High-Risk Outcomes in In Vitro Fertilization Pregnancies for Women of a Very Advanced Maternal Age: Insights from a Multi-Hospital Study in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Selection
2.2. IVF Procedures
2.3. Outcomes and Data Collection
2.4. Prenatal Care and Delivery
3. Statistical Analysis
4. Results
5. Discussion
5.1. Preeclampsia
5.2. Gestational Diabetes Mellitus
5.3. Preterm Labor
5.4. Low Birth Weight
5.5. Placental Abnormalities
5.6. Impact of Egg Donation on Outcomes Between Two Groups
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Athanasopoulou, P.; Giovanis, A.N.; Gdonteli, K. Health Tourism in Greece: The Fertility Center in Crete. In Strategic Innovative Marketing and Tourism; Kavoura, A., Borges-Tiago, T., Tiago, F., Eds.; Springer Proceedings in Business and Economics; Springer Nature: Cham, Switzerland, 2024; pp. 141–146. Available online: https://link.springer.com/10.1007/978-3-031-51038-0_16 (accessed on 28 November 2024).
- Sugai, S.; Haino, K.; Yoshihara, K.; Nishijima, K. Pregnancy outcomes at maternal age over 45 years: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100885. [Google Scholar] [CrossRef] [PubMed]
- Thilaganathan, B. Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet. Gynecol. 2018, 51, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Sacha, C.R.; Harris, A.L.; James, K.; Basnet, K.; Freret, T.S.; Yeh, J.; Kaimal, A.; Souter, I.; Roberts, D.J. Placental pathology in live births conceived with in vitro fertilization after fresh and frozen embryo transfer. Am. J. Obstet. Gynecol. 2020, 222, 360.e1–360.e16. [Google Scholar] [CrossRef] [PubMed]
- Masoudian, P.; Nasr, A.; de Nanassy, J.; Fung-Kee-Fung, K.; Bainbridge, S.A.; El Demellawy, D. Oocyte donation pregnancies and the risk of preeclampsia or gestational hypertension: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 328–339. [Google Scholar] [CrossRef]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Women’s Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Yoon, S.S.S. Clinical Outcomes in High-Risk Pregnancies Due to Advanced Maternal Age. J. Women’s Health 2021, 30, 160–167. [Google Scholar] [CrossRef]
- Thomopoulos, C.; Tsioufis, C.; Michalopoulou, H.; Makris, T.; Papademetriou, V.; Stefanadis, C. Assisted reproductive technology and pregnancy-related hypertensive complications: A systematic review. J. Hum. Hypertens. 2013, 27, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Fujiwara, T.; Suzuki, T.; Jwa, S.C.; Taniguchi, K.; Yamanobe, Y.; Kozuka, K.; Sago, H. Is in vitro fertilization associated with preeclampsia? A propensity score matched study. BMC Pregnancy Childbirth 2014, 14, 69. [Google Scholar] [CrossRef]
- Xiong, F.; Hu, L.; Zhang, Y.; Xiao, X. Correlation of hypertensive disorders in pregnancy with procedures of in vitro fertilization and pregnancy outcomes. Exp. Ther. Med. 2017, 14, 5405–5410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Chinnasamy, T.; Lifson, M.A.; Inci, F.; Demirci, U. Flexible Substrate-Based Devices for Point-of-Care Diagnostics. Trends Biotechnol. 2016, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E.; Luke, B.; Tobias, M.; Gopal, D.; Hornstein, M.D.; Diop, H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil. Steril. 2015, 103, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Kroener, L.; Wang, E.; Pisarska, M. Predisposing Factors to Abnormal First Trimester Placentation and the Impact on Fetal Outcomes. Semin. Reprod. Med. 2015, 34, 027–035. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Ling, Z.; Hou, X.; Fan, Y.; Xie, K.; Shen, R. In vitro fertilization is associated with the onset and progression of preeclampsia. Placenta 2020, 89, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Sibai, B.M. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am. J. Obstet. Gynecol. 2006, 195, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Chughtai, A.A.; Farquhar, C.M.; Pollock, W.; Lui, K.; Sullivan, E.A. Increased incidence of gestational hypertension and preeclampsia after assisted reproductive technology treatment. Fertil. Steril. 2016, 105, 920–926.e2. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.E.; Ray, J.G.; Chan, W.S. Maternal body mass index and the risk of preeclampsia: A systematic overview. Epidemiology 2003, 14, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.E. What are the metabolic precursors which increase the risk of pre-eclampsia and how could these be investigated further. Placenta 2017, 60, 110–114. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, B.; Li, N.; Wang, X.; Xu, B.; Li, Y. Relationship between advanced maternal age and decline of endometrial receptivity: A systematic review and meta-analysis. Aging 2023, 15, 2460–2472. [Google Scholar] [CrossRef]
- Cleary-Goldman, J.; Malone, F.D.; Vidaver, J.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Eddleman, K.A.; Klugman, S.M.; Dugoff, L.; et al. Impact of Maternal Age on Obstetric Outcome. Obstet. Gynecol. 2005, 105 Pt 1, 983–990. [Google Scholar] [CrossRef]
- Crawford, N.M.; Steiner, A.Z. Age-related Infertility. Obstet. Gynecol. Clin. N. Am. 2015, 42, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ethics Committee of the American Society for Reproductive Medicine; Ethics Committee of the American Society for Reproductive Medicine. Oocyte or embryo donation to women of advanced reproductive age: An Ethics Committee opinion. Fertil. Steril. 2016, 106, e3–e7. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar]
- Sun, M.; Luo, M.; Wang, T.; Wei, J.; Zhang, S.; Shu, J.; Zhong, T.; Liu, Y.; Chen, Q.; Zhu, P.; et al. Effect of the interaction between advanced maternal age and pre-pregnancy BMI on pre-eclampsia and GDM in Central China. BMJ Open Diabetes Res. Care 2023, 11, e003324. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Wang, Y.; Wang, H.; Ding, X.; Li, G. Maternal gestational diabetes in singleton pregnancies conceived by ART may be modified by periconceptional B vitamins. Front. Nutr. 2022, 9, 1069911. [Google Scholar] [CrossRef] [PubMed]
- Vaajala, M.; Liukkonen, R.; Ponkilainen, V.; Mattila, V.M.; Kekki, M.; Kuitunen, I. In vitro fertilization increases the odds of gestational diabetes: A nationwide register-based cohort study. Acta Diabetol. 2023, 60, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef]
- WHO. WHO: Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253. [Google Scholar]
- Esposito, G.; Mauri, P.A.; Cipriani, S.; Franchi, M.; Corrao, G.; Parazzini, F. The role of maternal age on the risk of preterm birth among singletons and multiples: A retrospective cohort study in Lombardy, Northern Italy. BMC Pregnancy Childbirth 2022, 22, 234. [Google Scholar]
- Gondane, P.; Kumbhakarn, S.; Maity, P.; Kapat, K. Recent Advances and Challenges in the Early Diagnosis and Treatment of Preterm Labor. Bioengineering 2024, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Baker, P.N.; Tong, C. The updated understanding of advanced maternal age. Fundam. Res. 2023, 4, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Köck, K.; Köck, F.; Klein, K.; Bancher-Todesca, D.; Helmer, H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J. Matern-Fetal Neonatal Med. 2010, 23, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Parnell, B.; Nelson-Piercy, C.; Seed, P.T.; Poston, L.; Chappell, L.C. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 2014, 348, g2301. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35 Pt A, 6492–6500. [Google Scholar]
- Aradhya, S.; Tegunimataka, A.; Kravdal, Ø.; Martikainen, P.; Myrskylä, M.; Barclay, K.; Goisis, A. Maternal age and the risk of low birthweight and pre-term delivery: A pan-Nordic comparison. Int. J. Epidemiol. 2023, 52, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Toutain, J.; Prochazkova-Carlotti, M.; Cappellen, D.; Jarne, A.; Chevret, E.; Ferrer, J.; Idrissi, Y.; Pelluard, F.; Carles, D.; Maugey-Laulon, B.; et al. Reduced Placental Telomere Length during Pregnancies Complicated by Intrauterine Growth Restriction. Tian X (Cindy), editor. PLoS ONE 2013, 8, e54013. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Monet, B.; Ducruet, T.; Chaillet, N.; Audibert, F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE 2018, 13, e0191002. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Maiti, K.; Dedman, L.; Smith, R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am. J. Obstet. Gynecol. 2018, 218, S762–S773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiong, L.; Jin, H.; Yu, J.; Li, X.; Fu, H.; Wen, L.; Qi, H.; Tong, C.; Saffery, R.; et al. Advanced maternal age causes premature placental senescence and malformation via dysregulated α-Klotho expression in trophoblasts. Aging Cell 2021, 20, e13417. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, K.G.; Garcia, É.M.; Santos Neto, E.T.D.; Gama, S.G.N.D. Advanced maternal age and its association with placenta praevia and placental abruption: A meta-analysis. Cad. Saúde Pública 2018, 34, e00206116. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Fu, Y.; Shi, H.; Li, R.; Zhao, Y.; Wang, Y.; Qiao, J. Placental Abnormalities and Placenta-Related Complications Following In-Vitro Fertilization: Based on National Hospitalized Data in China. Front. Endocrinol. 2022, 13, 924070. [Google Scholar] [CrossRef]
- Gundogan, F.; Bianchi, D.W.; Scherjon, S.A.; Roberts, D.J. Placental pathology in egg donor pregnancies. Fertil. Steril. 2010, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Caradeux, J.; Fernández, B.; Ávila, F.; Valenzuela, A.; Mondión, M.; Figueras, F. Pregnancies through oocyte donation. A mini review of pathways involved in placental dysfunction. Front. Med. 2024, 11, 1338516. [Google Scholar] [CrossRef] [PubMed]
- Keukens, A.; van Wely, M.; van der Meulen, C.; Mochtar, M.H. Pre-eclampsia in pregnancies resulting from oocyte donation, natural conception or IVF: A systematic review and meta-analysis. Hum. Reprod. 2022, 37, 586–599. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Sunkara, S.K.; Antonisamy, B.; Kamath, M.S. Higher risk of preterm birth and low birth weight following oocyte donation: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 60–67. [Google Scholar] [CrossRef]
- Moreno-Sepulveda, J.; Checa, M.A. Risk of adverse perinatal outcomes after oocyte donation: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2019, 36, 2017–2037. [Google Scholar] [CrossRef] [PubMed]
- Storgaard, M.; Loft, A.; Bergh, C.; Wennerholm, U.B.; Söderström-Anttila, V.; Romundstad, L.B.; Aittomaki, K.; Oldereid, N.; Forman, J.; Pinborg, A. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 561–572. [Google Scholar] [CrossRef]
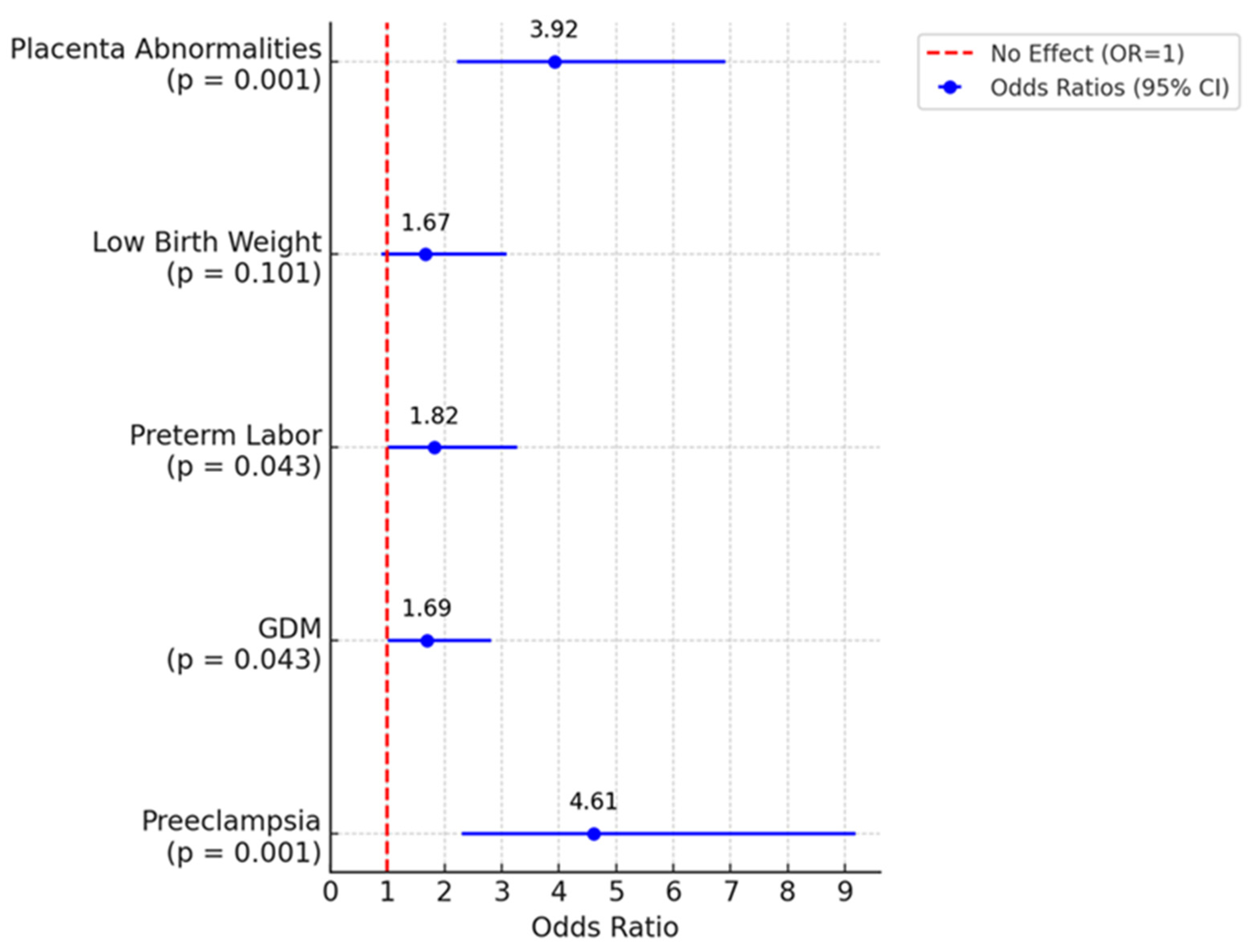
| Age | ||||||
|---|---|---|---|---|---|---|
| Below 50 Years | Above 50 Years | |||||
| n = 296 | % | n = 97 | % | OR (95% CI) | p | |
| Preeclampsia | 18 | 6.10% | 20 | 23.00% | 4.61 (2.31–9.19)) | <0.001 |
| Gestational diabetes | 73 | 24.70% | 31 | 35.60% | 1.69 (1.01–2.82) | 0.043 |
| Preterm labor | 44 | 14.90% | 21 | 24.10% | 1.82 (1.01–3.27) | 0.043 |
| Low birth weight | 40 | 13.50% | 18 | 20.70% | 1.67 (0.90–3.09) | 0.101 |
| Placenta abnormalities | 35 | 11.80% | 30 | 34.50% | 3.92 (2.22–6.91) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukopoulos, T.; Zikopoulos, A.; Kolibianakis, E.; Vatopoulou, A.; Gkrozou, F.; Sotiriou, S.; Zachariou, A.; Skentou, C. High-Risk Outcomes in In Vitro Fertilization Pregnancies for Women of a Very Advanced Maternal Age: Insights from a Multi-Hospital Study in Greece. J. Clin. Med. 2025, 14, 1323. https://doi.org/10.3390/jcm14041323
Loukopoulos T, Zikopoulos A, Kolibianakis E, Vatopoulou A, Gkrozou F, Sotiriou S, Zachariou A, Skentou C. High-Risk Outcomes in In Vitro Fertilization Pregnancies for Women of a Very Advanced Maternal Age: Insights from a Multi-Hospital Study in Greece. Journal of Clinical Medicine. 2025; 14(4):1323. https://doi.org/10.3390/jcm14041323
Chicago/Turabian StyleLoukopoulos, Themistoklis, Athanasios Zikopoulos, Efstratios Kolibianakis, Anastasia Vatopoulou, Fani Gkrozou, Sotirios Sotiriou, Athanasios Zachariou, and Charikleia Skentou. 2025. "High-Risk Outcomes in In Vitro Fertilization Pregnancies for Women of a Very Advanced Maternal Age: Insights from a Multi-Hospital Study in Greece" Journal of Clinical Medicine 14, no. 4: 1323. https://doi.org/10.3390/jcm14041323
APA StyleLoukopoulos, T., Zikopoulos, A., Kolibianakis, E., Vatopoulou, A., Gkrozou, F., Sotiriou, S., Zachariou, A., & Skentou, C. (2025). High-Risk Outcomes in In Vitro Fertilization Pregnancies for Women of a Very Advanced Maternal Age: Insights from a Multi-Hospital Study in Greece. Journal of Clinical Medicine, 14(4), 1323. https://doi.org/10.3390/jcm14041323






