 2024, 38, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Girotra, S.; Tang, Y.; Al-Araji, R.; Nallamothu, B.K.; McNally, B. Outcomes for Out-of-Hospital Cardiac Arrest in the United States During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2021, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, C.; Park, Y.; Won, M. Comparison of out-of-hospital cardiac arrests during the COVID-19 pandemic with those before the pandemic: An updated systematic review and meta-analysis. Front. Public Health 2023, 11, 1180511. [Google Scholar] [CrossRef]
- Baldi, E.; Cortegiani, A.; Savastano, S. Cardiac arrest and coronavirus disease 2019. Curr. Opin. Crit. Care 2022, 28, 237–243. [Google Scholar] [CrossRef]
- Gaspari, M.D.; Fedeli, U.; Saia, M.; Carturan, E.; Pilichou, K.; Corrado, D.; Thiene, G.; Rizzo, S.; Basso, C. Rate and Cause of Sudden Cardiac Death in the Young During the COVID-19 Pandemic and Vaccination. Circulation 2023, 148, 2069–2071. [Google Scholar] [CrossRef]
- Terzic, C.M.; Medina-Inojosa, B.J. Cardiovascular Complications of Coronavirus Disease-2019. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Introduction to the HCUP National Inpatient Sample (NIS). Available online: https://hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2020.jsp (accessed on 30 December 2023).
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar]
- Chu, Y.-T.; Ng, Y.-Y.; Wu, S.-C. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Serv. Res. 2010, 10, 140. [Google Scholar] [CrossRef]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P.; AMIS Plus Investigators. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef]
- Rozen, G.; Elbaz-Greener, G.; Marai, I.; Heist, E.K.; Ruskin, J.N.; Carasso, S.; Birati, E.Y.; Amir, O. The relationship between the body mass index and in-hospital mortality in patients admitted for sudden cardiac death in the United States. Clin. Cardiol. 2021, 44, 1673–1682. [Google Scholar] [CrossRef]
- Khan, A.J.; Jan Liao, C.; Kabir, C.; Hallak, O.; Samee, M.; Potts, S.; Klein, L.W. Etiology and Determinants of In-Hospital Survival in Patients Resuscitated After Out-of-Hospital Cardiac Arrest in an Urban Medical Center. Am. J. Cardiol. 2020, 130, 78–84. [Google Scholar] [CrossRef]
- Shiga, T.; Kohro, T.; Yamasaki, H.; Aonuma, K.; Suzuki, A.; Ogawa, H.; Hagiwara, N.; Yamazaki, T.; Nagai, R.; Kasanuki, H. Body Mass Index and Sudden Cardiac Death in Japanese Patients After Acute Myocardial Infarction: Data From the JCAD Study and HIJAMI-II Registry. J. Am. Heart Assoc. 2018, 7, e008633. [Google Scholar] [CrossRef]
- Matinrazm, S.; Ladejobi, A.; Pasupula, D.K.; Javed, A.; Durrani, A.; Ahmad, S.; Munir, M.B.; Adelstein, E.; Jain, S.K.; Saba, S. Effect of body mass index on survival after sudden cardiac arrest. Clin. Cardiol. 2018, 41, 46–50. [Google Scholar] [CrossRef]
- Gupta, T.; Kolte, D.; Mohananey, D.; Khera, S.; Goel, K.; Mondal, P.; Aronow, W.S.; Jain, D.; Cooper, H.A.; Iwai, S.; et al. Relation of Obesity to Survival After In-Hospital Cardiac Arrest. Am. J. Cardiol. 2016, 118, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Karam, N.; Jost, D.; Perrot, D.; Frattini, B.; Derkenne, C.; Sharifzadehgan, A.; Waldmann, V.; Beganton, F.; Narayanan, K.; et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: A population-based, observational study. Lancet Public Health 2020, 5, e437–e443. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.E.; Masuda, Y.; Tan, D.J.H.; Liu, N.; Morrison, L.J.; Ong, M.E.H.; Blewer, A.L.; Ho, A.F.W. Impact of the COVID-19 pandemic on the epidemiology of out-of-hospital cardiac arrest: A systematic review and meta-analysis. Ann. Intensive Care 2021, 11, 169. [Google Scholar] [CrossRef]
- Husain, A.A.; Rai, U.; Sarkar, A.K.; Chandrasekhar, V.; Hashmi, M.F. Out-of-Hospital Cardiac Arrest during the COVID-19 Pandemic: A Systematic Review. Healthcare 2023, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Uy-Evanado, A.; Chugh, H.S.; Sargsyan, A.; Nakamura, K.; Mariani, R.; Hadduck, K.; Salvucci, A.; Jui, J.; Chugh, S.S.; Reinier, K. Out-of-Hospital Cardiac Arrest Response and Outcomes During the COVID-19 Pandemic. JACC Clin. Electrophysiol. 2021, 7, 6–11. [Google Scholar] [CrossRef]
- Chugh, H.S.; Sargsyan, A.; Nakamura, K.; Uy-Evanado, A.; Dizon, B.; Norby, F.L.; Young, C.; Hadduck, K.; Jui, J.; Shepherd, D.; et al. Sudden cardiac arrest during the COVID-19 pandemic: A two-year prospective evaluation in a North American community. Heart Rhythm 2023, 20, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Giudicessi, J.R. Excess out-of-hospital sudden deaths during the COVID-19 pandemic: A direct or indirect effect of SARS-CoV-2 infections? Heart Rhythm 2021, 18, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Ichim, C.; Pavel, V.; Mester, P.; Schmid, S.; Todor, S.B.; Stoia, O.; Anderco, P.; Kandulski, A.; Müller, M.; Heumann, P.; et al. Assessing Key Factors Influencing Successful Resuscitation Outcomes in Out-of-Hospital Cardiac Arrest (OHCA). J. Clin. Med. 2024, 13, 7399. [Google Scholar] [CrossRef] [PubMed]
- Mountantonakis, S.E.; Makker, P.; Saleh, M.; Coleman, K.M.; Husk, G.; Jauhar, R.; Singh, V.; Epstein, L.M.; Kuvin, J. Increased Inpatient Mortality for Cardiovascular Patients During the First Wave of the COVID-19 Epidemic in New York. J. Am. Heart Assoc. 2021, 10, e020255. [Google Scholar] [CrossRef]
- Liu, J.Z.; Counts, C.R.; Drucker, C.J.; Emert, J.M.; Murphy, D.L.; Schwarcz, L.; Kudenchuk, P.J.; Sayre, M.R.; Rea, T.D. Acute SARS-CoV-2 Infection and Incidence and Outcomes of Out-of-Hospital Cardiac Arrest. JAMA Netw. Open 2023, 6, e2336992. [Google Scholar] [CrossRef]
- Sultanian, P.; Lundgren, P.; Strömsöe, A.; Aune, S.; Bergström, G.; Hagberg, E.; Hollenberg, J.; Lindqvist, J.; Djärv, T.; Castelheim, A.; et al. Cardiac arrest in COVID-19: Characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur. Heart J. 2021, 42, 1094–1106. [Google Scholar] [CrossRef]
- Yang, K.-C.; Lee, B.; Ahn, Y.-Y.; Perry, B.L. Use of and Comorbidities Associated With Diagnostic Codes for COVID-19 in US Health Insurance Claims. JAMA Netw. Open 2021, 4, e2124643. [Google Scholar] [CrossRef]
- van Dongen, L.H.; Blom, M.T.; de Haas, S.C.M.; van Weert, H.; Elders, P.; Tan, H.; Investigators, E.-N. Higher chances of survival to hospital admission after out-of-hospital cardiac arrest in patients with previously diagnosed heart disease. Open Heart 2021, 8, e001805. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: A prospective cohort in UK Biobank. Cardiovasc. Res. 2023, 119, 1718–1727. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Olshansky, B.; Chung, M.K.; Gordon, S.; Joglar, J.A.; Marcus, G.M.; Mar, P.L.; Russo, A.M.; Srivatsa, U.N.; Wan, E.Y.; et al. Cardiac Arrhythmias and Autonomic Dysfunction Associated With COVID-19: A Scientific Statement From the American Heart Association. Circulation 2024, 150, e449–e465. [Google Scholar] [CrossRef] [PubMed]
- Varney, J.A.; Dong, V.S.; Tsao, T.; Sabir, M.S.; Rivera, A.T.; Ghula, S.; Moriles, K.E.; Cherukuri, M.L.; Fazal, R.; Azevedo, C.B.; et al. COVID-19 and arrhythmia: An overview. J. Cardiol. 2022, 79, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Kyuno, D.; Tateno, M.; Ono, Y.; Magara, K.; Takasawa, K.; Takasawa, A.; Osanai, M. Common pathological findings in the heart in COVID-19-related sudden death cases: An autopsy case series. Heliyon 2023, 9, e20564. [Google Scholar] [CrossRef]
- Daoudi, S.; John, K.; Chalhoub, F.; Chee, J.; Infeld, M.; Elbaz-Greener, G.; Homoud, M.; Ruskin, J.N.; Heist, E.K.; Madias, C.; et al. Nationwide Trends in Hospitalizations for Atrial Fibrillation and Flutter in the United States before and during the Outbreak of the COVID-19 Pandemic. J. Clin. Med. 2024, 13, 4883. [Google Scholar] [CrossRef] [PubMed]


| Year | |||||
|---|---|---|---|---|---|
| 2016–2019 | 2020 | Total | p Value 1 | ||
| Patients, n | Unweighted | 6226 (avg) | 5717 | 30,620 | 0.066 |
| Weighted | 31,129 | 28,585 | 153,100 | ||
| Primary diagnosis, % | VT | 9.7% | 10.7% | 9.9% | 0.041 * |
| VF | 42.9% | 43.7% | 43.0% | 0.26 | |
| SCA | 47.4% | 45.6% | 47.0% | 0.019 * | |
| Age group, % | 18–44 | 10.7% | 11.4% | 10.8% | 0.007 * |
| 45–59 | 23.9% | 23.7% | 23.8% | ||
| 60–74 | 38.3% | 39.7% | 38.6% | ||
| 75 and older | 27.2% | 25.2% | 26.8% | ||
| Gender, % | Male | 62.8% | 62.3% | 62.7% | 0.40 |
| Female | 37.2% | 37.7% | 37.3% | ||
| Race, % | White | 66.7% | 66.4% | 66.6% | 0.12 |
| Black | 19.1% | 19.1% | 19.1% | ||
| Hispanic | 7.9% | 7.4% | 7.8% | ||
| Asian/Pacific Islander | 2.7% | 2.6% | 2.6% | ||
| Native American | 0.6% | 0.8% | 0.6% | ||
| Other | 3.2% | 3.7% | 3.2% | ||
| Comorbidities, % | Hypertension | 68.3% | 68.4% | 68.4% | 0.98 |
| Congestive Heart Failure | 28.8% | 30.3% | 29.1% | 0.029 * | |
| Diabetes | 34.1% | 32.6% | 33.8% | 0.026 * | |
| Renal Failure | 28.6% | 28.8% | 28.7% | 0.84 | |
| Ischemic Heart Disease | 23.7% | 22.0% | 23.3% | 0.009 * | |
| Acute Coronary Syndrome | 14.7% | 18.5% | 15.4% | <0.001 * | |
| Peripheral Vascular Disease | 7.1% | 6.3% | 6.9% | 0.070 | |
| Cardiac Pacemaker | 3.5% | 3.0% | 3.4% | 0.047 * | |
| Implantable Cardiac Defibrillator | 11.7% | 11.0% | 11.6% | 0.16 | |
| Non-morbid obesity | 8.9% | 9.9% | 9.1% | 0.012 * | |
| Morbid obesity | 7.9% | 8.4% | 8.0% | 0.23 | |
| Deyo-CCI, % | 0 | 15.7% | 15.5% | 15.6% | 0.22 |
| 1 | 21.7% | 20.7% | 21.5% | ||
| 2 or higher | 62.7% | 63.8% | 62.9% | ||
| Primary expected payer, % | Medicare | 55.5% | 52.5% | 54.9% | 0.0016 * |
| Medicaid | 13.2% | 14.3% | 13.4% | ||
| Private | 23.7% | 24.5% | 23.8% | ||
| Self-pay | 4.4% | 5.1% | 4.6% | ||
| No Charge | 0.2% | 0.3% | 0.3% | ||
| Other | 3.0% | 3.3% | 3.0% | ||
| Median household income, % | 0 to 25th percentile | 30.7% | 30.7% | 30.7% | 0.93 |
| 26th to 50th percentile | 26.1% | 26.5% | 26.2% | ||
| 51st to 75th percentile | 23.5% | 23.4% | 23.4% | ||
| Hospital status, % | Rural | 5.3% | 4.6% | 5.1% | <0.001 * |
| Urban nonteaching | 20.7% | 17.5% | 20.0% | ||
| Urban teaching | 74.1% | 77.9% | 74.9% | ||
| Hospital region, % | Northeast | 16.4% | 15.0% | 16.2% | 0.080 |
| Midwest | 22.6% | 22.8% | 22.6% | ||
| South | 41.5% | 42.6% | 41.7% | ||
| West | 19.5% | 19.6% | 19.5% | ||
| Hospital bedsize, % | Small | 16.3% | 18.9% | 16.8% | <0.001 * |
| Medium | 29.4% | 28.7% | 29.3% | ||
| Large | 54.3% | 52.4% | 53.9% | ||
| Year | |||||
|---|---|---|---|---|---|
| 2016–2019 | 2020 | Total | p Value 1 | ||
| In-hospital interventions, % | Coronary catheterization | 25.2% | 23.9% | 25.0% | 0.04 * |
| Pacemaker implantation | 1.1% | 0.9% | 1.0% | 0.38 | |
| ICD insertion | 15.8% | 16.9% | 16.0% | 0.02 * | |
| Mortality (total and by diagnosis), % | VT | 28.8% | 28.7% | 28.7% | 0.99 |
| VF | 23.8% | 24.5% | 23.9% | 0.44 | |
| SCA | 73.2% | 73.5% | 73.2% | 0.77 | |
| Total | 47.7% | 47.3% | 47.6% | 0.66 | |
| Disposition of patient, % | Routine | 47.9% | 48.5% | 48.0% | <0.001 * |
| Transfer to Short-term Hospital | 12.1% | 10.4% | 11.8% | ||
| Transfer other | 26.1% | 24.6% | 25.9% | ||
| Home Health Care | 12.7% | 15.3% | 13.2% | ||
| Against Medical Advice | 1.0% | 1.0% | 1.0% | ||
| Discharge alive, unknown destination | 0.2% | 0.2% | 0.2% | ||
| Length of stay, mean days | 5.71 | 6.08 | 5.78 | 0.008 * | |
| Total charges, mean dollars | 122,659 | 144,786 | 127,096 | <0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoudi, S.; Furer, A.; John, K.; Chalhoub, F.; Chee, J.; Infeld, M.; Elbaz-Greener, G.; Homoud, M.; Udelson, J.; Madias, C.; et al. Nationwide Trends in Hospitalizations for Sudden Cardiac Arrest Before and During the COVID Outbreak. J. Clin. Med. 2025, 14, 7517. https://doi.org/10.3390/jcm14217517
Daoudi S, Furer A, John K, Chalhoub F, Chee J, Infeld M, Elbaz-Greener G, Homoud M, Udelson J, Madias C, et al. Nationwide Trends in Hospitalizations for Sudden Cardiac Arrest Before and During the COVID Outbreak. Journal of Clinical Medicine. 2025; 14(21):7517. https://doi.org/10.3390/jcm14217517
Chicago/Turabian StyleDaoudi, Sarah, Ariel Furer, Kevin John, Fadi Chalhoub, Jennifer Chee, Margaret Infeld, Gabby Elbaz-Greener, Munther Homoud, James Udelson, Christopher Madias, and et al. 2025. "Nationwide Trends in Hospitalizations for Sudden Cardiac Arrest Before and During the COVID Outbreak" Journal of Clinical Medicine 14, no. 21: 7517. https://doi.org/10.3390/jcm14217517
APA StyleDaoudi, S., Furer, A., John, K., Chalhoub, F., Chee, J., Infeld, M., Elbaz-Greener, G., Homoud, M., Udelson, J., Madias, C., & Rozen, G. (2025). Nationwide Trends in Hospitalizations for Sudden Cardiac Arrest Before and During the COVID Outbreak. Journal of Clinical Medicine, 14(21), 7517. https://doi.org/10.3390/jcm14217517







