Mitral Valve Prolapse in Athletes: Prevalence, Arrhythmic Associations, and Clinical Implications—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
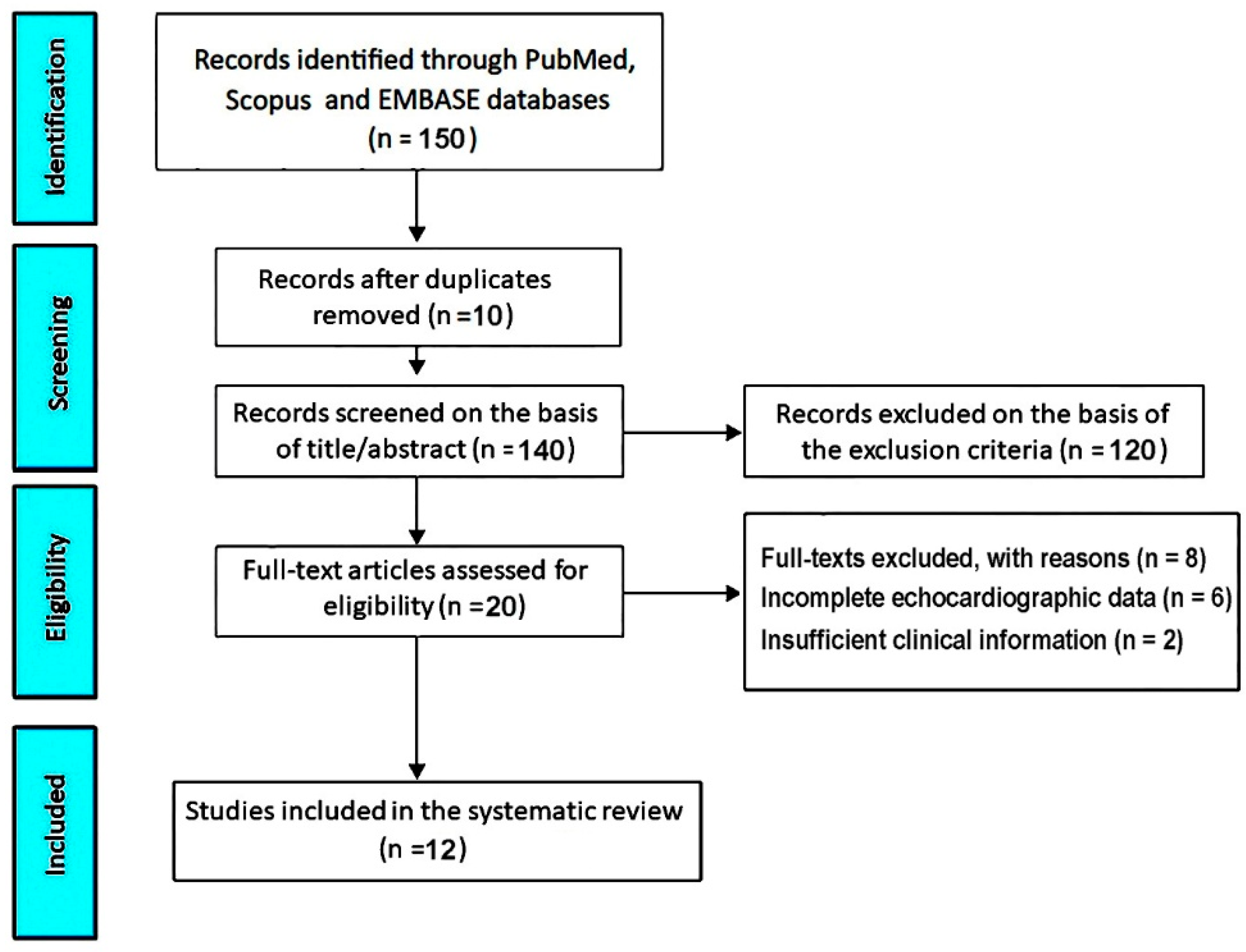
3.1. Study Selection
3.2. Clinical Data
3.3. ECG and Arrhythmia Data
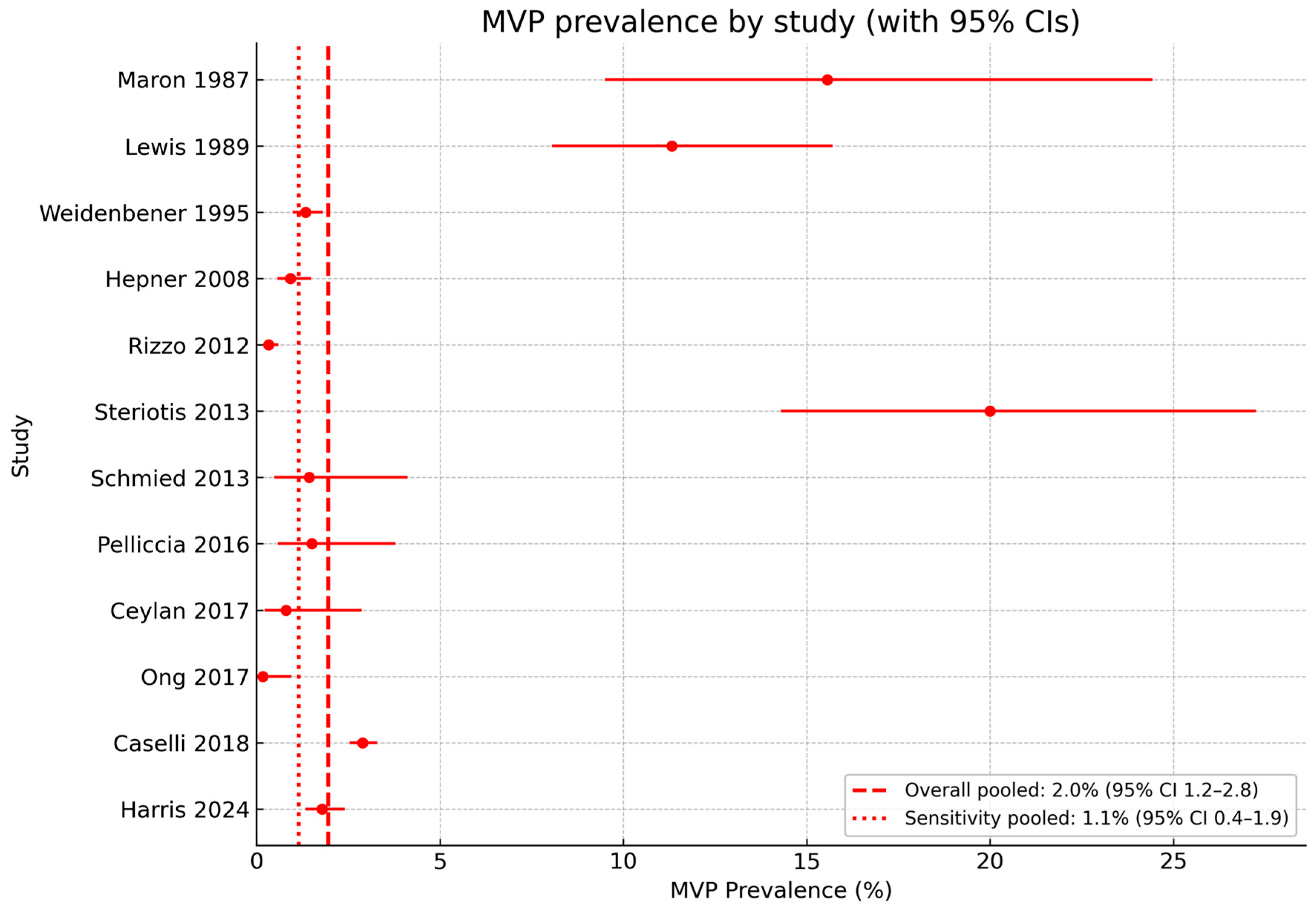
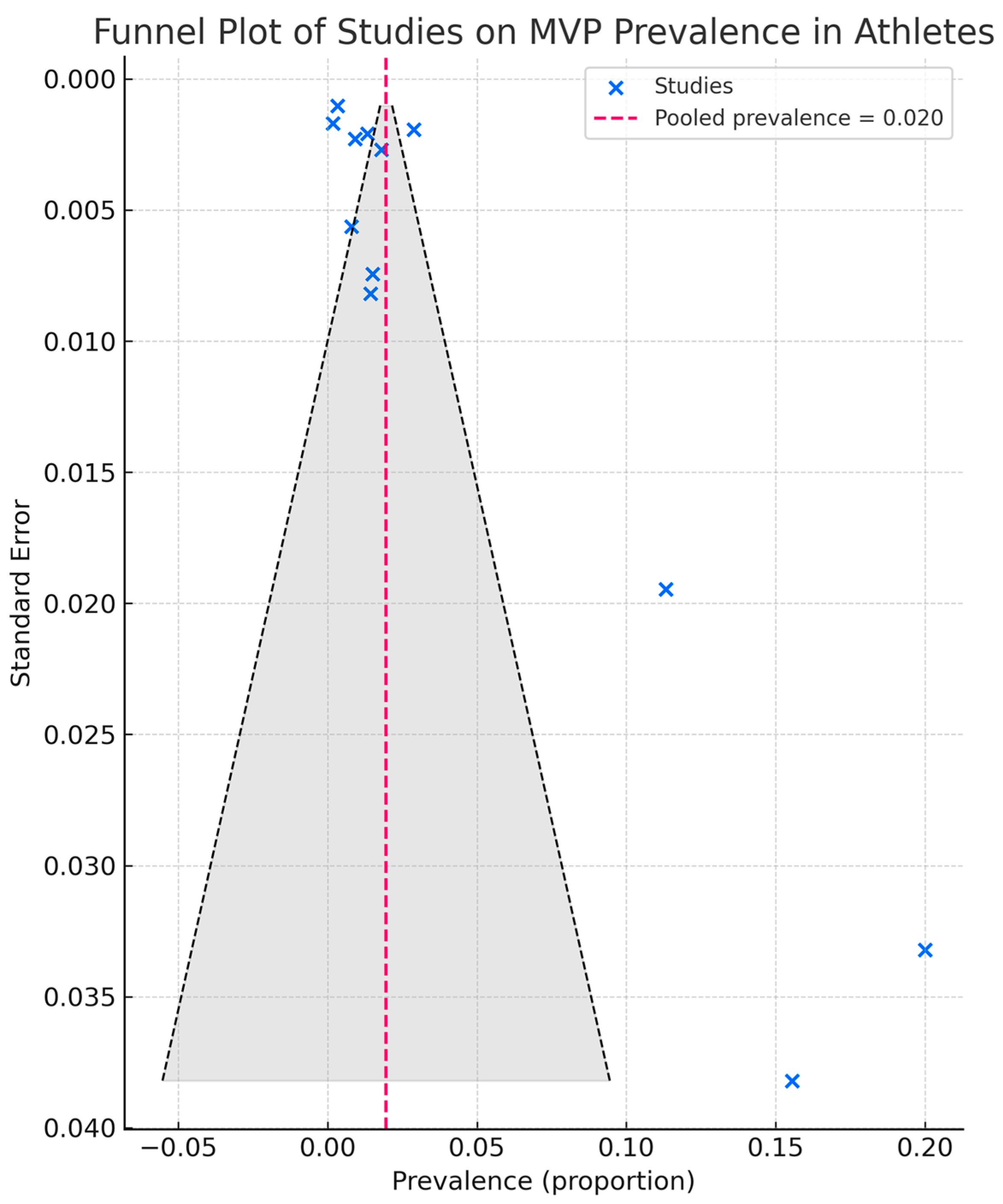
3.4. Echocardiographic Data
3.5. Follow-Up Data
3.6. Risk of Bias Assessment
4. Discussion
4.1. Interpretation of the Main Findings
4.2. Evidence Gaps and Quality Considerations
4.3. Comparison Between MVP Prevalence in Athletes and in the General Population
4.4. Clinical Implications for Screening, Eligibility, and Surveillance in Athletes with MVP
4.5. Chest Shape Assessment in Athletes
4.6. Limitations of the Included Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population: The benign nature of echocardiographic features in the Framingham Heart Study. J. Am. Coll. Cardiol. 2002, 40, 1298–1304. [Google Scholar] [CrossRef]
- Parwani, P.; Avierinos, J.F.; Levine, R.A.; Delling, F.N. Mitral Valve Prolapse: Multimodality Imaging and Genetic Insights. Prog. Cardiovasc. Dis. 2017, 60, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller, F.A., Jr.; Ilstrup, D.M.; Tajik, A.J. Echocardiographically documented mitral-valve prolapse. Long–term follow–up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Hawkins, I.; Kramer-Fox, R.; Lutas, E.M.; Hammond, I.W.; Spitzer, M.C.; Hochreiter, C.; Roberts, R.B.; Belkin, R.N.; Kligfield, P.; et al. Complications of mitral valve prolapse. Disproportionate occurrence in men and older patients. Am. J. Med. 1986, 81, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Düren, D.R.; Becker, A.E.; Dunning, A.J. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: A prospective study. J. Am. Coll. Cardiol. 1988, 11, 42–47. [Google Scholar] [CrossRef]
- Wilcken, D.E.; Hickey, A.J. Lifetime risk for patients with mitral valve prolapse of developing severe valve regurgitation requiring surgery. Circulation 1988, 78, 10–14. [Google Scholar] [CrossRef]
- Marks, A.R.; Choong, C.Y.; Sanfilippo, A.J.; Ferré, M.; Weyman, A.E. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N. Engl. J. Med. 1989, 320, 1031–1036. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.T.; Maalouf, J.; Asirvatham, S.J.; Michelena, H.I.; Enriquez-Sarano, M. Presentation and outcome of arrhythmic mitral valve prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Maron, B.J.; Friedman, R.A.; Kligfield, P.; Levine, B.D.; Viskin, S.; Chaitman, B.R.; Okin, P.M.; Saul, J.P.; Salberg, L.; Van Hare, G.F.; et al. Assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12–25 years of age): A scientific statement from the American Heart Association and the American College of Cardiology. J. Am. Coll. Cardiol. 2014, 64, 1479–1514. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2019 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Vriz, O.; Landi, I.; Eltayeb, A.; Limongelli, G.; Mos, L.; Delise, P.; Bossone, E.; D’ Andrea, A. Mitral Valve Prolapse and Sudden Cardiac Death in Athletes at High Risk. Curr. Cardiol. Rev. 2023, 19, e201222212066. [Google Scholar] [CrossRef]
- Markiewicz, W.; Stoner, J.; London, E.; Hunt, S.A.; Popp, R.L. Mitral valve prolapse in one hundred presumably healthy young females. Circulation 1976, 53, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Procacci, P.M.; Savran, S.V.; Schreiter, S.L.; Bryson, A.L. Prevalence of clinical mitral-valve prolapse in 1169 young women. N. Engl. J. Med. 1976, 294, 1086–1088. [Google Scholar] [CrossRef]
- McLarin, C.; Arensberg, D.; Felner, J.M.; Schlant, R.C. Echocardiographically determined mitral valve prolapse in male patients. South. Med. J. 1979, 72, 1416–1417. [Google Scholar] [CrossRef]
- Wann, L.S.; Grove, J.R.; Hess, T.R.; Glisch, L.; Ptacin, M.J.; Hughes, C.V.; Gross, C.M. Prevalence of mitral prolapse by two dimensional echocardiography in healthy young women. Br. Heart J. 1983, 49, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.D.; Garrison, R.J.; Devereux, R.B.; Castelli, W.P.; Anderson, S.J.; Levy, D.; McNamara, P.M.; Stokes, J., 3rd; Kannel, W.B.; Feinleib, M. Mitral valve prolapse in the general population. 1. Epidemiologic features: The Framingham Study. Am. Heart J. 1983, 106, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Bryhn, M.; Persson, S. The prevalence of mitral valve prolapse in healthy men and women in Sweden. An echocardiographic study. Acta Med. Scand. 1984, 215, 157–160. [Google Scholar] [CrossRef]
- Lee, W.R.; Sheikh, M.U.; Lee, K.J. Prevalence of mitral valve prolapse in presumably healthy Korean adults. Clin. Cardiol. 1985, 8, 356–358. [Google Scholar] [CrossRef]
- Warth, D.C.; King, M.E.; Cohen, J.M.; Tesoriero, V.L.; Marcus, E.; Weyman, A.E. Prevalence of mitral valve prolapse in normal children. J. Am. Coll. Cardiol. 1985, 5, 1173–1177. [Google Scholar] [CrossRef]
- Levine, R.A.; Stathogiannis, E.; Newell, J.B.; Harrigan, P.; Weyman, A.E. Reconsideration of echocardiographic standards for mitral valve prolapse: Lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J. Am. Coll. Cardiol. 1988, 11, 1010–1019. [Google Scholar] [CrossRef]
- Levine, R.A.; Handschumacher, M.D.; Sanfilippo, A.J.; Hagege, A.A.; Harrigan, P.; Marshall, J.E.; Weyman, A.E. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989, 80, 589–598. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Bruno, A.; Lombardo, M.; Muti, P. Echocardiographic assessment of mitral valve prolapse prevalence before and after the year 1999: A systematic review. J. Clin. Med. 2024, 13, 6160. [Google Scholar] [CrossRef] [PubMed]
- Compagnucci, P.; Selimi, A.; Cipolletta, L.; Volpato, G.; Gasperetti, A.; Valeri, Y.; Parisi, Q.; Curcio, A.; Natale, A.; Dello Russo, A.; et al. Arrhythmic Mitral Valve Prolapse and Sports Activity: Pathophysiology, Risk Stratification, and Sports Eligibility Assessment. J. Clin. Med. 2024, 13, 1350. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Bodison, S.A.; Wesley, Y.E.; Tucker, E.; Green, K.J. Results of screening a large group of intercollegiate competitive athletes for cardiovascular disease. J. Am. Coll. Cardiol. 1987, 10, 1214–1221. [Google Scholar] [CrossRef]
- Lewis, J.F.; Maron, B.J.; Diggs, J.A.; Spencer, J.E.; Mehrotra, P.P.; Curry, C.L. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominantly black population of collegiate athletes. Am. J. Cardiol. 1989, 64, 1029–1033. [Google Scholar] [CrossRef]
- Weidenbener, E.J.; Krauss, M.D.; Waller, B.F.; Taliercio, C.P. Incorporation of screening echocardiography in the preparticipation exam. Clin. J. Sport Med. 1995, 5, 86–89. [Google Scholar] [CrossRef]
- Hepner, A.D.; Morrell, H.; Greaves, S.; Greaves, J.; Movahed, M.R. Prevalence of mitral valvar prolapse in young athletes. Cardiol. Young 2008, 18, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Spataro, A.; Cecchetelli, C.; Quaranta, F.; Livrieri, S.; Sperandii, F.; Cifra, B.; Borrione, P.; Pigozzi, F. Structural cardiac disease diagnosed by echocardiography in asymptomatic young male soccer players: Implications for pre-participation screening. Br. J. Sports Med. 2012, 46, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Schmied, C.; Di Paolo, F.M.; Zerguini, A.Y.; Dvorak, J.; Pelliccia, A. Screening athletes for cardiovascular disease in Africa: A challenging experience. Br. J. Sports Med. 2013, 47, 579–584. [Google Scholar] [CrossRef]
- Steriotis, A.K.; Nava, A.; Rigato, I.; Mazzotti, E.; Daliento, L.; Thiene, G.; Basso, C.; Corrado, D.; Bauce, B. Noninvasive cardiac screening in young athletes with ventricular arrhythmias. Am. J. Cardiol. 2013, 111, 557–562. [Google Scholar] [CrossRef]
- Pelliccia, A.; Quattrini, F.M.; Squeo, M.R.; Caselli, S.; Culasso, F.; Link, M.S.; Spataro, A.; Bernardi, M. Cardiovascular diseases in Paralympic athletes. Br. J. Sports Med. 2016, 50, 1075–1080. [Google Scholar] [CrossRef]
- Ceylan, Ö.; Meşe, T.; Gürsu, A.H. Using cardiovascular imaging modalities to determine cardiac disorders before starting sports activities. Turk. Kardiyol. Dern. Ars. 2017, 45, 160–166. [Google Scholar] [CrossRef]
- Ong, G.; Connelly, K.A.; Goodman, J.; Leong-Poi, H.; Evangelista, V.; Levitt, K.; Gledhill, N.; Jamnik, V.; Gledhill, S.; Yan, A.T.; et al. Echocardiographic Assessment of Young Male Draft-Eligible Elite Hockey Players Invited to the Medical and Fitness Combine by the National Hockey League. Am. J. Cardiol. 2017, 119, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Mango, F.; Clark, J.; Pandian, N.G.; Corrado, D.; Autore, C.; Pelliccia, A. Prevalence and Clinical Outcome of Athletes with Mitral Valve Prolapse. Circulation 2018, 137, 2080–2082. [Google Scholar] [CrossRef]
- Harris, K.M.; Mackey-Bojack, S.; Fisher, G.; Nwaudo, D.; Maron, B.J. Arrhythmogenic Mitral Valve Prolapse Revisited: A Not Uncommon Cause of Youthful Sudden Death in Athletes and Women. Am. J. Med. 2025, 138, 156–160. [Google Scholar] [CrossRef]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef]
- Battaglia, V.; Santangelo, G.; Bursi, F.; Simeoli, P.; Guazzi, M. Arrhythmogenic Mitral Valve Prolapse and Sudden Cardiac Death: An Update and Current Perspectives. Curr. Probl. Cardiol. 2023, 48, 101724. [Google Scholar] [CrossRef]
- Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Athanasiou, A.; Moris, D.; Damaskos, C.; Garmpis, N.; Voudris, V. Mitral valve prolapse: An underestimated cause of sudden cardiac death—A current review of the literature. J. Thorac. Dis. 2017, 9, 5390–5398. [Google Scholar] [CrossRef]
- Zilberszac, R.; Gleiss, A.; Massetti, M.; Wisser, W.; Binder, T.; Gabriel, H.; Rosenhek, R. Left atrial size predicts outcome in severe but asymptomatic mitral regurgitation. Sci. Rep. 2023, 13, 3892. [Google Scholar] [CrossRef] [PubMed]
- Tastet, L.; Lim, L.J.; Bibby, D.; Hu, G.; Cristin, L.; Rich, A.H.; Jhawar, R.; Fang, Q.; Arya, F.; Delling, F.N. Primary Atriopathy in Mitral Valve Prolapse: Echocardiographic Evidence and Clinical Implications. Circ. Cardiovasc. Imaging 2024, 17, e016319. [Google Scholar] [CrossRef]
- Petek, B.J.; Baggish, A.L. Valvular Heart Disease in Athletes. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Uhm, J.S.; Suh, Y.J.; Kim, M.; Kim, I.S.; Jin, M.N.; Cho, M.S.; Yu, H.T.; Kim, T.H.; Hong, Y.J.; et al. Usefulness of Cardiac Magnetic Resonance Images for Prediction of Sudden Cardiac Arrest in Patients with Mitral Valve Prolapse: A Multicenter Retrospective Cohort Study. BMC Cardiovasc. Disord. 2021, 21, 546. [Google Scholar] [CrossRef]
- Opriș, D.R.; Harpa, M.M.; Anitei, D.E.; Calburean, P.; Rudzik, R. Mitral Valve Prolapse and Sudden Cardiac Death—A Puzzle with Missing Pieces: Review of the Literature and Case Report. Med. Sci. 2025, 13, 185. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Versaci, F.; Frati, G.; Sciarra, L. How to manage an athlete with mitral valve prolapse. Eur. J. Prev. Cardiol. 2021, 28, 1110–1117. [Google Scholar] [CrossRef]
- Pistelli, L.; Vetta, G.; Parlavecchio, A.; Crea, P.; Parisi, F.; Magnocavallo, M.; Caminiti, R.; Frea, S.; Vairo, A.; Desalvo, P.; et al. Arrhythmic risk profile in mitral valve prolapse: A systematic review and metanalysis of 1715 patients. J. Cardiovasc. Electrophysiol. 2024, 35, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Schiavon, M.; Thiene, G. Screening for hypertrophic cardiomyopathy in young athletes. N. Engl. J. Med. 1998, 339, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baggish, A.L.; Levine, B.D.; Ackerman, M.J.; Day, S.M.; Dineen, E.H.; Guseh, J.S., II; La Gerche, A.; Lampert, R.; Martinez, M.W.; et al. Clinical Considerations for Competitive Sports Participation for Athletes with Cardiovascular Abnormalities: A Scientific Statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2025, 85, 1059–1108. [Google Scholar] [CrossRef]
- Chatrath, N.; Papadakis, M. Physical activity and exercise recommendations for patients with valvular heart disease. Heart 2022, 108, 1938–1944. [Google Scholar] [CrossRef]
- Segreti, A.; Celeski, M.; Monticelli, L.M.; Perillo, A.; Crispino, S.P.; Di Gioia, G.; Cammalleri, V.; Fossati, C.; Mega, S.; Papalia, R.; et al. Mitral and Tricuspid Valve Disease in Athletes. J. Clin. Med. 2023, 12, 3562. [Google Scholar] [CrossRef]
- Han, H.C.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral Valve Prolapse and Sudden Cardiac Death: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef]
- Cristin, L.; Tastet, L.; Shah, D.J.; Miller, M.A.; Delling, F.N. Multimodality Imaging of Arrhythmic Risk in Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2025, 18, e017313. [Google Scholar] [CrossRef]
- Oxborough, D.; George, K.; Cooper, R.; Bhatia, R.; Ramcharan, T.; Zaidi, A.; Gati, S.; Prakash, K.; Rakhit, D.; Robinson, S.; et al. Echocardiography in the cardiac assessment of young athletes: A 2025 guideline from the British Society of Echocardiography (endorsed by Cardiac Risk in the Young). Echo Res. Pract. 2025, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anzà, C. A New modified anthropometric haller index obtained without radiological exposure. Int. J. Cardiovasc. Imaging 2018, 34, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M. The relationship between mitral valve prolapse and thoracic skeletal abnormalities in clinical practice: A systematic review. J. Cardiovasc. Med. 2024, 25, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Influence of chest conformation on myocardial strain parameters in healthy subjects with mitral valve prolapse. Int. J. Cardiovasc. Imaging 2021, 37, 1009–1022. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Muti-Schünemann, G.E.U.; Rispoli, G.A.; Lombardo, M.; Muti, P. Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review. J. Clin. Med. 2025, 14, 2277. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Prognostic value of modified Haller index in patients with suspected coronary artery disease referred for exercise stress echocardiography. J. Cardiovasc. Echogr. 2021, 31, 85–95. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Population (Size) | Av. Age (yrs) (% Males) | Study Design | MVP Diagnostic Criteria | MVP Cases (%) | VAs (%) | SVAs (%) | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Maron B.J. et al. USA (1987) [28] | Intercollegiate Athletes (90) | 19.3 (71) | P | M-mode: ≥3 mm late systolic or pansystolic displacement of one/both mitral leaflets posterior to line of closure. 2D echo: displacement of mitral leaflets beyond annular plane into LA in long-axis view or apical 4-chambers views. | 14 (15.6) | 1 (1.1) | NR | Echocardiography improves MVP detection; auscultation alone often misleading. |
| Lewis J.F. et al. USA (1989) [29] | Preparticipation echo screening (football) (265) | 19 (83) | P | M-mode: ≥3 mm late systolic or pansystolic displacement of one/both mitral leaflets posterior to line of closure. 2D echo: displacement of mitral leaflets beyond annular plane into LA in long-axis view or apical 4-chambers views. | 30 (11) | NR | NR | Feasibility of large-scale echo screening; MVP generally benign in athletes. |
| Weidenbener E.J. et al. USA (1995) [30] | Athletic (2997) | NS | R | Parasternal long- and short-axis views chosen as screening views | 40 (1.3) | NR | NR | Recommended adding echocardiography to pre-participation exam despite cost concerns. |
| Hepner A.D. et al. USA (2008) [31] | Young athletes (1742) | 17.5 (67.3) | R | Use of multiple echocardiographic cross sections (rather than the long-axis view alone) | 16 (0.9) | NR | NR | MVP in young athletes usually asymptomatic with favorable short-term outcome. |
| Rizzo M. et al. Italy (2012) [32] | Youth soccer players (3100) | 11 (100) | P | Single or bileaflet systolic protrusion ≥2 mm beyond the long-axis annular plane into LA | 10 (0.32) | NR | NR | Echocardiography detected MVP cases undiagnosed by ECG or clinical exam. |
| Schmied C. et al. Switzerland (2013) [33] | FIFA elite footballers (210) | 18.6 (100) | P | Leaflet thickening >5 mm + systolic displacement >2 mm in parasternal long-axis view | 3 (1.4) | 0(0.0) | 0 (0.0) | Applied strict criteria; identified MVP in African athletes without major complications. |
| Steriotis A.K. et al. Italy (2013) [34] | Young athletes with ventricular arrhythmias (145) | 17.3 (73) | P | Single or bileaflet systolic protrusion ≥2 mm beyond the long-axis annular plane into LA | 29 (20) | 145 (100) | NR | Found MVP associated with ventricular arrhythmias in competitive athletes. |
| Pelliccia A. et al. Italy (2016) [35] | Paralympic athletes (267) | 35 (76) | P | Single or bileaflet systolic protrusion ≥2 mm beyond the long-axis annular plane into LA | 4 (1.5) | 5 (1.9) | 4 (1.5) | MVP was rare; overall cardiac abnormalities infrequent in Paralympic athletes. |
| Ceylan Ö et al. Turkey (2017) [36] | Youth athletes (football, basketball, volleyball, swimming) (250) | 13 (89.6) | P | Single or bileaflet systolic protrusion ≥2 mm beyond the long-axis annular plane into LA | 2 (0.8) | 1 (0.4) | 1 (0.4) | Pediatric cardiology screening revealed MVP and other conditions requiring restrictions. |
| Ong G. et al. Canada (2017) [37] | Draft-eligible elite hockey players (592) | 18 (NS) | P | Single or bileaflet systolic protrusion ≥2 mm beyond the long-axis annular plane into LA | 1 (0.2) | NR | NR | Echocardiography in elite hockey players occasionally revealed benign MVP. |
| Caselli S. et al. Italy (2018) [38] | Competitive athletes (7449) | 30 (67) | P | Single or bileaflet systolic protrusion ≥2 mm beyond the long-axis annular plane into LA | 215 (2.9) | 62 (0.8) | 1 (0.01) | MVP prognosis usually benign; adverse outcomes linked to arrhythmias or regurgitation. |
| Harris K.M. et al. USA (2024) [39] | US autopsy SCD registry (2406) | 22 (51) | R | Pathologic criteria: bileaflet myxomatous involvement, chordal thickening/elongation, interstitial or replacement LV fibrosis | 43 (1.8) | NR | NR | Autopsies revealed arrhythmogenic MVP with fibrosis as cause of sudden death. |
| Study Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality (Total Quality Score) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maron B.J. et al. (1987) [28] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Lewis J.F. et al. (1989) [29] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Weidenbener E.J. et al. (1995) [30] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Hepner A.D. et al. (2008) [31] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Rizzo M. et al. (2012) [32] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Schmied C. et al. (2013) [33] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Steriotis A.K. et al. (2013) [34] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Pelliccia A. et al. (2016) [35] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Ceylan Ö. et al. (2017) [36] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Ong G. et al. (2017) [37] | Yes | Yes | NR | Yes | No | No | No | No | Yes | No | Yes | NR | NR | No | 5 |
| Caselli S. et al. (2018) [38] | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | No | Yes | NR | Yes | No | 8 |
| Harris K.M. et al. (2024) [39] | Yes | Yes | NR | Yes | No | No | NR | No | Yes | No | Yes | NR | NR | No | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Baravelli, M. Mitral Valve Prolapse in Athletes: Prevalence, Arrhythmic Associations, and Clinical Implications—A Systematic Review. J. Clin. Med. 2025, 14, 7475. https://doi.org/10.3390/jcm14217475
Sonaglioni A, Nicolosi GL, Lombardo M, Baravelli M. Mitral Valve Prolapse in Athletes: Prevalence, Arrhythmic Associations, and Clinical Implications—A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7475. https://doi.org/10.3390/jcm14217475
Chicago/Turabian StyleSonaglioni, Andrea, Gian Luigi Nicolosi, Michele Lombardo, and Massimo Baravelli. 2025. "Mitral Valve Prolapse in Athletes: Prevalence, Arrhythmic Associations, and Clinical Implications—A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7475. https://doi.org/10.3390/jcm14217475
APA StyleSonaglioni, A., Nicolosi, G. L., Lombardo, M., & Baravelli, M. (2025). Mitral Valve Prolapse in Athletes: Prevalence, Arrhythmic Associations, and Clinical Implications—A Systematic Review. Journal of Clinical Medicine, 14(21), 7475. https://doi.org/10.3390/jcm14217475