Premature Ovarian Insufficiency and Diminished Ovarian Reserve: From Diagnosis to Current Management and Treatment
Abstract
1. Introduction
2. Premature Ovarian Insufficiency
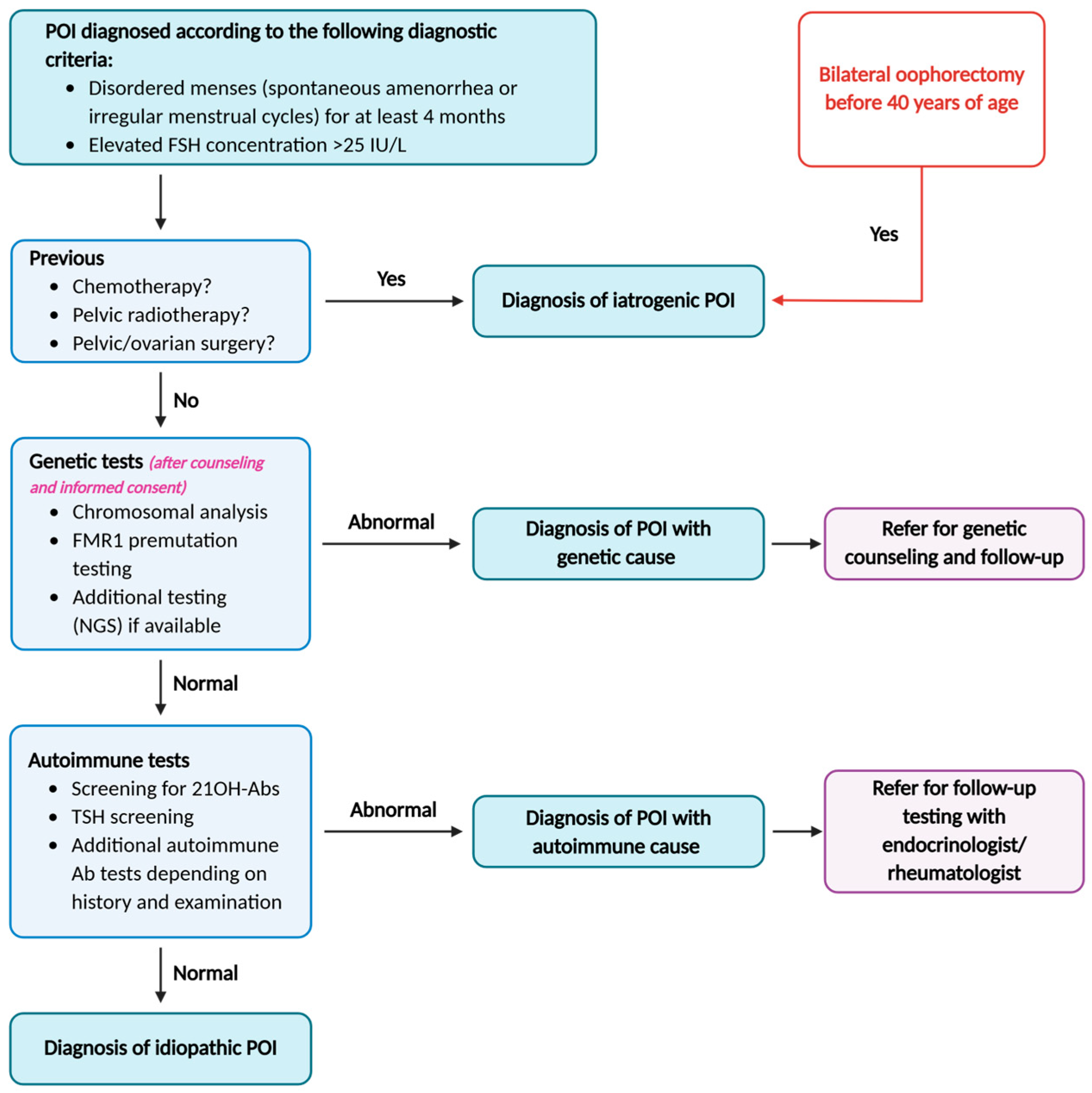
2.1. Diagnostic Criteria
2.2. Etiologies
2.2.1. Iatrogenic Causes of POI
2.2.2. Non-Iatrogenic Causes of POI
Genetic and Chromosomal Disorders
Immune Disorders
3. Diminished Ovarian Reserve
3.1. Diagnostic Criteria
3.2. Etiologies
3.2.1. Iatrogenic Causes of DOR
3.2.2. Non-Iatrogenic Causes of DOR
Genetic and Chromosomal Disorders
Immune Disorders
Environmental Causes of DOR

4. Therapeutic Options and Management
4.1. Fertility Preservation Strategies When POI or DOR Are Anticipated
4.2. POI Management: Hormone Replacement Therapy
4.3. DOR Management: Improving Ovarian Stimulation Protocols
4.3.1. Dehydroepiandrosterone (DHEA) and Testosterone
4.3.2. High-Dose Gonadotropins vs. Mild Stimulation
4.4. Potential Therapeutic Options to Boost the Ovarian Reserve
4.4.1. In Vitro Activation
4.4.2. Platelet-Rich Plasma Injections
4.4.3. Bone Marrow Stem Cell Transplantation
4.4.4. Hope from Experimental Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, W.H.B.; Kelsey, T.W. Human Ovarian Reserve from Conception to the Menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Anderson, R.A.; Wallace, W.H.B. Antimüllerian Hormone, the Assessment of the Ovarian Reserve, and the Reproductive Outcome of the Young Patient with Cancer. Fertil. Steril. 2013, 99, 1469–1475. [Google Scholar] [CrossRef]
- Lin, C.; Jing, M.; Zhu, W.; Tu, X.; Chen, Q.; Wang, X.; Zheng, Y.; Zhang, R. The Value of Anti-Müllerian Hormone in the Prediction of Spontaneous Pregnancy: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 695157. [Google Scholar] [CrossRef]
- Yeganeh, L.; Giri, R.; Flanagan, M.; Panay, N.; Anderson, R.A.; Bennie, A.; Cedars, M.; Davies, M.; Ee, C.; Gravholt, C.H.; et al. Evidence-Based Guideline: Premature Ovarian Insufficiency. Fertil. Steril. 2025, 123, 221–236. [Google Scholar] [CrossRef]
- Yeganeh, L.; Vermeulen, N.; Ee, C.; Teede, H.; Vincent, A.J. Lifestyle Management in Menopause: A Systematic Review of Women with Premature Ovarian Insufficiency. Clin. Endocrinol. 2025. early view. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.H.; Mertens, A.C.; Spencer, J.B.; Manatunga, A.K.; Howards, P.P. Menses Resumption after Cancer Treatment-Induced Amenorrhea Occurs Early or Not at All. Fertil. Steril. 2016, 105, 765–772.e4. [Google Scholar] [CrossRef] [PubMed]
- Kashi, O.; Meirow, D. Overactivation or Apoptosis: Which Mechanisms Affect Chemotherapy-Induced Ovarian Reserve Depletion? Int. J. Mol. Sci. 2023, 24, 16291. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical Fibrosis and Blood-Vessels Damage in Human Ovaries Exposed to Chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633. [Google Scholar] [CrossRef]
- Shai, D.; Aviel-Ronen, S.; Spector, I.; Raanani, H.; Shapira, M.; Gat, I.; Roness, H.; Meirow, D. Ovaries of Patients Recently Treated with Alkylating Agent Chemotherapy Indicate the Presence of Acute Follicle Activation, Elucidating Its Role among Other Proposed Mechanisms of Follicle Loss. Fertil. Steril. 2021, 115, 1239–1249. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Thomson, A.B.; Kelsey, T.W. The Radiosensitivity of the Human Oocyte. Hum. Reprod. 2003, 18, 117–121. [Google Scholar] [CrossRef]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H.B. Cancer Treatment and Gonadal Function: Experimental and Established Strategies for Fertility Preservation in Children and Young Adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Gillet, N.; Smets, M.; Bassil, S.; Casanas-Roux, F. Large Ovarian Endometriomas. Hum. Reprod. 1996, 11, 641–645. [Google Scholar] [CrossRef]
- Muzii, L.; Panici, P.B. Combined Technique of Excision and Ablation for the Surgical Treatment of Ovarian Endometriomas: The Way Forward? Reprod. Biomed. Online 2010, 20, 300–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muzii, L.; Marana, R.; Angioli, R.; Bianchi, A.; Cucinella, G.; Vignali, M.; Benedetti Panici, P.; Busacca, M. Histologic Analysis of Specimens from Laparoscopic Endometrioma Excision Performed by Different Surgeons: Does the Surgeon Matter? Fertil. Steril. 2011, 95, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Kjer, J.J. Ovarian Damage Due to Cyst Removal: A Comparison of Endometriomas and Dermoid Cysts. Acta Obs. Gynecol. Scand. 2016, 95, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.; van der Coelen, S.; Dolmans, M.-M. Gene Expression Analysis of Ovarian Follicles and Stromal Cells in Girls with Turner Syndrome. Mol. Hum. Reprod. 2024, 30, gaae043. [Google Scholar] [CrossRef]
- Peek, R.; Nadesapillai, S.; Thi Nguyen, T.Y.; Vassart, S.; Smeets, D.; van de Zande, G.; Camboni, A.; Braat, D.; van der Velden, J.; Donnez, J.; et al. Assessment of Folliculogenesis in Ovarian Tissue from Young Patients with Turner Syndrome Using a Murine Xenograft Model. Fertil. Steril. 2023, 120, 371–381. [Google Scholar] [CrossRef]
- van der Coelen, S.; van der Velden, J.; Nadesapillai, S.; Braat, D.; Peek, R.; Fleischer, K. Navigating Fertility Dilemmas across the Lifespan in Girls with Turner Syndrome—A Scoping Review. Hum. Reprod. Update 2024, 30, 383–409. [Google Scholar] [CrossRef]
- Le Poulennec, T.; Dubreuil, S.; Grynberg, M.; Chabbert-Buffet, N.; Sermondade, N.; Fourati, S.; Siffroi, J.-P.; Héron, D.; Bachelot, A. Ovarian Reserve in Patients with FMR1 Gene Premutation and the Role of Fertility Preservation. Ann. Endocrinol. 2024, 85, 269–275. [Google Scholar] [CrossRef]
- Rosario, R.; Stewart, H.L.; Choudhury, N.R.; Michlewski, G.; Charlet-Berguerand, N.; Anderson, R.A. Evidence for a Fragile X Messenger Ribonucleoprotein 1 (FMR1) mRNA Gain-of-Function Toxicity Mechanism Contributing to the Pathogenesis of Fragile X-Associated Premature Ovarian Insufficiency. FASEB J. 2022, 36, e22612. [Google Scholar] [CrossRef]
- Friedman-Gohas, M.; Orvieto, R.; Michaeli, A.; Aizer, A.; Kirshenbaum, M.; Cohen, Y. Dysregulation of Anti-Mullerian Hormone Expression Levels in Mural Granulosa Cells of FMR1 Premutation Carriers. Sci. Rep. 2021, 11, 14139. [Google Scholar] [CrossRef]
- Nie, L.; Wang, X.; Wang, S.; Hong, Z.; Wang, M. Genetic Insights into the Complexity of Premature Ovarian Insufficiency. Reprod. Biol. Endocrinol. 2024, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Camboni, A.; Dolmans, M.M. Immune System Regulation of Physiological and Pathological Aspects of the Ovarian Follicle Pool throughout the Female Reproductive Lifespan. Hum. Reprod. 2025, 40, 12–22. [Google Scholar] [CrossRef]
- Hviid, A.; Rubin, S.; Mühlemann, K. Mumps. Lancet 2008, 371, 932–944. [Google Scholar] [CrossRef]
- Shim, H.M.; Hwang, J.Y.; Lee, K.M.; Kim, Y.; Jeong, D.; Roh, J.; Choi, H.; Hwang, J.H.; Park, H. Coxsackievirus B3 Infection Reduces Female Mouse Fertility. Exp. Anim. 2015, 64, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, M.; Orvieto, R. Premature Ovarian Insufficiency (POI) and Autoimmunity-an Update Appraisal. J. Assist. Reprod. Genet. 2019, 36, 2207–2215. [Google Scholar] [CrossRef]
- Szeliga, A.; Calik-Ksepka, A.; Maciejewska-Jeske, M.; Grymowicz, M.; Smolarczyk, K.; Kostrzak, A.; Smolarczyk, R.; Rudnicka, E.; Meczekalski, B. Autoimmune Diseases in Patients with Premature Ovarian Insufficiency—Our Current State of Knowledge. Int. J. Mol. Sci. 2021, 22, 2594. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Hua, S.; Du, J.; Chen, X.; Liu, X.; Ma, X.; Liang, X.; Yang, Y. Investigation of the Causal Relationship Between Autoimmune Diseases and Premature Ovarian Insufficiency. Reprod. Sci. 2025, 32, 176–186. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B. Ovarian Reserve Testing: A User’s Guide. Am. J. Obs. Gynecol. 2017, 217, 129–140. [Google Scholar] [CrossRef]
- Greene, A.D.; Patounakis, G.; Segars, J.H. Genetic Associations with Diminished Ovarian Reserve: A Systematic Review of the Literature. J. Assist. Reprod. Genet. 2014, 31, 935–946. [Google Scholar] [CrossRef]
- Pastore, L.M.; Christianson, M.S.; Stelling, J.; Kearns, W.G.; Segars, J.H. Reproductive Ovarian Testing and the Alphabet Soup of Diagnoses: DOR, POI, POF, POR, and FOR. J. Assist. Reprod. Genet. 2018, 35, 17–23. [Google Scholar] [CrossRef]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and Interpreting Measures of Ovarian Reserve: A Committee Opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Park, S.U.; Walsh, L.; Berkowitz, K.M. Mechanisms of Ovarian Aging. Reproduction 2021, 162, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Pampanini, V.; Hassan, J.; Oliver, E.; Stukenborg, J.-B.; Damdimopoulou, P.; Jahnukainen, K. Fertility Preservation for Prepubertal Patients at Risk of Infertility: Present Status and Future Perspectives. Horm. Res. Paediatr. 2021, 93, 599–608. [Google Scholar] [CrossRef]
- Barasoain, M.; Barrenetxea, G.; Huerta, I.; Télez, M.; Carrillo, A.; Pérez, C.; Criado, B.; Arrieta, I. Study of FMR1 Gene Association with Ovarian Dysfunction in a Sample from the Basque Country. Gene 2013, 521, 145–149. [Google Scholar] [CrossRef]
- Eslami, H.; Eslami, A.; Favaedi, R.; Asadpour, U.; Zari Moradi, S.; Eftekhari-Yazdi, P.; Madani, T.; Shahhoseini, M.; Mohseni Meybodi, A. Epigenetic Aberration of FMR1 Gene in Infertile Women with Diminished Ovarian Reserve. Cell J. 2018, 20, 78–83. [Google Scholar] [CrossRef]
- Liu, M.-N.; Zhang, K.; Xu, T.-M. The Role of BMP15 and GDF9 in the Pathogenesis of Primary Ovarian Insufficiency. Hum. Fertil. 2021, 24, 325–332. [Google Scholar] [CrossRef]
- Jordan, P.; Verebi, C.; Hervé, B.; Perol, S.; Bernard, V.; Karila, D.; Jali, E.; Brac de la Perrière, A.; Grouthier, V.; Jonard-Catteau, S.; et al. Revisiting GDF9 Variants in Primary Ovarian Insufficiency: A Shift from Dominant to Recessive Pathogenicity? Gene 2024, 927, 148734. [Google Scholar] [CrossRef] [PubMed]
- Hervé, B.; Verebi, C.; Bonnier, M.; Jordan, P.; Stroe, D.; Voisin, C.; Hill, D.; Sarfati, C.; Lansiaux, M.; Terral, D.; et al. From Dominant Assumptions to Recessive Trait: Rethinking BMP15 in Ovarian Dysfunction. Reprod. Sci. 2025, 32, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Titus, S.; Moy, F.; Ginsburg, E.S.; Oktay, K. Ovarian Aging in Women with BRCA Germline Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 3839–3847. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Di Micco, R.; Zuber, V.; Taghavi, K.; Bianchini, G.; Bellaminutti, S.; Meani, F.; Graffeo, R.; Candiani, M.; Mueller, M.D.; et al. Ovarian Reserve of Women with and without BRCA Pathogenic Variants: A Systematic Review and Meta-Analysis. Breast 2021, 60, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Lambertini, M.; Lee, D.-Y.; Wang, E.; Clatot, F.; Karlan, B.Y.; Demeestere, I.; Bang, H.; Oktay, K. Association of Germline BRCA Pathogenic Variants with Diminished Ovarian Reserve: A Meta-Analysis of Individual Patient-Level Data. J. Clin. Oncol. 2021, 39, 2016–2024. [Google Scholar] [CrossRef]
- Zhu, Q.; Ma, H.; Wang, J.; Liang, X. Understanding the Mechanisms of Diminished Ovarian Reserve: Insights from Genetic Variants and Regulatory Factors. Reprod. Sci. 2024, 31, 1521–1532. [Google Scholar] [CrossRef]
- Wang, E.T.; Pisarska, M.D.; Bresee, C.; Chen, Y.-D.I.; Lester, J.; Afshar, Y.; Alexander, C.; Karlan, B.Y. BRCA1 Germline Mutations May Be Associated with Reduced Ovarian Reserve. Fertil. Steril. 2014, 102, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Kim, J.Y.; Barad, D.; Babayev, S.N. Association of BRCA1 Mutations with Occult Primary Ovarian Insufficiency: A Possible Explanation for the Link between Infertility and Breast/Ovarian Cancer Risks. J. Clin. Oncol. 2010, 28, 240–244. [Google Scholar] [CrossRef]
- Oktay, K.; Turan, V.; Titus, S.; Stobezki, R.; Liu, L. BRCA Mutations, DNA Repair Deficiency, and Ovarian Aging. Biol. Reprod. 2015, 93, 67. [Google Scholar] [CrossRef]
- Lawrenz, B.; Henes, J.; Henes, M.; Neunhoeffer, E.; Schmalzing, M.; Fehm, T.; Kïtter, I. Impact of Systemic Lupus Erythematosus on Ovarian Reserve in Premenopausal Women: Evaluation by Using Anti-Muellerian Hormone. Lupus 2011, 20, 1193–1197. [Google Scholar] [CrossRef]
- Gao, H.; Ma, J.; Wang, X.; Lv, T.; Liu, J.; Ren, Y.; Li, Y.; Zhang, Y. Preliminary Study on the Changes of Ovarian Reserve, Menstruation, and Lymphocyte Subpopulation in Systemic Lupus Erythematosus (SLE) Patients of Childbearing Age. Lupus 2018, 27, 445–453. [Google Scholar] [CrossRef]
- Angley, M.; Spencer, J.B.; Lim, S.S.; Howards, P.P. Anti-Müllerian Hormone in African-American Women with Systemic Lupus Erythematosus. Lupus Sci. Med. 2020, 7, e000439. [Google Scholar] [CrossRef]
- Silva, C.A.; Yamakami, L.Y.S.; Aikawa, N.E.; Araujo, D.B.; Carvalho, J.F.; Bonfá, E. Autoimmune Primary Ovarian Insufficiency. Autoimmun. Rev. 2014, 13, 427–430. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, Mechanisms and Clinical Perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Li, Y. Modern Epigenetics Methods in Biological Research. Methods 2021, 187, 104–113. [Google Scholar] [CrossRef]
- Nilsson, E.; Larsen, G.; Manikkam, M.; Guerrero-Bosagna, C.; Savenkova, M.I.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE 2012, 7, e36129. [Google Scholar] [CrossRef]
- Zama, A.M.; Uzumcu, M. Epigenetic Effects of Endocrine-Disrupting Chemicals on Female Reproduction: An Ovarian Perspective. Front. Neuroendocr. 2010, 31, 420–439. [Google Scholar] [CrossRef]
- Richardson, M.C.; Guo, M.; Fauser, B.C.J.M.; Macklon, N.S. Environmental and Developmental Origins of Ovarian Reserve. Hum. Reprod. Update 2014, 20, 353–369. [Google Scholar] [CrossRef]
- Ding, T.; Yan, W.; Zhou, T.; Shen, W.; Wang, T.; Li, M.; Zhou, S.; Wu, M.; Dai, J.; Huang, K.; et al. Endocrine Disrupting Chemicals Impact on Ovarian Aging: Evidence from Epidemiological and Experimental Evidence. Environ. Pollut. 2022, 305, 119269. [Google Scholar] [CrossRef]
- Tian, T.; Hao, Y.; Wang, Y.; Xu, X.; Long, X.; Yan, L.; Zhao, Y.; Qiao, J. Mixed and Single Effects of Endocrine Disrupting Chemicals in Follicular Fluid on Likelihood of Diminished Ovarian Reserve: A Case-Control Study. Chemosphere 2023, 330, 138727. [Google Scholar] [CrossRef]
- Messerlian, C.; Souter, I.; Gaskins, A.J.; Williams, P.L.; Ford, J.B.; Chiu, Y.-H.; Calafat, A.M.; Hauser, R. Earth Study Team Urinary Phthalate Metabolites and Ovarian Reserve among Women Seeking Infertility Care. Hum. Reprod. 2016, 31, 75–83. [Google Scholar] [CrossRef]
- Hammarstrand, S.; Jakobsson, K.; Andersson, E.; Xu, Y.; Li, Y.; Olovsson, M.; Andersson, E.M. Perfluoroalkyl Substances (PFAS) in Drinking Water and Risk for Polycystic Ovarian Syndrome, Uterine Leiomyoma, and Endometriosis: A Swedish Cohort Study. Environ. Int. 2021, 157, 106819. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) and Their Effects on the Ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef]
- Sharara, F.I.; Beatse, S.N.; Leonardi, M.R.; Navot, D.; Scott, R.T. Cigarette Smoking Accelerates the Development of Diminished Ovarian Reserve as Evidenced by the Clomiphene Citrate Challenge Test. Fertil. Steril. 1994, 62, 257–262. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Jadoul, P.; Gilliaux, S.; Amorim, C.A.; Luyckx, V.; Squifflet, J.; Donnez, J.; Van Langendonckt, A. A Review of 15 Years of Ovarian Tissue Bank Activities. J. Assist. Reprod. Genet. 2013, 30, 305–314. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Smith, A.G.; Kelsey, T.W.; Edgar, A.E.; Anderson, R.A. Fertility Preservation for Girls and Young Women with Cancer: Population-Based Validation of Criteria for Ovarian Tissue Cryopreservation. Lancet Oncol. 2014, 15, 1129–1136. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Donnez, J. Fertility Preservation in Women for Medical and Social Reasons: Oocytes vs Ovarian Tissue. Best Pract. Res. Clin. Obs. Gynaecol. 2021, 70, 63–80. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Halachmi, S.; Peretz, B.A.; Shmuel, Z.; Golan, D.; Makler, A.; Brandes, J.M. Premature Ovarian Failure--the Prognostic Application of Autoimmunity on Conception after Ovulation Induction. Fertil. Steril. 1993, 59, 750–755. [Google Scholar] [CrossRef]
- Corenblum, B.; Rowe, T.; Taylor, P.J. High-Dose, Short-Term Glucocorticoids for the Treatment of Infertility Resulting from Premature Ovarian Failure. Fertil. Steril. 1993, 59, 988–991. [Google Scholar] [CrossRef]
- Panay, N.; Anderson, R.A.; Nappi, R.E.; Vincent, A.J.; Vujovic, S.; Webber, L.; Wolfman, W. Premature Ovarian Insufficiency: An International Menopause Society White Paper. Climacteric 2020, 23, 426–446. [Google Scholar] [CrossRef]
- Yelland, S.; Steenson, S.; Creedon, A.; Stanner, S. The Role of Diet in Managing Menopausal Symptoms: A Narrative Review. Nutr. Bull. 2023, 48, 43–65. [Google Scholar] [CrossRef]
- Craciunas, L.; Zdoukopoulos, N.; Vinayagam, S.; Mohiyiddeen, L. Hormone Therapy for Uterine and Endometrial Development in Women with Premature Ovarian Insufficiency. Cochrane Database Syst. Rev. 2022, 10, CD008209. [Google Scholar] [CrossRef]
- Rozenberg, S.; Di Pietrantonio, V.; Vandromme, J.; Gilles, C. Menopausal Hormone Therapy and Breast Cancer Risk. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101577. [Google Scholar] [CrossRef]
- Cedars, M.I. Managing Poor Ovarian Response in the Patient with Diminished Ovarian Reserve. Fertil. Steril. 2022, 117, 655–656. [Google Scholar] [CrossRef]
- Conforti, A.; Carbone, L.; Di Girolamo, R.; Iorio, G.G.; Guida, M.; Campitiello, M.R.; Ubaldi, F.M.; Rienzi, L.; Vaiarelli, A.; Cimadomo, D.; et al. Therapeutic Management in Women with a Diminished Ovarian Reserve: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Fertil. Steril. 2025, 123, 457–476. [Google Scholar] [CrossRef]
- Sen, A.; Prizant, H.; Light, A.; Biswas, A.; Hayes, E.; Lee, H.-J.; Barad, D.; Gleicher, N.; Hammes, S.R. Androgens Regulate Ovarian Follicular Development by Increasing Follicle Stimulating Hormone Receptor and microRNA-125b Expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3008–3013. [Google Scholar] [CrossRef]
- Laird, M.; Thomson, K.; Fenwick, M.; Mora, J.; Franks, S.; Hardy, K. Androgen Stimulates Growth of Mouse Preantral Follicles In Vitro: Interaction with Follicle-Stimulating Hormone and With Growth Factors of the TGFβ Superfamily. Endocrinology 2017, 158, 920–935. [Google Scholar] [CrossRef]
- Fujibe, Y.; Baba, T.; Nagao, S.; Adachi, S.; Ikeda, K.; Morishita, M.; Kuno, Y.; Suzuki, M.; Mizuuchi, M.; Honnma, H.; et al. Androgen Potentiates the Expression of FSH Receptor and Supports Preantral Follicle Development in Mice. J. Ovarian Res. 2019, 12, 31. [Google Scholar] [CrossRef]
- Devillers, M.M.; François, C.M.; Chester, M.; Corre, R.; Cluzet, V.; Giton, F.; Cohen-Tannoudji, J.; Guigon, C.J. Androgen Receptor Signaling Regulates Follicular Growth and Steroidogenesis in Interaction with Gonadotropins in the Ovary during Mini-Puberty in Mice. Front. Endocrinol. 2023, 14, 1130681. [Google Scholar] [CrossRef]
- Kara, M.; Aydin, T.; Aran, T.; Turktekin, N.; Ozdemir, B. Does Dehydroepiandrosterone Supplementation Really Affect IVF-ICSI Outcome in Women with Poor Ovarian Reserve? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 173, 63–65. [Google Scholar] [CrossRef]
- Yeung, T.W.Y.; Chai, J.; Li, R.H.W.; Lee, V.C.Y.; Ho, P.C.; Ng, E.H.Y. A Randomized, Controlled, Pilot Trial on the Effect of Dehydroepiandrosterone on Ovarian Response Markers, Ovarian Response, and In Vitro Fertilization Outcomes in Poor Responders. Fertil. Steril. 2014, 102, 108–115.e1. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, H.-F.; Li, L.; Xin, A.-J.; Sun, Y.-J.; Sun, X.-X. Effects of Dehydroepiandrosterone on Embryo Quality and Follicular Fluid Markers in Patients with Diminished Ovarian Reserves. Reprod. Dev. Med. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Narkwichean, A.; Maalouf, W.; Baumgarten, M.; Polanski, L.; Raine-Fenning, N.; Campbell, B.; Jayaprakasan, K. Efficacy of Dehydroepiandrosterone (DHEA) to Overcome the Effect of Ovarian Ageing (DITTO): A Proof of Principle Double Blinded Randomized Placebo Controlled Trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 39–48. [Google Scholar] [CrossRef]
- Kim, C.-H.; Howles, C.M.; Lee, H.-A. The Effect of Transdermal Testosterone Gel Pretreatment on Controlled Ovarian Stimulation and IVF Outcome in Low Responders. Fertil. Steril. 2011, 95, 679–683. [Google Scholar] [CrossRef]
- Saharkhiz, N.; Zademodares, S.; Salehpour, S.; Hosseini, S.; Nazari, L.; Tehrani, H.G. The Effect of Testosterone Gel on Fertility Outcomes in Women with a Poor Response in in Vitro Fertilization Cycles: A Pilot Randomized Clinical Trial. J. Res. Med. Sci. 2018, 23, 3. [Google Scholar] [CrossRef]
- Hoang, Q.H.; Ho, H.S.; Do, H.T.; Nguyen, T.V.; Nguyen, H.P.; Le, M.T. Therapeutic Effect of Prolonged Testosterone Pretreatment in Women with Poor Ovarian Response: A Randomized Control Trial. Reprod. Med. Biol. 2021, 20, 305–312. [Google Scholar] [CrossRef]
- Klinkert, E.R.; Broekmans, F.J.M.; Looman, C.W.N.; Habbema, J.D.F.; te Velde, E.R. Expected Poor Responders on the Basis of an Antral Follicle Count Do Not Benefit from a Higher Starting Dose of Gonadotrophins in IVF Treatment: A Randomized Controlled Trial*. Hum. Reprod. 2005, 20, 611–615. [Google Scholar] [CrossRef]
- van Tilborg, T.C.; Oudshoorn, S.C.; Eijkemans, M.J.C.; Mochtar, M.H.; van Golde, R.J.T.; Hoek, A.; Kuchenbecker, W.K.H.; Fleischer, K.; de Bruin, J.P.; Groen, H.; et al. Individualized FSH Dosing Based on Ovarian Reserve Testing in Women Starting IVF/ICSI: A Multicentre Trial and Cost-Effectiveness Analysis. Hum. Reprod. 2017, 32, 2485–2495. [Google Scholar] [CrossRef]
- Youssef, M.A.; van Wely, M.; Al-Inany, H.; Madani, T.; Jahangiri, N.; Khodabakhshi, S.; Alhalabi, M.; Akhondi, M.; Ansaripour, S.; Tokhmechy, R.; et al. A Mild Ovarian Stimulation Strategy in Women with Poor Ovarian Reserve Undergoing IVF: A Multicenter Randomized Non-Inferiority Trial. Hum. Reprod. 2017, 32, 112–118. [Google Scholar] [CrossRef]
- Humaidan, P.; Chin, W.; Rogoff, D.; D’Hooghe, T.; Longobardi, S.; Hubbard, J.; Schertz, J. Efficacy and Safety of Follitropin Alfa/Lutropin Alfa in ART: A Randomized Controlled Trial in Poor Ovarian Responders. Hum. Reprod. 2017, 32, 544–555. [Google Scholar] [CrossRef]
- Tosun, S.A.; Ozkaya, E.; Aru, B.; Yanikkaya Demirel, G.; Cogendez, E.; Sipahi, M. Does LH Supplementation in Poor Responders Affect Granulosa Cells Apoptosis Rate in ART? A Prospective Randomised Controlled Trial. J. Obstet. Gynaecol. 2022, 42, 133–138. [Google Scholar] [CrossRef]
- Massin, N.; Abdennebi, I.; Porcu-Buisson, G.; Chevalier, N.; Descat, E.; Piétin-Vialle, C.; Goro, S.; Brussieux, M.; Pinto, M.; Pasquier, M.; et al. The BISTIM Study: A Randomized Controlled Trial Comparing Dual Ovarian Stimulation (Duostim) with Two Conventional Ovarian Stimulations in Poor Ovarian Responders Undergoing IVF. Hum. Reprod. 2023, 38, 927–937. [Google Scholar] [CrossRef]
- Choe, S.-A.; Kim, M.J.; Lee, H.J.; Kim, J.; Chang, E.M.; Kim, J.W.; Park, H.M.; Lyu, S.W.; Lee, W.S.; Yoon, T.K.; et al. Increased Proportion of Mature Oocytes with Sustained-Release Growth Hormone Treatment in Poor Responders: A Prospective Randomized Controlled Study. Arch. Gynecol. Obs. 2018, 297, 791–796. [Google Scholar] [CrossRef]
- Lee, Y.-X.; Shen, M.-S.; Tzeng, C.-R. Low Dose Growth Hormone Adjuvant Treatment with Ultra-Long Ovarian Stimulation Protocol in Poor Responders Showed Non-Inferior Pregnancy Outcome Compared with Normal Responders. Front. Endocrinol. 2019, 10, 892. [Google Scholar] [CrossRef]
- Lee, H.N.; Chang, E.M. Primordial Follicle Activation as New Treatment for Primary Ovarian Insufficiency. Clin. Exp. Reprod. Med. 2019, 46, 43–49. [Google Scholar] [CrossRef]
- Fraison, E.; Crawford, G.; Casper, G.; Harris, V.; Ledger, W. Pregnancy Following Diagnosis of Premature Ovarian Insufficiency: A Systematic Review. Reprod. Biomed. Online 2019, 39, 467–476. [Google Scholar] [CrossRef]
- Devenutto, L.; Quintana, R.; Quintana, T. In vitro Activation of Ovarian Cortex and Autologous Transplantation: A Novel Approach to Primary Ovarian Insufficiency and Diminished Ovarian Reserve. Hum. Reprod. Open 2020, 2020, hoaa046. [Google Scholar] [CrossRef]
- Vo, K.C.T.; Kawamura, K. In Vitro Activation Early Follicles: From the Basic Science to the Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 3785. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawamura, N.; Hsueh, A.J.W. Activation of Dormant Follicles: A New Treatment for Premature Ovarian Failure? Curr. Opin. Obs. Gynecol. 2016, 28, 217–222. [Google Scholar] [CrossRef]
- Kawamura, K.; Ishizuka, B.; Hsueh, A.J.W. Drug-Free in-Vitro Activation of Follicles for Infertility Treatment in Poor Ovarian Response Patients with Decreased Ovarian Reserve. Reprod. Biomed. Online 2020, 40, 245–253. [Google Scholar] [CrossRef]
- Lunding, S.A.; Pors, S.E.; Kristensen, S.G.; Landersoe, S.K.; Jeppesen, J.V.; Flachs, E.M.; Pinborg, A.; Macklon, K.T.; Pedersen, A.T.; Andersen, C.Y.; et al. Biopsying, Fragmentation and Autotransplantation of Fresh Ovarian Cortical Tissue in Infertile Women with Diminished Ovarian Reserve. Hum. Reprod. 2019, 34, 1924–1936. [Google Scholar] [CrossRef]
- Ferreri, J.; Fàbregues, F.; Calafell, J.M.; Solernou, R.; Borrás, A.; Saco, A.; Manau, D.; Carmona, F. Drug-Free in-Vitro Activation of Follicles and Fresh Tissue Autotransplantation as a Therapeutic Option in Patients with Primary Ovarian Insufficiency. Reprod. Biomed. Online 2020, 40, 254–260. [Google Scholar] [CrossRef]
- Méndez, M.; Fabregues, F.; Ferreri, J.; Calafell, J.M.; Villarino, A.; Otero, J.; Farre, R.; Carmona, F. Biomechanical Characteristics of the Ovarian Cortex in POI Patients and Functional Outcomes after Drug-Free IVA. J. Assist. Reprod. Genet. 2022, 39, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Grin, L.; Berkovitz-Shperling, R.; Goldstein, G.; Michailov, Y.; Gemer, O.; Anteby, E.; Kawamura, K.; Saar-Ryss, B.; Friedler, S. Drug-Free in Vitro Activation of Ovarian Follicles and Fresh Tissue Autotransplantation in Patients with Poor Ovarian Response and Premature Ovarian Insufficiency. F&S Sci. 2025, 6, 303–311. [Google Scholar] [CrossRef]
- Steiner, A.Z. Evidence That Biopsying, Fragmentation and Auto-Transplantation of Ovarian Tissue Should Be Abandoned as a Treatment of Diminished Ovarian Reserve. Hum. Reprod. 2019, 34, 1853–1854. [Google Scholar] [CrossRef]
- Griesinger, G.; Fauser, B.C.J.M. Drug-Free in-Vitro Activation of Ovarian Cortex; Can It Really Activate the ‘Ovarian Gold Reserve’? Reprod. Biomed. Online 2020, 40, 187–189. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Korun, Z.E.U.; Herlihy, N.; Scott, R.T.; Tiras, B.; Seli, E. Ovarian Reserve Parameters and IVF Outcomes in 510 Women with Poor Ovarian Response (POR) Treated with Intraovarian Injection of Autologous Platelet Rich Plasma (PRP). Aging 2022, 14, 2513–2523. [Google Scholar] [CrossRef]
- Melo, P.; Navarro, C.; Jones, C.; Coward, K.; Coleman, L. The Use of Autologous Platelet-Rich Plasma (PRP) versus No Intervention in Women with Low Ovarian Reserve Undergoing Fertility Treatment: A Non-Randomized Interventional Study. J. Assist. Reprod. Genet. 2020, 37, 855–863. [Google Scholar] [CrossRef]
- Herlihy, N.S.; Cakiroglu, Y.; Whitehead, C.; Reig, A.; Tiras, B.; Scott, R.T.; Seli, E. Effect of Intraovarian Platelet-Rich Plasma Injection on IVF Outcomes in Women with Poor Ovarian Response: The PROVA Randomized Controlled Trial. Hum. Reprod. 2024, 39, 1495–1503. [Google Scholar] [CrossRef]
- Molinaro, P.; Ballester, A.; Garcia-Velasco, J.A.; Muñoz, M.; Herraiz, S. Impact of Bilateral Intraovarian Platelet-Rich Plasma in Women with Poor Ovarian Response or Primary Ovarian Insufficiency: A Retrospective Study. Fertil. Steril. 2025, 124, 496–505. [Google Scholar] [CrossRef]
- Éliás, M.; Kónya, M.; Kekk, Z.; Turan, C.; das Virgens, I.P.A.; Tóth, R.; Keszthelyi, M.; Hegyi, P.; Várbíró, S.; Sipos, M. Platelet-Rich Plasma (PRP) Treatment of the Ovaries Significantly Improves Fertility Parameters and Reproductive Outcomes in Diminished Ovarian Reserve Patients: A Systematic Review and Meta-Analysis. J. Ovarian Res. 2024, 17, 104. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Maleki, F.; Hajizadeh-Sharafabad, F.; Ghasemnejad-Berenji, H. Evaluation of Intraovarian Injection of Platelet-Rich Plasma for Enhanced Ovarian Function and Reproductive Success in Women with POI and POR: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2025, 30, 610. [Google Scholar] [CrossRef]
- Herraiz, S.; Buigues, A.; Díaz-García, C.; Romeu, M.; Martínez, S.; Gómez-Seguí, I.; Simón, C.; Hsueh, A.J.; Pellicer, A. Fertility Rescue and Ovarian Follicle Growth Promotion by Bone Marrow Stem Cell Infusion. Fertil. Steril. 2018, 109, 908–918.e2. [Google Scholar] [CrossRef]
- Pellicer, N.; Cozzolino, M.; Diaz-García, C.; Galliano, D.; Cobo, A.; Pellicer, A.; Herraiz, S. Ovarian Rescue in Women with Premature Ovarian Insufficiency: Facts and Fiction. Reprod. Biomed. Online 2023, 46, 543–565. [Google Scholar] [CrossRef]
- Park, H.-S.; Chugh, R.M.; Seok, J.; Cetin, E.; Mohammed, H.; Siblini, H.; Liakath Ali, F.; Ghasroldasht, M.M.; Alkelani, H.; Elsharoud, A.; et al. Comparison of the Therapeutic Effects between Stem Cells and Exosomes in Primary Ovarian Insufficiency: As Promising as Cells but Different Persistency and Dosage. Stem Cell Res. Ther. 2023, 14, 165. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Li, X.; Wang, Q.; Xie, J.; Fu, X. Heat Shock Pretreatment of Mesenchymal Stem Cells for Inhibiting the Apoptosis of Ovarian Granulosa Cells Enhanced the Repair Effect on Chemotherapy-Induced Premature Ovarian Failure. Stem Cell Res. Ther. 2018, 9, 240. [Google Scholar] [CrossRef]
- Huang, B.; Ding, C.; Zou, Q.; Lu, J.; Wang, W.; Li, H. Human Amniotic Fluid Mesenchymal Stem Cells Improve Ovarian Function During Physiological Aging by Resisting DNA Damage. Front. Pharmacol. 2020, 11, 272. [Google Scholar] [CrossRef]
- Luo, Q.; Tang, Y.; Jiang, Z.; Bao, H.; Fu, Q.; Zhang, H. hUCMSCs Reduce Theca Interstitial Cells Apoptosis and Restore Ovarian Function in Premature Ovarian Insufficiency Rats through Regulating NR4A1-Mediated Mitochondrial Mechanisms. Reprod. Biol. Endocrinol. 2022, 20, 125. [Google Scholar] [CrossRef]
- Polonio, A.M.; García-Velasco, J.A.; Herraiz, S. Stem Cell Paracrine Signaling for Treatment of Premature Ovarian Insufficiency. Front. Endocrinol. 2021, 11, 626322. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jia, Z.; Zhang, F.; Han, C.; Zhao, L.; Jia, Y.; Cui, M. HGF-Modified Human Umbilical Cord Mesenchymal Stem Cells Rescue Impaired Ovarian Reserve Function in Chemotherapy-Induced POI Rats by Improving Angiogenesis While Decreasing Apoptosis and Fibrosis in the Ovary. Tissue Cell 2023, 82, 102121. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes Derived from Human Adipose Mesenchymal Stem Cells Improve Ovary Function of Premature Ovarian Insufficiency by Targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miR-144-5p Improves Rat Ovarian Function after Chemotherapy-Induced Ovarian Failure by Targeting PTEN. Lab. Investig. 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Zheng, J.; Tian, Y.; Zhang, H.; Tan, Y.; Li, Q.; Zhang, J.; Huang, X. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Improve Ovarian Function and Proliferation of Premature Ovarian Insufficiency by Regulating the Hippo Signaling Pathway. Front. Endocrinol. 2021, 12, 711902. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhang, L.; Zhang, P.; Xu, Y.; Wang, J.; Zhao, X.; Dai, Z.; Zhou, H.; Zhao, S.; Fan, A. Human UC-MSC-Derived Exosomes Facilitate Ovarian Renovation in Rats with Chemotherapy-Induced Premature Ovarian Insufficiency. Front. Endocrinol. 2023, 14, 1205901. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houeis, L.; Donnez, J.; Dolmans, M.-M. Premature Ovarian Insufficiency and Diminished Ovarian Reserve: From Diagnosis to Current Management and Treatment. J. Clin. Med. 2025, 14, 7473. https://doi.org/10.3390/jcm14217473
Houeis L, Donnez J, Dolmans M-M. Premature Ovarian Insufficiency and Diminished Ovarian Reserve: From Diagnosis to Current Management and Treatment. Journal of Clinical Medicine. 2025; 14(21):7473. https://doi.org/10.3390/jcm14217473
Chicago/Turabian StyleHoueis, Lara, Jacques Donnez, and Marie-Madeleine Dolmans. 2025. "Premature Ovarian Insufficiency and Diminished Ovarian Reserve: From Diagnosis to Current Management and Treatment" Journal of Clinical Medicine 14, no. 21: 7473. https://doi.org/10.3390/jcm14217473
APA StyleHoueis, L., Donnez, J., & Dolmans, M.-M. (2025). Premature Ovarian Insufficiency and Diminished Ovarian Reserve: From Diagnosis to Current Management and Treatment. Journal of Clinical Medicine, 14(21), 7473. https://doi.org/10.3390/jcm14217473







