The Heart’s Small Molecules: The Importance of MicroRNAs in Cardiovascular Health
Abstract
1. Introduction
2. Methods
Systematic Literature Search and Study Selection
3. Regulation of Apoptosis in Ischemia/Reperfusion Injury by MicroRNAs
4. MicroRNA Biomarkers in Coronary Artery Disease
5. MicroRNA-Mediated Regulation in Cardiac Hypertrophy
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Rat | miR-101 ↓→ Rab1a ↓→ fetal gene expression, protein synthesis, and cell size ↓ | [98] | Mouse and Human | miR-133 ↓→ RhoA, Cdc42, Nelf-A ↑→ cardiac hypertrophy ↑ | [99] | Human and Mouse | miR-499 ↑→ target mRNAs ↓ (incl. Akt, MAPKs). - | [100] |
| Rat and Mouse | miR-145 ↑→ GATA6 ↓→ ANF, BNP, β-MHC ↓; ERK1/2, JNK, Akt-GSK3β ↓ | [101] | Mouse | miR-155 ↑→ TP53INP1 ↓→ fibroblast proliferation/myofibroblast transition ↑→ cardiac remodeling ↑ | [102] | Mouse and Human | miR-199b ↑→ Dyrk1a ↓→ NFAT activity ↑→ hypertrophy & fibrosis ↑ | [97] |
| Rat | High glucose → miR-150 ↓→ p300 ↑→ hypertrophy ↑; PKCβ2 mediates miR-150 downregulation. | [103] | Rat | LPA → miR-23a ↑→ LPA1 ↓→hypertrophy ↑; LPA3 mediates miR-23a upregulation via PI3K/AKT. | [96] | Mouse | miR-133a ↓ in TAC and ISO; overexpression →QT prolongation, reduced fibrosis. | [104] |
| Mouse and Rat | Isoproterenol/aldosterone →miR-9 ↓, NFATc3 ↑→ myocardin ↑→ cardiac hypertrophy ↑ | [95] | Rat | miR-350 ↑→ MAPK11/14, MAPK8/9 ↓→ p38 & JNK ↓→ NFATc3 ↑→hypertrophy & apoptosis ↑ | [105] | Human, Mouse and Rat | miR-21 ↑→ Spry1 ↓→ CTGF, lysyl oxidase, Rac1-GTP ↑→ atrial fibrosis ↑ | [106] |
| Mouse and Rat | miR-328 ↑→ SERCA2a ↓→ intracellular Ca2+ ↑→ calcineurin ↑→ NFATc3 ↑→ hypertrophy ↑; | [107] | Mouse and Human | miR-199b ↑→ Dyrk1a ↓→ NFAT activity ↑→ hypertrophy & fibrosis ↑ | [97] | Mouse | miR-27b ↑→ PPAR-γ ↓→ hypertrophy & dysfunction ↑; TGF-β1 ↓ miR-27b | [108] |
6. MicroRNA Contributions to the Molecular Basis of Cardiac Arrhythmias
7. Prognostic Relevance of MicroRNA Expression in Heart Failure
8. MicroRNA Profiles and Fibrotic Pathways in Valvular Heart Disease
9. MicroRNA–Gene Interactions in Cardiomyopathies
10. Developmental MicroRNA Dysregulation in Congenital Heart Diseases
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Axtell, M.J. Evolution of microRNAs and their targets: Are all microRNAs biologically relevant? Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2008, 1779, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kabekkodu, S.P.; Shukla, V.; Varghese, V.K.; D’Souza, J.; Chakrabarty, S.; Satyamoorthy, K. Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. 2018, 93, 1955–1986. [Google Scholar] [CrossRef] [PubMed]
- Latronico, M.V.; Condorelli, G. MicroRNAs and cardiac pathology. Nat. Rev. Cardiol. 2009, 6, 419–429. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X. The function of miRNA in cardiac hypertrophy. Cell Mol. Life Sci. 2012, 69, 3561–3570. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xie, F.; Li, C.; Zhang, B.; Nichols, R.L.; Pan, X. Identification and characterization of microRNAs in the plant parasitic root-knot nematode Meloidogyne incognita using deep sequencing. Funct. Integr. Genomics 2016, 16, 127–142. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef]
- Moreno-Moya, J.M.; Vilella, F.; Simon, C. MicroRNA: Key gene expression regulators. Fertil. Steril. 2014, 101, 1516–1523. [Google Scholar] [CrossRef]
- Farazi, T.A.; Spitzer, J.I.; Morozov, P.; Tuschl, T. miRNAs in human cancer. J. Pathol. 2011, 223, 102–115. [Google Scholar] [CrossRef]
- Li, H.; Zhan, J.; Chen, C.; Wang, D. MicroRNAs in cardiovascular diseases. Med. Rev. 2022, 2, 140–168. [Google Scholar] [CrossRef]
- Sharma, S.; Artner, T.; Preissner, K.T.; Lang, I.M. Nucleic acid liquid biopsies in cardiovascular disease: Cell-free RNA liquid biopsies in cardiovascular disease. Atherosclerosis 2024, 398, 118584. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Singhanat, K.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Roles of melatonin and its receptors in cardiac ischemia-reperfusion injury. Cell Mol. Life Sci. 2018, 75, 4125–4149. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Song, W.; Liang, R.; Teng, L.; Zhang, M.; Zhang, J.; Zhu, L. MicroRNA as a Potential Biomarker and Treatment Strategy for Ischemia-Reperfusion Injury. Int. J. Genom. 2021, 2021, 9098145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Toan, S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rao, S.; Yang, M.; Ma, C.; Hong, F.; Yang, S. Role of Mitochondrial Pathways in Cell Apoptosis during He-Patic Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 2357. [Google Scholar] [CrossRef]
- Orellana-Urzua, S.; Briones-Valdivieso, C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential Role of Natural Antioxidants in Countering Reperfusion Injury in Acute Myocardial Infarction and Ischemic Stroke. Antioxidants 2023, 12, 1760. [Google Scholar] [CrossRef]
- Chen, K.Y.; Liu, Z.; Lu, J.H.; Yang, S.Y.; Hu, X.Y.; Liang, G.Y. The Function of Circular RNAs in Myocardial Ischemia-Reperfusion Injury: Underlying Mechanisms and Therapeutic Advancement. Cardiovasc. Drugs Ther. 2025, 39, 875–886. [Google Scholar] [CrossRef]
- Liu, N.B.; Wu, M.; Chen, C.; Fujino, M.; Huang, J.S.; Zhu, P.; Li, X.K. Novel Molecular Targets Participating in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Cardiol. Res. Pract. 2019, 2019, 6935147. [Google Scholar] [CrossRef]
- Wei, C.; Li, L.; Gupta, S. NF-κB-mediated miR-30b regulation in cardiomyocytes cell death by targeting Bcl-2. Mol. Cell Biochem. 2014, 387, 135–141. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, G.; Liang, Z.; Liu, X.; Li, T.; Fan, J.; Bai, J.; Wang, Y. MicroRNA-15b enhances hypoxia/reoxygenation-induced apoptosis of cardiomyocytes via a mitochondrial apoptotic pathway. Apoptosis 2014, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jiang, H.; Chen, S.; Zhang, H. Inhibition of microRNA-101 attenuates hypoxia/reoxygenation-induced apoptosis through induction of autophagy in H9c2 cardiomyocytes. Mol. Med. Rep. 2015, 11, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, L.; Kim, I.; Sun, P.; Gupta, S. NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free. Radic. Res. 2014, 48, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, Y.; Hou, L.; Ju, J.; Li, X.; Du, N.; Guan, X.; Liu, Z.; Zhang, T.; Qin, W. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J. Mol. Cell Cardiol. 2014, 75, 111–121. [Google Scholar] [CrossRef]
- Rane, S.; He, M.; Sayed, D.; Vashistha, H.; Malhotra, A.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F.; Abdellatif, M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ. Res. 2009, 104, 879–886. [Google Scholar] [CrossRef]
- Qian, L.; Van Laake, L.W.; Huang, Y.; Liu, S.; Wendland, M.F.; Srivastava, D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J. Exp. Med. 2011, 208, 549–560. [Google Scholar] [CrossRef]
- Fang, J.; Song, X.-W.; Tian, J.; Chen, H.-Y.; Li, D.-F.; Wang, J.-F.; Ren, A.-J.; Yuan, W.-J.; Lin, L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 2012, 17, 410–423. [Google Scholar] [CrossRef]
- He, B.; Xiao, J.; Ren, A.-J.; Zhang, Y.-F.; Zhang, H.; Chen, M.; Xie, B.; Gao, X.-G.; Wang, Y.-W. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J. Biomed. Sci. 2011, 18, 22. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J. Mol. Cell Cardiol. 2009, 47, 5–14. [Google Scholar] [CrossRef]
- Roca-Alonso, L.; Castellano, L.; Mills, A.; Dabrowska, A.; Sikkel, M.; Pellegrino, L.; Jacob, J.; Frampton, A.; Krell, J.; Coombes, R. Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in β-adrenergic signaling and enhances apoptosis. Cell Death Dis. 2015, 6, e1754. [Google Scholar] [CrossRef]
- Zhao, F.; Li, B.; Wei, Y.-z.; Zhou, B.; Wang, H.; Chen, M.; Gan, X.-D.; Wang, Z.-H.; Xiong, S.-X. MicroRNA-34a regulates high glucose-induced apoptosis in H9c2 cardiomyocytes. J. Huazhong Univ. Sci. Technol. [Med. Sci. ] 2013, 33, 834–839. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, K.; Li, A. microRNA-21 protects against ischemia-reperfusion and hypoxia-reperfusion-induced cardiocyte apoptosis via the phosphatase and tensin homolog/Akt-dependent mechanism. Mol. Med. Rep. 2014, 9, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, G.; Chen, L.; Guo, J.; Liu, Y. Downregulation of microRNA-100 protects H2O2-induced apoptosis in neonatal cardiomyocytes. Int. J. Clin. Exp. Patho 2015, 8, 5491–5496. [Google Scholar]
- Yu, X.-Y.; Song, Y.-H.; Geng, Y.-J.; Lin, Q.-X.; Shan, Z.-X.; Lin, S.-G.; Li, Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem. Biophys. Res. Commun. 2008, 376, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Donath, S.; Li, Y.; Qin, D.; Prabhakar, B.S.; Li, P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010, 6, e1000795. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, J.; Sun, Y.; Wu, Z.; Liu, Z.; Huang, G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int. Heart J. 2009, 50, 377–387. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, H.G.; Zhang, K.K.; Ma, D.; Yang, G.; Zheng, Z.L.; Liu, K.; Yu, B.; Zhai, C.L.; Yang, S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. Febs. Lett. 2014, 588, 3137–3146. [Google Scholar] [CrossRef]
- Cha, M.J.; Jang, J.K.; Ham, O.; Song, B.W.; Lee, S.Y.; Lee, C.Y.; Park, J.H.; Lee, J.; Seo, H.H.; Choi, E.; et al. MicroRNA-145 suppresses ROS-induced Ca overload of cardiomyocytes by targeting CaMKIIδ. Biochem. Biophys. Res. Commun. 2013, 435, 720–726. [Google Scholar] [CrossRef]
- Frank, D.; Gantenberg, J.; Boomgaarden, I.; Kuhn, C.; Will, R.; Jarr, K.U.; Eden, M.; Kramer, K.; Luedde, M.; Mairbäurl, H.; et al. MicroRNA-20a inhibits stress-induced cardiomyocyte apoptosis involving its novel target Egln3/PHD3. J. Mol. Cell Cardiol. 2012, 52, 711–717. [Google Scholar] [CrossRef]
- Zhang, B.S.; Zhou, M.; Li, C.B.; Zhou, J.X.; Li, H.Q.; Zhu, D.; Wang, Z.; Chen, A.Q.; Zhao, Q. MicroRNA-92a Inhibition Attenuates Hypoxia/Reoxygenation-Induced Myocardiocyte Apoptosis by Targeting. PLoS ONE 2014, 9, e100298. [Google Scholar] [CrossRef]
- Ferrone, M.; Ciccarelli, M.; Varzideh, F.; Kansakar, U.; Guerra, G.; Cerasuolo, F.A.; Buonaiuto, A.; Fiordelisi, A.; Venga, E.; Esposito, M.; et al. Endothelial microRNAs in INOCA patients with diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 268. [Google Scholar] [CrossRef]
- Thupakula, S.; Nimmala, S.S.R.; Ravula, H.; Chekuri, S.; Padiya, R. Emerging biomarkers for the detection of cardiovascular diseases. Egypt Heart J. 2022, 74, 77. [Google Scholar] [CrossRef]
- Shao, C.; Wang, J.; Tian, J.; Tang, Y.D. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv. Exp. Med. Biol. 2020, 1177, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Kadar, A.; Glasz, T. Development of atherosclerosis and plaque biology. Cardiovasc. Surg. 2001, 9, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C. Stable angina pectoris: Definition, clinical presentation and pathophysiologic mechanisms. In Essentials in Stable Angina Pectoris; Springer: Berlin/Heidelberg, Germany, 2016; pp. 15–35. [Google Scholar]
- Braunwald, E.; Antman, E.M.; Beasley, J.W.; Califf, R.M.; Cheitlin, M.D.; Hochman, J.S.; Jones, R.H.; Kereiakes, D.; Kupersmith, J.; Levin, T.N. ACC/AHA guidelines for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J. Am. Coll. Cardiol. 2000, 36, 970–1062. [Google Scholar] [CrossRef]
- Fine, J.J.; Valois, R.F.; Kammermann, S.K. Behavioral, Clinical, and Coronary Artery Calcium Screening for Prevention and Treatment of Subclinical Atherosclerosis: Implications for Health Education. Am. J. Health Educ. 2025, 56, 329–355. [Google Scholar] [CrossRef]
- Roth, G.A.; Nguyen, G.; Forouzanfar, M.H.; Mokdad, A.H.; Naghavi, M.; Murray, C.J. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation 2015, 132, 1270–1282. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The global burden of cardiovascular diseases and risks: A compass for global action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Hata, J.; Kiyohara, Y. Epidemiology of stroke and coronary artery disease in Asia. Circ. J. 2013, 77, 1923–1932. [Google Scholar] [CrossRef]
- Ahmed, M.; Javaid, H.; Shafiq, A.; Nadeem, Z.A.; Ahsan, A.; Nofal, A.; Ahmed, R.; Alam, M.; Fudim, M.; Fonarow, G.C.; et al. Trends and Disparities in Coronary Artery Disease and Obesity-Related Mortality in the United States From 1999–2022. Endocrinol. Diabetes. Metab. 2024, 7, e70010. [Google Scholar] [CrossRef]
- Mensah, G.A.; Mokdad, A.H.; Ford, E.; Narayan, K.M.V.; Giles, W.H.; Vinicor, F.; Deedwania, P.C. Obesity, metabolic syndrome, and type 2 diabetes: Emerging epidemics and their cardiovascular implications. Cardiol. Clin. 2004, 22, 485–504. [Google Scholar] [CrossRef]
- Balakumar, P.; Maung, U.K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2016, 113, 600–609. [Google Scholar] [CrossRef]
- Wang, X.; Lian, Y.; Wen, X.; Guo, J.; Wang, Z.; Jiang, S.; Hu, Y. Expression of miR-126 and its potential function in coronary artery disease. Afr. Health Sci. 2017, 17, 474–480. [Google Scholar] [CrossRef][Green Version]
- Kumar, D.; Narang, R.; Sreenivas, V.; Rastogi, V.; Bhatia, J.; Saluja, D.; Srivastava, K. Circulatory miR-133b and miR-21 as Novel Biomarkers in Early Prediction and Diagnosis of Coronary Artery Disease. Genes 2020, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, N.; Fei, Z.; Qiu, J.; Ma, D.; Liu, X.; Cai, G.; Li, S. Analysis of plasma miR-208a and miR-370 expression levels for early diagnosis of coronary artery disease. Biomed. Rep. 2016, 5, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.D.; Cho, M.; Cai, X.P.; Cheng, J.; Jing, X.; Cen, J.M.; Liu, X.; Yang, X.L.; Suh, Y. A common variant in pre-miR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutat. Res. Mol. Mech. Mutagen. 2014, 761, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Hou, J.; Xuan, W.; Zhang, C.; Meng, X. The relationship of plasma miR-503 and coronary collateral circulation in patients with coronary artery disease. Life Sci. 2018, 207, 145–151. [Google Scholar] [CrossRef]
- Fan, J.L.; Zhang, L.; Bo, X.H. MiR-126 on mice with coronary artery disease by targeting S1PR2. Eur. Rev. Med. Pharmacol. 2020, 24, 893–904. [Google Scholar] [CrossRef]
- Han, H.; Qu, G.; Han, C.; Wang, Y.; Sun, T.; Li, F.; Wang, J.; Luo, S. MiR-34a, miR-21 and miR-23a as potential biomarkers for coronary artery disease: A pilot microarray study and confirmation in a 32 patient cohort. Exp. Mol. Med. 2015, 47, e138. [Google Scholar] [CrossRef]
- Lu, H.Q.; Liang, C.; He, Z.Q.; Fan, M.; Wu, Z.G. Circulating miR-214 is associated with the severity of coronary artery disease. J. Geriatr. Cardiol. 2013, 10, 34–38. [Google Scholar]
- Vindis, C.; Faccini, J.; Ruidavets, J.-B.; Cordelier, P.; Martins, F.; Maoret, J.-J.; Ferrieres, J.; Elbaz, M. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Atherosclerosis 2017, 263, E277. [Google Scholar] [CrossRef][Green Version]
- Sung, J.H.; Kim, S.H.; Yang, W.I.; Kim, W.J.; Moon, J.Y.; Kim, I.J.; Cha, D.H.; Cho, S.Y.; Kim, J.O.; Kim, K.A.; et al. miRNA polymorphisms (miR-146a, miR-149, miR-196a2 and miR-499) are associated with the risk of coronary artery disease. Mol. Med. Rep. 2016, 14, 2328–2342. [Google Scholar] [CrossRef]
- Gao, W.; He, H.W.; Wang, Z.M.; Zhao, H.; Lian, X.Q.; Wang, Y.S.; Zhu, J.; Yan, J.J.; Zhang, D.G.; Yang, Z.J.; et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012, 11, 55. [Google Scholar] [CrossRef]
- Andiappan, R.; Govindan, R.; Ramasamy, T.; Poomarimuthu, M. Circulating miR-133a-3p and miR-451a as potential biomarkers for diagnosis of coronary artery disease. Acta Cardiol. 2024, 79, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, Y.; Song, D.; Liu, B. Reduced Plasma miR-146a Is a Predictor of Poor Coronary Collateral Circulation in Patients with Coronary Artery Disease. Biomed. Res. Int. 2016, 2016, 4285942. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.R.; Gupta, H.; Bhushan, S.; Singh, A.; Roy, A.; Saini, N. Role of miR-128-3p and miR-195-5p as biomarkers of coronary artery disease in Indians: A pilot study. Sci. Rep.-Uk 2024, 14, 11881. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, X.; Liu, M.; Yun, L.; Cong, B. Diagnostic value of cardiac miR-126-5p, miR-134-5p, and miR-499a-5p in coronary artery disease-induced sudden cardiac death. Front. Cardiovasc. Med. 2022, 9, 944317. [Google Scholar] [CrossRef]
- Chen, B.R.; Luo, L.Y.; Wei, X.L.; Gong, D.; Jin, L.Z. Altered Plasma miR-144 as a Novel Biomarker for Coronary Artery Disease. Ann. Clin. Lab Sci. 2018, 48, 440–445. [Google Scholar]
- Kumar, D.; Narang, R.; Saluja, D.; Srivastava, K. Functional Association of miR-133b and miR-21 Through Novel Gene Targets ATG5, LRP6 and SGPP1 in Coronary Artery Disease. Mol. Diagn. Ther. 2022, 26, 655–664. [Google Scholar] [CrossRef]
- Elgebaly, S.A.; Christenson, R.H.; Kandil, H.; Ibrahim, M.; Rizk, H.; El-Khazragy, N.; Rashed, L.; Yacoub, B.; Eldeeb, H.; Ali, M.M.; et al. Nourin-Dependent miR-137 and miR-106b: Novel Biomarkers for Early Diagnosis of Myocardial Ischemia in Coronary Artery Disease Patients. Diagnostics 2021, 11, 703. [Google Scholar] [CrossRef]
- Takahashi, Y.; Satoh, M.; Minami, Y.; Tabuchi, T.; Itoh, T.; Nakamura, M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin. Sci. (Lond) 2010, 119, 395–405. [Google Scholar] [CrossRef]
- Ding, H.; Chen, W.; Chen, X. Serum miR-96-5p is a novel and non-invasive marker of acute myocardial infarction associated with coronary artery disease. Bioengineered 2022, 13, 3930–3943. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhong, W.; Zhang, Q.; Zhang, Q.; Yu, Z.; Wu, H. Circulating microRNA expression profiling and bioinformatics analysis of patients with coronary artery disease by RNA sequencing. J. Clin. Lab Anal. 2020, 34, e23020. [Google Scholar] [CrossRef]
- Hosseinpor, S.; Khalvati, B.; Safari, F.; Mirzaei, A.; Hosseini, E. The association of plasma levels of miR-146a, miR-27a, miR-34a, and miR-149 with coronary artery disease. Mol. Biol. Rep. 2022, 49, 3559–3567. [Google Scholar] [CrossRef]
- Mayr, B.; Muller, E.E.; Schafer, C.; Droese, S.; Schonfelder, M.; Niebauer, J. Exercise-induced changes in miRNA expression in coronary artery disease. Clin. Chem. Lab. Med. 2021, 59, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, A.; Zahedmehr, A.; Arabian, M.; Shakerian, F.; Maleki, M.; Oveisee, M.; Malakootian, M. MiR-6721-5p as a natural regulator of Meta-VCL is upregulated in the serum of patients with coronary artery disease. Non-Coding. Rna Res. 2025, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, T.; Ye, W.; Wang, J.Y.; Zhou, J.P.; Li, Z.Y.; Li, C.C. Circulating miR-182-5p and miR-5187-5p as biomarkers for the diagnosis of unprotected left main coronary artery disease. J. Thorac. Dis. 2019, 11, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Song, M.A.; Choe, D.; Xiao, D.; Foster, G.; Zhang, L. Early Detection of Coronary Artery Disease by Micro-RNA Analysis in Asymptomatic Patients Stratified by Coronary CT Angiography. Diagnostics 2020, 10, 875. [Google Scholar] [CrossRef]
- Li, H.; Gao, F.; Wang, X.; Wu, J.; Lu, K.; Liu, M.; Li, R.; Ding, L.; Wang, R. Circulating microRNA-378 levels serve as a novel biomarker for assessing the severity of coronary stenosis in patients with coronary artery disease. Biosci. Rep. 2019, 39, BSR20182016. [Google Scholar] [CrossRef]
- Azar Bahadori, R.; Shabani, D.; Arjmandrad, E.; Kazerani, M.; Rohani, M.; Ramazani Karim, Z.; Ali-Kheyl, M.; Nosratabadi, R.; Pourghadamyari, H.; Zaemi, M.A. Circulating miRNA-106b-5p As a Potential Biomarker for Coronary Artery Disease. Int. J. Mol. Cell Med. 2024, 13, 325–336. [Google Scholar] [CrossRef]
- Kanasniece, E.; Dauksaite, V.; Vilne, B.; Gailite, L.; Caunite, L.; Érglis, A.; Trusinskis, K. Circulating plasma microRNA expression profile: Potential minimally invasive biomarker for early detection of coronary artery disease development. Mol. Biol. Rep. 2025, 52, 927. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, S.; Liu, D.H.; Chen, L. Clinical Significance of MicroRNA-299-3p in Coronary Artery Disease Based on Bioinformatics Analysis. Cell Biochem. Biophys. 2024, 82, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Iacobescu, L.; Ciobanu, A.O.; Macarie, R.; Vadana, M.; Ciortan, L.; Tucureanu, M.M.; Butoi, E.; Simionescu, M.; Vinereanu, D. Diagnostic and Prognostic Role of Circulating microRNAs in Patients with Coronary Artery Disease-Impact on Left Ventricle and Arterial Function. Curr. Issues Mol. Biol. 2024, 46, 8499–8511. [Google Scholar] [CrossRef] [PubMed]
- Iusupova, A.O.; Pakhtusov, N.N.; Slepova, O.A.; Khabarova, N.V.; Privalova, E.V.; Bure, I.V.; Nemtsova, M.V.; Belenkov, Y.N. MiRNA-34a, miRNA-145, and miRNA-222 Expression, Matrix Metalloproteinases, TNF-alpha and VEGF in Patients with Different Phenotypes of Coronary Artery Disease. Int. J. Mol. Sci. 2024, 25, 12978. [Google Scholar] [CrossRef]
- Dogan, N.; Ozuynuk-Ertugrul, A.S.; Balkanay, O.O.; Yildiz, C.E.; Guclu-Geyik, F.; Kirsan, C.B.; Coban, N. Examining the effects of coronary artery disease- and mitochondrial biogenesis-related genes’ and microRNAs’ expression levels on metabolic disorders in epicardial adipose tissue. Gene 2024, 895, 147988. [Google Scholar] [CrossRef]
- Cordes, K.R.; Srivastava, D. MicroRNA regulation of cardiovascular development. Circ. Res. 2009, 104, 724–732. [Google Scholar] [CrossRef]
- Wu, J.-J.; Jin, J.; Li, Y.-H.; Wang, C.; Bai, J.; Jiang, Q.-J.; He, T.-X.; Nie, S.-J.; Li, D.-J.; Qu, L.-F. LncRNA FGF7-5 and lncRNA GLRX3 together inhibits the formation of carotid plaque via regulating the miR-2681-5p/ERCC4 axis in atherosclerosis. Cell Cycle 2023, 22, 165–182. [Google Scholar] [CrossRef]
- Wu, H.K.; Hui, Y.P.; Qian, X.K.; Wang, X.T.; Xu, J.W.; Wang, F.; Pan, S.S.; Chen, K.Y.; Liu, Z.; Gao, W.L.; et al. Exosomes derived from mesenchymal stem cells ameliorate impaired glucose metabolism in myocardial Ischemia/reperfusion injury through miR-132-3p/PTEN/AKT pathway. Cell Cycle 2024, 23, 893–912. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, F.; Zhang, F.; Lu, B. The potential roles of exosomes in pathological cardiomyocyte hypertrophy mechanisms and therapy: A review. Medicine 2024, 103, e37994. [Google Scholar] [CrossRef]
- Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.T. Overview of MicroRNAs in Cardiac Hypertrophy, Fibrosis, and Apoptosis. Int. J. Mol. Sci. 2016, 17, 749. [Google Scholar] [CrossRef]
- Caturano, A.; Vetrano, E.; Galiero, R.; Salvatore, T.; Docimo, G.; Epifani, R.; Alfano, M.; Sardu, C.; Marfella, R.; Rinaldi, L.; et al. Cardiac Hypertrophy: From Pathophysiological Mechanisms to Heart Failure Development. Rev. Cardiovasc. Med. 2022, 23, 165. [Google Scholar] [CrossRef]
- Wang, K.; Long, B.; Zhou, J.; Li, P.F. miR-9 and NFATc3 Regulate Myocardin in Cardiac Hypertrophy. J. Biol. Chem. 2010, 285, 11903–11912. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Y.; Wang, F.; Hou, J.; Cong, X.; Hu, S.; Chen, X. Reciprocal regulation of miR-23a and lysophosphatidic acid receptor signaling in cardiomyocyte hypertrophy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- da Costa Martins, P.A.; Salic, K.; Gladka, M.M.; Armand, A.-S.; Leptidis, S.; El Azzouzi, H.; Hansen, A.; Coenen-de Roo, C.J.; Bierhuizen, M.F.; Van Der Nagel, R. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010, 12, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.X.; Yuan, M.H.; Zhou, R.S.; Bai, Q.R.; Zhang, W.; Zhang, M.; Huang, Y.; Shi, L. MicroRNA-101 Inhibits Rat Cardiac Hypertrophy by Targeting Rab1a. J. Cardiovasc. Pharm. 2015, 65, 357–363. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Hu, Y.; Eschenbacher, W.H.; Dorn, L.E.; Dorn, G.W., 2nd. Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ. Res. 2012, 111, 521–531. [Google Scholar] [CrossRef]
- Li, R.T.; Yan, G.J.; Zhang, Q.; Jiang, Y.; Sun, H.X.; Hu, Y.L.; Sun, J.X.; Xu, B. miR-145 inhibits isoproterenol-induced cardiomyocyte hypertrophy by targeting the expression and localization of GATA6. Febs. Lett. 2013, 587, 1754–1761. [Google Scholar] [CrossRef]
- He, W.; Huang, H.; Xie, Q.; Wang, Z.; Fan, Y.; Kong, B.; Huang, D.; Xiao, Y. MiR-155 knockout in fibroblasts improves cardiac remodeling by targeting tumor protein p53-inducible nuclear protein 1. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 423–435. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Zhou, B.; Su, H.; Liu, Y.H.; Du, C. miR-150 regulates high glucose-induced cardiomyocyte hypertrophy by targeting the transcriptional co-activator p300. Exp. Cell Res. 2013, 319, 173–184. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Wang, W.; Tu, Y.; Eschenbacher, W.H.; Dorn, L.E.; Condorelli, G.; Diwan, A.; Nerbonne, J.M.; Dorn, G.W. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ. Res. 2010, 106, 166–175. [Google Scholar] [CrossRef]
- Ge, Y.; Pan, S.; Guan, D.; Yin, H.; Fan, Y.; Liu, J.; Zhang, S.; Zhang, H.; Feng, L.; Wang, Y. MicroRNA-350 induces pathological heart hypertrophy by repressing both p38 and JNK pathways. Bba-Mol Basis Dis. 2013, 1832, 1–10. [Google Scholar] [CrossRef]
- Adam, O.; Löhfelm, B.; Thum, T.; Gupta, S.K.; Puhl, S.-L.; Schäfers, H.-J.; Böhm, M.; Laufs, U. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res. Cardiol. 2012, 107, 278. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Gao, X.; Zhang, R.; Zhang, Y.; Liang, H.; Xu, C.; Du, W.; Zhang, Y.; Liu, X. MicroRNA-328 as a regulator of cardiac hypertrophy. Int. J. Cardiol. 2014, 173, 268–276. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Zhang, Y.; Xiao, H.; Sun, Q.; Hou, N.; Guo, S.L.; Wang, Y.L.; Fan, K.J.; Zhan, D.W.; et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2012, 22, 516–527. [Google Scholar] [CrossRef]
- Padeletti, L.; Bagliani, G. General Introduction, Classification, and Electrocardiographic Diagnosis of Cardiac Arrhythmias. Card. Electrophysiol. Clin. 2017, 9, 345–363. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Burashnikov, A. Mechanisms of cardiac arrhythmia. In Electrical Diseases of the Heart: Volume 1: Basic Foundations and Primary Electrical Diseases; Springer: Berlin/Heidelberg, Germany, 2013; pp. 93–128. [Google Scholar]
- Singh, B.N. Current antiarrhythmic drugs: An overview of mechanisms of action and potential clinical utility. J. Cardiovasc. Electrophysiol. 1999, 10, 283–301. [Google Scholar] [CrossRef]
- Wit, A.L.; Rosen, M.R. Pathophysiologic mechanisms of cardiac arrhythmias. Am. Heart J. 1983, 106, 798–811. [Google Scholar] [CrossRef]
- Tribulova, N.; Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Barancik, M. New aspects of pathogenesis of atrial fibrillation: Remodelling of intercalated discs. J. Physiol. Pharmacol. 2015, 66, 625–634. [Google Scholar]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Yamamoto, C.; Trayanova, N.A. Atrial fibrillation: Insights from animal models, computational modeling, and clinical studies. EBioMedicine 2022, 85, 104310. [Google Scholar] [CrossRef]
- Xintarakou, A.; Tzeis, S.; Psarras, S.; Asvestas, D.; Vardas, P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation facts and gaps. Europace 2020, 22, 342–351. [Google Scholar] [CrossRef]
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global epidemiology of atrial fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef]
- Chugh, S.S.; Roth, G.A.; Gillum, R.F.; Mensah, G.A. Global burden of atrial fibrillation in developed and developing nations. Glob Heart 2014, 9, 113–119. [Google Scholar] [CrossRef]
- Ferro, A.; Segreti, A.; Crispino, S.P.; Cricco, R.; Di Cristo, A.; Ciancio, M.; Gurrieri, F.; Ussia, G.P.; Grigioni, F. Exploring the Role of Genetic and Genomic Factors in Therapeutic Response to Heart Failure: A Comprehensive Analytical Review. Genes 2025, 16, 801. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Santulli, G.; Iaccarino, G.; De Luca, N.; Trimarco, B.; Condorelli, G. Atrial fibrillation and microRNAs. Front. Physiol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Su, X.; Liang, H.; Wang, H.; Chen, G.; Jiang, H.; Wu, Q.; Liu, T.; Liu, Q.; Yu, T.; Gu, Y.; et al. Over-expression of microRNA-1 causes arrhythmia by disturbing intracellular trafficking system. Sci. Rep.-Uk 2017, 7, 46259. [Google Scholar] [CrossRef]
- Lu, Y.M.; Hou, S.X.; Huang, D.M.; Luo, X.H.; Zhang, J.C.; Chen, J.; Xu, W.P. Expression profile analysis of circulating microRNAs and their effects on ion channels in Chinese atrial fibrillation patients. Int. J. Clin. Exp. Med. 2015, 8, 845–853. [Google Scholar]
- Jin, Y.; Zhou, T.Y.; Cao, J.N.; Feng, Q.T.; Fu, Y.J.; Xu, X.; Yang, C.J. MicroRNA-206 Downregulates Connexin43 in Cardiomyocytes to Induce Cardiac Arrhythmias in a Transgenic Mouse Model. Heart Lung Circ. 2019, 28, 1755–1761. [Google Scholar] [CrossRef]
- Shan, H.; Li, X.; Pan, Z.; Zhang, L.; Cai, B.; Zhang, Y.; Xu, C.; Chu, W.; Qiao, G.; Li, B. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br. J. Pharmacol. 2009, 158, 1227–1235. [Google Scholar] [CrossRef]
- Barana, A.; Matamoros, M.; Dolz-Gaiton, P.; Perez-Hernandez, M.; Amoros, I.; Nunez, M.; Sacristan, S.; Pedraz, A.; Pinto, A.; Fernandez-Aviles, F.; et al. Chronic atrial fibrillation increases microRNA-21 in human atrial myocytes decreasing L-type calcium current. Circ. Arrhythm. Electrophysiol. 2014, 7, 861–868. [Google Scholar] [CrossRef]
- Cheng, W.L.; Kao, Y.H.; Chao, T.F.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. MicroRNA-133 suppresses ZFHX3-dependent atrial remodelling and arrhythmia. Acta Physiol. 2019, 227, e13322. [Google Scholar] [CrossRef]
- Hedley, P.L.; Carlsen, A.L.; Christiansen, K.M.; Kanters, J.K.; Behr, E.R.; Corfield, V.A.; Christiansen, M. MicroRNAs in cardiac arrhythmia: DNA sequence variation of MiR-1 and MiR-133A in long QT syndrome. Scand. J. Clin. Lab. Investig. 2014, 74, 485–491. [Google Scholar] [CrossRef][Green Version]
- Chiang, D.Y.; Kongchan, N.; Beavers, D.L.; Alsina, K.M.; Voigt, N.; Neilson, J.R.; Jakob, H.; Martin, J.F.; Dobrev, D.; Wehrens, X.H.; et al. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ. Arrhythm. Electrophysiol. 2014, 7, 1214–1222. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, T.; Feng, Q.; Yang, J.; Cao, J.; Xu, X.; Yang, C. Inhibition of MicroRNA-206 Ameliorates Ischemia-Reperfusion Arrhythmia in a Mouse Model by Targeting Connexin43. J. Cardiovasc. Transl. Res. 2020, 13, 584–592. [Google Scholar] [CrossRef]
- Takahashi, K.; Sasano, T.; Sugiyama, K.; Kurokawa, J.; Tamura, N.; Soejima, Y.; Sawabe, M.; Isobe, M.; Furukawa, T. High-fat diet increases vulnerability to atrial arrhythmia by conduction disturbance via miR-27b. J. Mol. Cell Cardiol. 2016, 90, 38–46. [Google Scholar] [CrossRef]
- Moric-Janiszewska, E.; Smolik, S.; Morka, A.; Szydlowski, L.; Kapral, M. Expression levels of serum circulating microRNAs in pediatric patients with ventricular and supraventricular arrhythmias. Adv. Med. Sci. 2021, 66, 411–417. [Google Scholar] [CrossRef]
- Esfandyari, D.; Idrissou, B.M.G.; Hennis, K.; Avramopoulos, P.; Dueck, A.; El-Battrawy, I.; Gruter, L.; Meier, M.A.; Nager, A.C.; Ramanujam, D.; et al. MicroRNA-365 regulates human cardiac action potential duration. Nat. Commun. 2022, 13, 220. [Google Scholar] [CrossRef]
- Osbourne, A.; Calway, T.; Broman, M.; McSharry, S.; Earley, J.; Kim, G.H. Downregulation of connexin43 by microRNA-130a in cardiomyocytes results in cardiac arrhythmias. J. Mol. Cell Cardiol. 2014, 74, 53–63. [Google Scholar] [CrossRef]
- Blanco, R.R.; Austin, H.; Vest, R.N., 3rd; Valadri, R.; Li, W.; Lassegue, B.; Song, Q.; London, B.; Dudley, S.C.; Bloom, H.L.; et al. Angiotensin receptor type 1 single nucleotide polymorphism 1166A/C is associated with malignant arrhythmias and altered circulating miR-155 levels in patients with chronic heart failure. J. Card. Fail 2012, 18, 717–723. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Li, Z.; Fu, G. miR-1231 exacerbates arrhythmia by targeting calciumchannel gene CACNA2D2 in myocardial infarction. Am. J. Transl. Res. 2017, 9, 1822–1833. [Google Scholar]
- Xu, X.; Zhang, Q.; Song, H.; Ao, Z.; Li, X.; Cheng, C.; Shi, M.; Fu, F.; Sun, C.; Liu, Y.; et al. Effects of artemisinin on ventricular arrhythmias in response to left ventricular afterload increase and microRNA expression profiles in Wistar rats. PeerJ 2018, 6, e6110. [Google Scholar] [CrossRef]
- Yang, B.; Lin, H.; Xiao, J.; Lu, Y.; Luo, X.; Li, B.; Zhang, Y.; Xu, C.; Bai, Y.; Wang, H.; et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007, 13, 486–491. [Google Scholar] [CrossRef]
- Yi, J.; Chen, K.; Cao, Y.; Wen, C.; An, L.; Tong, R.; Wu, X.; Gao, H. Up-regulated novel-miR-17 promotes hypothermic reperfusion arrhythmias by negatively targeting Gja1 and mediating activation of the PKC/c-Jun signaling pathway. J. Mol. Cell Cardiol. 2024, 193, 1–10. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Wang, N.; Pan, Z.; Gao, X.; Zhang, F.; Zhang, Y.; Shan, H.; Luo, X.; Bai, Y.; et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation 2010, 122, 2378–2387. [Google Scholar] [CrossRef]
- Harling, L.; Lambert, J.; Ashrafian, H.; Darzi, A.; Gooderham, N.J.; Athanasiou, T. Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur. J. Cardio.-Thorac 2017, 51, 73–78. [Google Scholar] [CrossRef]
- Joseph, S.; Silbiger, V.N.; Fatah, M.; Chatterjee, D.; Rosa Neta, A.P.; Sacilotto, L.; D’Arezzo Pessente, G.; Darrieux, F.; Scanavacca, M.I.; Krieger, J.E.; et al. Diagnostic and prognostic significance of miRNA-15a-5p, 16–5p, and 92a-3p in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2025, in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Chu, W.; Wang, B.; Zhang, J.; Zhao, M.; Li, X.; Li, B.; Lu, Y.; Yang, B. Tanshinone IIA inhibits miR-1 expression through p38 MAPK signal pathway in post-infarction rat cardiomyocytes. Cell Physiol. Biochem. 2011, 26, 991–998. [Google Scholar] [CrossRef]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Wu, P.; Li, H.; Zhang, Y.; Feng, K.; Huang, H.; Zhang, J.; Lai, Y.; Pei, J.; et al. Burden and Temporal Trends of Valvular Heart Disease-Related Heart Failure From 1990 to 2019 and Projection Up to 2030 in Group of 20 Countries: An Analysis for the Global Burden of Disease Study 2019. J. Am. Heart Assoc. 2024, 13, e036462. [Google Scholar] [CrossRef]
- Oikonomou, E.; Siasos, G.; Tousoulis, D.; Kokkou, E.; Genimata, V.; Zisimos, K.; Latsios, G.; Stefanadis, C. Diagnostic and therapeutic potentials of microRNAs in heart failure. Curr. Top. Med. Chem. 2013, 13, 1548–1558. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wong, L.L.; Liew, O.W.; Richards, A.M. Heart Failure with Reduced Ejection Fraction (HFrEF) and Preserved Ejection Fraction (HFpEF): The Diagnostic Value of Circulating MicroRNAs. Cells 2019, 8, 1651. [Google Scholar] [CrossRef]
- Fayyaz, A.U.; Eltony, M.; Prokop, L.J.; Koepp, K.E.; Borlaug, B.A.; Dasari, S.; Bois, M.C.; Margulies, K.B.; Maleszewski, J.J.; Wang, Y. Pathophysiological insights into HFpEF from studies of human cardiac tissue. Nat. Rev. Cardiol. 2025, 22, 90–104. [Google Scholar] [CrossRef]
- Stoicescu, L.; Crisan, D.; Morgovan, C.; Avram, L.; Ghibu, S. Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. Int. J. Mol. Sci. 2024, 25, 794. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Saghiv, M.S.; Sagiv, M.S. Cardiovascular Function. In Basic Exercise Physiology: Clinical and Laboratory Perspectives; Springer: Berlin/Heidelberg, Germany, 2020; pp. 285–369. [Google Scholar]
- Endo, K.; Naito, Y.; Ji, X.; Nakanishi, M.; Noguchi, T.; Goto, Y.; Nonogi, H.; Ma, X.; Weng, H.; Hirokawa, G.; et al. MicroRNA 210 as a biomarker for congestive heart failure. Biol. Pharm. Bull. 2013, 36, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Batkai, S.; Beermann, J.; Bar, C.; Pfanne, A.; Thum, S.; Magnoli, M.; Balconi, G.; Nicolosi, G.L.; Tavazzi, L.; et al. Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur. J. Heart Fail 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Taubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Wang, X.; Song, C.; Zhou, X.; Han, X.; Li, J.; Wang, Z.; Shang, H.; Liu, Y.; Cao, H. Mitochondria Associated MicroRNA Expression Profiling of Heart Failure. Biomed. Res. Int. 2017, 2017, 4042509. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, Q.; Zhou, X.; Li, J.; Li, Y.; Zhang, L.; Zhou, Q.; Tang, B. Circulating miRNA-21 is a promising biomarker for heart failure. Mol. Med. Rep. 2017, 16, 7766–7774. [Google Scholar] [CrossRef]
- Tian, C.; Hu, G.; Gao, L.; Hackfort, B.T.; Zucker, I.H. Extracellular vesicular MicroRNA-27a* contributes to cardiac hypertrophy in chronic heart failure. J. Mol. Cell Cardiol. 2020, 143, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Zou, R.; Zhou, L.; Lim, J.Y.; Phua, D.C.Y.; Liu, C.; Chong, J.P.C.; Ng, J.Y.X.; Liew, O.W.; Chan, S.P.; et al. Combining Circulating MicroRNA and NT-proBNP to Detect and Categorize Heart Failure Subtypes. J. Am. Coll. Cardiol. 2019, 73, 1300–1313. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Y.J.; Sara, J.D.; Liu, L.J.; Liu, L.P.; Zhao, X.; Gao, H. Circulating MicroRNA-145 is Associated with Acute Myocardial Infarction and Heart Failure. Chinese Med. J.-Peking 2017, 130, 51–56. [Google Scholar] [CrossRef]
- Dong, S.; Ma, W.; Hao, B.; Hu, F.; Yan, L.; Yan, X.; Wang, Y.; Chen, Z.; Wang, Z. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int. J. Clin. Exp. Patho. 2014, 7, 565–574. [Google Scholar]
- Cardin, S.; Guasch, E.; Luo, X.; Naud, P.; Le Quang, K.; Shi, Y.; Tardif, J.C.; Comtois, P.; Nattel, S. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ. Arrhythm. Electrophysiol. 2012, 5, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.Q.; Jiang, Z.M.; Zhao, Q.P.; Xin, F. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomed. Pharmacother. 2015, 71, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Melman, Y.F.; Shah, R.; Danielson, K.; Xiao, J.; Simonson, B.; Barth, A.; Chakir, K.; Lewis, G.D.; Lavender, Z.; Truong, Q.A.; et al. Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 2015, 131, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- van Almen, G.C.; Verhesen, W.; van Leeuwen, R.E.; van de Vrie, M.; Eurlings, C.; Schellings, M.W.; Swinnen, M.; Cleutjens, J.P.; van Zandvoort, M.A.; Heymans, S.; et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef]
- Scrutinio, D.; Conserva, F.; Passantino, A.; Iacoviello, M.; Lagioia, R.; Gesualdo, L. Circulating microRNA-150-5p as a novel biomarker for advanced heart failure: A genome-wide prospective study. J. Heart Lung Transpl. 2017, 36, 616–624. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Qin, F.; Bai, F.; Sun, C.; Liu, Q. Expression of serum microRNA-155 and its clinical importance in patients with heart failure after myocardial infarction. J. Int. Med. Res. 2019, 47, 6294–6302. [Google Scholar] [CrossRef]
- Yang, H.; Shan, L.; Gao, Y.; Li, L.; Xu, G.; Wang, B.; Yin, X.; Gao, C.; Liu, J.; Yang, W. MicroRNA-181b Serves as a Circulating Biomarker and Regulates Inflammation in Heart Failure. Dis. Markers 2021, 2021, 4572282. [Google Scholar] [CrossRef]
- Marketou, M.; Kontaraki, J.; Zacharis, E.; Maragkoudakis, S.; Fragkiadakis, K.; Kampanieris, E.; Plevritaki, A.; Savva, E.; Malikides, O.; Chlouverakis, G.; et al. Peripheral Blood MicroRNA-21 as a Predictive Biomarker for Heart Failure With Preserved Ejection Fraction in Old Hypertensives. Am. J. Hypertens 2024, 37, 298–305. [Google Scholar] [CrossRef]
- Verjans, R.; Peters, T.; Beaumont, F.J.; van Leeuwen, R.; van Herwaarden, T.; Verhesen, W.; Munts, C.; Bijnen, M.; Henkens, M.; Diez, J.; et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload-Induced Heart Failure. Hypertension 2018, 71, 280–288. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Mone, P.; Avvisato, R.; Varzideh, F.; De Gennaro, S.; Salemme, L.; Macina, G.; Kansakar, U.; Cioppa, A.; Frullone, S.; et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: Validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech. Ageing Dev. 2023, 212, 111818. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, D.; Huang, Q.; Wu, J.; Xiang, Y.; Ou, B. Serum microRNA-125b-5p expression in patients with dilated cardiomyopathy combined with heart failure and its effect on myocardial fibrosis. Scand. Cardiovasc. J. 2024, 58, 2373083. [Google Scholar] [CrossRef]
- Wang, G.; Wang, R.; Ruan, Z.; Liu, L.; Li, Y.; Zhu, L. MicroRNA-132 attenuated cardiac fibrosis in myocardial infarction-induced heart failure rats. Biosci. Rep. 2020, 40, BSR20201696. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yao, J.; Xiao, N.; Han, Y.; Wu, X.; Ci, C.; Chen, K.; Geng, X. DNA methyltransferase 1 (DNMT1) suppresses mitophagy and aggravates heart failure via the microRNA-152-3p/ETS1/RhoH axis. Lab. Investig. 2022, 102, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Arul, J.C.; Raja Beem, S.S.; Parthasarathy, M.; Kuppusamy, M.K.; Rajamani, K.; Silambanan, S. Association of microRNA-210-3p with NT-proBNP, sST2, and Galectin-3 in heart failure patients with preserved and reduced ejection fraction: A cross-sectional study. PLoS ONE 2025, 20, e0320365. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhao, H.; Li, L.; Zhang, Z.Z.; Jiang, N.; Yang, X.W.; Zhang, T.; Lian, B.W.; Liu, Y.K.; Zhang, C.; et al. Sirt1 improves heart failure through modulating the NF-κB p65/microRNA-155/BNDF signaling cascade. Aging-Us 2021, 13, 14482–14498. [Google Scholar] [CrossRef]
- Mi, S.; Huang, F.; Jiao, M.; Qian, Z.; Han, M.; Miao, Z.; Zhan, H. Inhibition of MEG3 ameliorates cardiomyocyte apoptosis and autophagy by regulating the expression of miRNA-129-5p in a mouse model of heart failure. Redox Rep. 2023, 28, 2224607. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Marzocco, S.; De Gennaro, S.; Famiglietti, M.; Macina, G. Empagliflozin improves the MicroRNA signature of endothelial dysfunction in patients with heart failure with preserved ejection fraction and diabetes. J. Pharmacol. Exp. Ther. 2023, 384, 116–122. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef]
- Spahillari, A.; Jackson, L.; Varrias, D.; Michelhaugh, S.A.; Januzzi, J.L.; Shahideh, B.; Daghfal, D.; Valkov, N.; Murtagh, G.; Das, S. MicroRNAs are associated with cardiac biomarkers, cardiac structure and function and incident outcomes in heart failure. ESC Heart Fail. 2024, 11, 1400–1410. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Y.; Duan, Y.; Li, B.; Xia, J.; Yu, S.; Zhang, G. Comprehensive microRNA profiling reveals potential augmentation of the IL1 pathway in rheumatic heart valve disease. BMC Cardiovasc. Disord. 2018, 18, 53. [Google Scholar] [CrossRef]
- Chambers, J.B.; Bridgewater, B. Epidemiology of valvular heart disease. In Valvular Heart Disease: A Companion to Braunwald’s Heart Disease, 4th ed.; Saunders: Philadelphia, PA, USA, 2020; pp. 1–13. [Google Scholar]
- Shelbaya, K.; Claggett, B.; Dorbala, P.; Skali, H.; Solomon, S.D.; Matsushita, K.; Konety, S.; Mosley, T.H.; Shah, A.M. Stages of Valvular Heart Disease Among Older Adults in the Community: The Atherosclerosis Risk in Communities Study. Circulation 2023, 147, 638–649. [Google Scholar] [CrossRef]
- Lansakara, M.; Unai, S. An overview of aortic valve anatomy: The current understanding. Indian J. Thorac. Cardiovasc. Surg. 2023, 39, 246–252. [Google Scholar] [CrossRef]
- Morciano, G.; Boncompagni, C.; Ramaccini, D.; Pedriali, G.; Bouhamida, E.; Tremoli, E.; Giorgi, C.; Pinton, P. Comprehensive Analysis of Mitochondrial Dynamics Alterations in Heart Diseases. Int. J. Mol. Sci. 2023, 24, 3414. [Google Scholar] [CrossRef]
- Perrucci, G.L.; Zanobini, M.; Gripari, P.; Songia, P.; Alshaikh, B.; Tremoli, E.; Poggio, P. Pathophysiology of Aortic Stenosis and Mitral Regurgitation. Compr. Physiol. 2017, 7, 799–818. [Google Scholar] [CrossRef]
- Ohukainen, P.; Ruskoaho, H.; Rysa, J. Cellular Mechanisms of Valvular Thickening in Early and Intermediate Calcific Aortic Valve Disease. Curr. Cardiol. Rev. 2018, 14, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, U.; Ozcelik, F.; Yildiz, M.; Budak, M. Lipoprotein(a) Gene Polymorphism Increases a Risk Factor for Aortic Valve Calcification. J. Cardiovasc. Dev. Dis. 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Small, A.M.; Yutzey, K.E.; Binstadt, B.A.; Voigts Key, K.; Bouatia-Naji, N.; Milan, D.; Aikawa, E.; Otto, C.M.; St Hilaire, C.; American Heart Association Council on Genomic and Precision Medicine; et al. Unraveling the Mechanisms of Valvular Heart Disease to Identify Medical Therapy Targets: A Scientific Statement From the American Heart Association. Circulation 2024, 150, e109–e128. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lei, S.; Luo, X.; He, J.; Fang, Y.; Yang, H.; Liu, Y.; Deng, C.Y.; Wu, S.; Xue, Y.M.; et al. Construction of Prediction Model for Atrial Fibrillation with Valvular Heart Disease Based on Machine Learning. Rev. Cardiovasc. Med. 2022, 23, 247. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Wang, J.; Wee, A.S.; Yong, Q.-W.; Tay, E.L.-W.; Woo, C.C.; Sorokin, V.; Richards, A.M.; Ling, L.-H. Differential microRNA expression profile in myxomatous mitral valve prolapse and fibroelastic deficiency valves. Int. J. Mol. Sci. 2016, 17, 753. [Google Scholar] [CrossRef]
- Fabiani, I.; Scatena, C.; Mazzanti, C.M.; Conte, L.; Pugliese, N.R.; Franceschi, S.; Lessi, F.; Menicagli, M.; De Martino, A.; Pratali, S. Micro-RNA-21 (biomarker) and global longitudinal strain (functional marker) in detection of myocardial fibrotic burden in severe aortic valve stenosis: A pilot study. J. Transl. Med. 2016, 14, 248. [Google Scholar] [CrossRef][Green Version]
- Jan, M.I.; Khan, R.A.; Ali, T.; Bilal, M.; Bo, L.; Sajid, A.; Malik, A.; Urehman, N.; Waseem, N.; Nawab, J. Interplay of mitochondria apoptosis regulatory factors and microRNAs in valvular heart disease. Arch. Biochem. Biophys. 2017, 633, 50–57. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.H.; Zhang, X.W.; Song, Z.G.; Han, L.; He, Y.Y.; Xu, Z.Y. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J. Thorac. Cardiov. Sur. 2014, 147, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Bagardi, M.; Ghilardi, S.; Zamarian, V.; Ceciliani, F.; Brambilla, P.G.; Lecchi, C. Circulating MiR-30b-5p is upregulated in Cavalier King Charles Spaniels affected by early myxomatous mitral valve disease. PLoS ONE 2022, 17, e0266208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lin, S.; Li, X.; Guo, D.; Wang, Y.; Hu, Y. Serum miR-222 is independently associated with atrial fibrillation in patients with degenerative valvular heart disease. Bmc Cardiovasc. Disord. 2021, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- Songia, P.; Chiesa, M.; Alfieri, V.; Massaiu, I.; Moschetta, D.; Myasoedova, V.; Valerio, V.; Fusini, L.; Gripari, P.; Zanobini, M.; et al. Putative Circulating MicroRNAs Are Able to Identify Patients with Mitral Valve Prolapse and Severe Regurgitation. Int. J. Mol. Sci. 2021, 22, 2102. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, N.; Han, Y.; Zhang, C.; Zhang, H. miRNA Expression Profiling Uncovers a Role of miR-139-5p in Regulating the Calcification of Human Aortic Valve Interstitial Cells. Front. Genet. 2021, 12, 722564. [Google Scholar] [CrossRef]
- Yang, V.K.; Tai, A.K.; Huh, T.P.; Meola, D.M.; Juhr, C.M.; Robinson, N.A.; Hoffman, A.M. Dysregulation of valvular interstitial cell let-7c, miR-17, miR-20a, and miR-30d in naturally occurring canine myxomatous mitral valve disease. PLoS ONE 2018, 13, e0188617. [Google Scholar] [CrossRef]
- Petrkova, J.; Borucka, J.; Kalab, M.; Klevcova, P.; Michalek, J.; Taborsky, M.; Petrek, M. Increased Expression of miR-146a in Valvular Tissue From Patients With Aortic Valve Stenosis. Front. Cardiovasc. Med. 2019, 6, 86. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Y.; Zheng, D.D.; Xu, H.X.; Wang, T.; Pan, M.; Shi, J.H.; Zhu, J.H. M1 macrophages promote aortic valve calcification mediated by microRNA-214/TWIST1 pathway in valvular interstitial cells. Am. J. Transl. Res. 2016, 8, 5773–5783. [Google Scholar]
- Yang, L.M.; Wu, D.W.; Li, M.Q.; Zhu, X.Y.; Tian, Y.K.; Chen, Z.; Li, M.B.; Zhang, H.; Liang, D.G. Upregulation of microRNA-195 ameliorates calcific aortic valve disease by inhibiting VWF via suppression of the p38-MAPK signaling pathway. Int. J. Cardiol. 2020, 309, 101–107. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.; Gu, Y.; Yan, Y.; Ding, X.; Zou, L.; Xu, Z.; Wang, G. MicroRNA-22 promoted osteogenic differentiation of valvular interstitial cells by inhibiting CAB39 expression during aortic valve calcification. Cell Mol. Life Sci. 2022, 79, 146. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, B.; Lovren, F.; Pan, Y.; Garg, V.; Quan, A.; Tang, G.; Singh, K.K.; Shukla, P.C.; Kalra, N.P.; Peterson, M.D. miRNA-141 is a novel regulator of BMP-2–mediated calcification in aortic stenosis. J. Thorac Cardiov. Sur. 2012, 144, 256–262.e252. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhai, Y.; Ao, L.; Fullerton, D.A.; Meng, X. MicroRNA-204 deficiency in human aortic valves elevates valvular osteogenic activity. Int. J. Mol. Sci. 2019, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, J.; Liu, M.; Huang, Y.; Zhou, T.; Jiang, N.; Hui, H.; Xu, K. RNA-sequencing of human aortic valves identifies that miR-629-3p and TAGLN miRNA-mRNA pair involving in calcified aortic valve disease. J. Physiol. Biochem. 2022, 78, 819–831. [Google Scholar] [CrossRef]
- Sanchez-Garcia, A.d.J.; Soule-Egea, M.; Fuentevilla-Alvarez, G.; Vargas-Alarcon, G.; Hernández-Mejia, B.I.; Martínez-Hernández, H.; Mora-Canela, S.L.; Santibanez-Escobar, F.; Ávila-Martinez, V.; Castrejón-Tellez, V. Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis. Int. J. Mol. Sci. 2025, 26, 779. [Google Scholar] [CrossRef]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Xiang, X.; Niepmann, S.T.; Sedaghat, A.; Tiyerili, V.; Chennupati, R.; Moore IV, J.B.; Boon, R.A. Circulating microRNA-122-5p is associated with a lack of improvement in left ventricular function after transcatheter aortic valve replacement and regulates viability of cardiomyocytes through extracellular vesicles. Circulation 2022, 146, 1836–1854. [Google Scholar] [CrossRef]
- Ohukainen, P.; Syväranta, S.; Näpänkangas, J.; Rajamäki, K.; Taskinen, P.; Peltonen, T.; Helske-Suihko, S.; Kovanen, P.T.; Ruskoaho, H.; Rysä, J. MicroRNA-125b and chemokine CCL4 expression are associated with calcific aortic valve disease. Ann. Med. 2015, 47, 423–429. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Guo, J.; Sun, W.; Ji, Y.; Wang, Y.; Liu, J.; Kong, X. Overexpression of microRNA-93-5p and microRNA-374a-5p suppresses the osteogenic differentiation and mineralization of human aortic valvular interstitial cells through the BMP2/Smad1/5/RUNX2 signaling pathway. J. Cardiovasc. Pharm. 2023, 82, 138–147. [Google Scholar] [CrossRef]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef]
- Otto, C.M.; Newby, D.E.; Hillis, G.S. Calcific Aortic Stenosis: A Review. JAMA 2024, 332, 2014–2026. [Google Scholar] [CrossRef]
- Bulut, H.I.; Arjomandi Rad, A.; Syrengela, A.A.; Ttofi, I.; Djordjevic, J.; Kaur, R.; Keiralla, A.; Krasopoulos, G. A Comprehensive Review of Management Strategies for Bicuspid Aortic Valve (BAV): Exploring Epidemiology, Aetiology, Aortopathy, and Interventions in Light of Recent Guidelines. J. Cardiovasc. Dev. Dis. 2023, 10, 398. [Google Scholar] [CrossRef]
- Thrumurthy, S.G.; Karthikesalingam, A.; Patterson, B.O.; Holt, P.J.; Thompson, M.M. The diagnosis and management of aortic dissection. BMJ 2011, 344, d8290. [Google Scholar] [CrossRef] [PubMed]
- Jabagi, H.; Levine, D.; Gharibeh, L.; Camillo, C.; Castillero, E.; Ferrari, G.; Takayama, H.; Grau, J.B. Implications of Bicuspid Aortic Valve Disease and Aortic Stenosis/Insufficiency as Risk Factors for Thoracic Aortic Aneurysm. Rev. Cardiovasc. Med. 2023, 24, 178. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; He, S.-Q.; Shan, S.-K.; Xu, F.; Wu, F.; Li, F.-X.-Z.; Zheng, M.-H.; Lei, L.-M.; Duan, J.-Y.; Wu, Y.-Y. Endothelial cells derived extracellular vesicles promote diabetic arterial calcification via circ_0008362/miR-1251-5p/Runx2 axial. Cardiovasc. Diabetol. 2024, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef]
- Rapezzi, C.; Aimo, A.; Barison, A.; Emdin, M.; Porcari, A.; Linhart, A.; Keren, A.; Merlo, M.; Sinagra, G. Restrictive cardiomyopathy: Definition and diagnosis. Eur. Heart J. 2022, 43, 4679–4693. [Google Scholar] [CrossRef]
- Marian, A.J. Hypertrophic cardiomyopathy: From genetics to treatment. Eur. J. Clin. Investig. 2010, 40, 360–369. [Google Scholar] [CrossRef]
- Pasipoularides, A. Morphomechanic phenotypic variability of sarcomeric cardiomyopathies: A multifactorial polygenic perspective. J. Mol. Cell Cardiol. 2019, 126, 23–35. [Google Scholar] [CrossRef]
- Borjesson, M.; Pelliccia, A. Incidence and aetiology of sudden cardiac death in young athletes: An international perspective. Br. J. Sports Med. 2009, 43, 644–648. [Google Scholar] [CrossRef]
- Ackerman, M.; Atkins, D.L.; Triedman, J.K. Sudden Cardiac Death in the Young. Circulation 2016, 133, 1006–1026. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Q.; Li, S.; Luo, J. Mesenchymal stem cells ameliorate inflammation and pyroptosis in diabetic cardiomyopathy via the miRNA-223-3p/NLRP3 pathway. Diabetol. Metab. Syndr. 2024, 16, 146. [Google Scholar] [CrossRef]
- Roncarati, R.; Viviani Anselmi, C.; Losi, M.A.; Papa, L.; Cavarretta, E.; Da Costa Martins, P.; Contaldi, C.; Saccani Jotti, G.; Franzone, A.; Galastri, L. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927. [Google Scholar] [CrossRef]
- Jiao, M.; You, H.-Z.; Yang, X.-Y.; Yuan, H.; Li, Y.-L.; Liu, W.-X.; Jin, M.; Du, J. Circulating microRNA signature for the diagnosis of childhood dilated cardiomyopathy. Sci. Rep. 2018, 8, 724. [Google Scholar] [CrossRef]
- Halkein, J.; Tabruyn, S.R.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.Q.N.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M.; et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013, 123, 2143–2154. [Google Scholar] [CrossRef]
- Li, X.; Du, N.; Zhang, Q.; Li, J.; Chen, X.; Liu, X.; Hu, Y.; Qin, W.; Shen, N.; Xu, C. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014, 5, e1479. [Google Scholar] [CrossRef]
- Fang, L.; Ellims, A.H.; Moore, X.L.; White, D.A.; Taylor, A.J.; Chin-Dusting, J.; Dart, A.M. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J. Transl. Med. 2015, 13, 314. [Google Scholar] [CrossRef]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Tabuchi, T.; Nakamura, M. Expression of microRNA-208 is Associated With Adverse Clinical Outcomes in Human Dilated Cardiomyopathy. J. Card. Fail. 2010, 16, 404–410. [Google Scholar] [CrossRef]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.-R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef]
- Kuster, D.W.; Mulders, J.; Ten Cate, F.J.; Michels, M.; Dos Remedios, C.G.; da Costa Martins, P.A.; van der Velden, J.; Oudejans, C.B. MicroRNA transcriptome profiling in cardiac tissue of hypertrophic cardiomyopathy patients with MYBPC3 mutations. J. Mol. Cell Cardiol. 2013, 65, 59–66. [Google Scholar] [CrossRef]
- Kuehl, U.; Lassner, D.; Gast, M.; Stroux, A.; Rohde, M.; Siegismund, C.; Wang, X.; Escher, F.; Gross, M.; Skurk, C. Differential cardiac microRNA expression predicts the clinical course in human enterovirus cardiomyopathy. Circ. Heart Fail. 2015, 8, 605–618. [Google Scholar] [CrossRef]
- Bonet, F.; Hernandez-Torres, F.; Ramos-Sanchez, M.; Quezada-Feijoo, M.; Bermudez-Garcia, A.; Daroca, T.; Alonso-Villa, E.; Garcia-Padilla, C.; Mangas, A.; Toro, R. Unraveling the Etiology of Dilated Cardiomyopathy through Differential miRNA-mRNA Interactome. Biomolecules 2024, 14, 524. [Google Scholar] [CrossRef]
- Sun, D.D.; Li, C.; Liu, J.L.; Wang, Z.G.; Liu, Y.; Luo, C.; Chen, Y.Y.; Wen, S.J. Expression Profile of microRNAs in Hypertrophic Cardiomyopathy and Effects of microRNA-20 in Inducing Cardiomyocyte Hypertrophy Through Regulating Gene. DNA Cell Biol. 2019, 38, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, U.; Budak, M.; Gurdogan, M.; Ozturk, G.; Yildiz, M.; Taylan, G.; Altay, S.; Yalta, K. KCNQ1 Polymorphism in the Context of Ischemic Cardiomyopathy: A Potential Key to Decision-Making for Device Implantation. Clin. Cardiol. 2025, 48, e70148. [Google Scholar] [CrossRef] [PubMed]
- Guha, K.; McDonagh, T.A. The epidemiology of heart failure. Oxf. Textb. Heart Fail. 2011, 3960, 19. [Google Scholar] [CrossRef]
- Bironaite, D.; Daunoravicius, D.; Bogomolovas, J.; Cibiras, S.; Vitkus, D.; Zurauskas, E.; Zasytyte, I.; Rucinskas, K.; Labeit, S.; Venalis, A.; et al. Molecular mechanisms behind progressing chronic inflammatory dilated cardiomyopathy. Bmc Cardiovasc. Disord. 2015, 15, 26. [Google Scholar] [CrossRef]
- Elsaka, O. Exploring Emerging and Innovative Biomarkers for the Assessment and Monitoring of Cardiovascular Disease Risk and Treatment Efficacy in Patients. APIK J. Intern. Med. 2025, 13, 94–101. [Google Scholar] [CrossRef]
- Guo, R.; Weingärtner, S.; Šiurytė, P.; Stoeck, C.T.; Füetterer, M.; Campbell-Washburn, A.E.; Suinesiaputra, A.; Jerosch-Herold, M.; Nezafat, R. Emerging Techniques in Cardiac Magnetic Resonance Imaging. J. Magn. Reson. Imaging 2022, 55, 1043–1059. [Google Scholar] [CrossRef]
- Belmonte, T.; Mangas, A.; Calderon-Dominguez, M.; Quezada-Feijoo, M.; Ramos, M.; Campuzano, O.; Gomez, S.; Pena, M.L.; Cubillos-Arango, A.M.; Dominguez, F.; et al. Peripheral microRNA panels to guide the diagnosis of familial cardiomyopathy. Transl. Res. 2020, 218, 1–15. [Google Scholar] [CrossRef]
- Bui, Q.M.; Ding, J.; Hong, K.N.; Adler, E.A. The Genetic Evaluation of Dilated Cardiomyopathy. Struct. Heart 2023, 7, 100200. [Google Scholar] [CrossRef]
- Bracamonte-Baran, W.; Cihakova, D. Cardiac Autoimmunity: Myocarditis. Adv. Exp. Med. Biol. 2017, 1003, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Pinamonti, B.; Brun, F.; Mestroni, L.; Sinagra, G. Arrhythmogenic right ventricular cardiomyopathy: From genetics to diagnostic and therapeutic challenges. World J. Cardiol. 2014, 6, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, E.I.; Procopio, A.; Scalise, S.; Esposito, C.; Nicoletta, G.; Santamaria, G.; De Angelis, M.T.; Dorn, T.; Moretti, A.; Laugwitz, K.L.; et al. Deciphering the Role of Wnt and Rho Signaling Pathway in iPSC-Derived ARVC Cardiomyocytes by In Silico Mathematical Modeling. Int. J. Mol. Sci. 2021, 22, 2004. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, F.; Chen, X.; Rao, M.; Zhang, N.N.; Chen, K.; Deng, H.T.; Song, J.P.; Hu, S.S. Comprehensive Myocardial Proteogenomics Profiling Reveals C/EBPα as the Key Factor in the Lipid Storage of ARVC. J. Proteome Res. 2017, 16, 2863–2876. [Google Scholar] [CrossRef]
- James, C.A.; Syrris, P.; van Tintelen, J.P.; Calkins, H. The role of genetics in cardiovascular disease: Arrhythmogenic cardiomyopathy. Eur. Heart J. 2020, 41, 1393–1400. [Google Scholar] [CrossRef]
- Gerull, B.; Brodehl, A. Insights Into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail. Rep. 2021, 18, 378–390. [Google Scholar] [CrossRef]
- Fakih, Y.; Al Sakan, M.; Fawaz, N.; El Ghazawi, A.; Fawaz, A.; Refaat, M.M. Restrictive Cardiomyopathy: A Comprehensive Literature Review. Ann. Clin. Cardiol. 2025, 7, 5–14. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, X.; Shan, H.; Wang, N.; Wang, J.; Ren, J.; Feng, S.; Xie, L.; Lu, C.; Yuan, Y. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway. Circulation 2012, 126, 840–850. [Google Scholar] [CrossRef]
- Hinkel, R.; Batkai, S.; Bahr, A.; Bozoglu, T.; Straub, S.; Borchert, T.; Viereck, J.; Howe, A.; Hornaschewitz, N.; Oberberger, L.; et al. AntimiR-132 Attenuates Myocardial Hypertrophy in an Animal Model of Percutaneous Aortic Constriction. J. Am. Coll. Cardiol. 2021, 77, 2923–2935. [Google Scholar] [CrossRef]
- Bueno Marinas, M.; Celeghin, R.; Cason, M.; Bariani, R.; Frigo, A.C.; Jager, J.; Syrris, P.; Elliott, P.M.; Bauce, B.; Thiene, G.; et al. A microRNA Expression Profile as Non-Invasive Biomarker in a Large Arrhythmogenic Cardiomyopathy Cohort. Int. J. Mol. Sci. 2020, 21, 1536. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Mangas, A.; Belmonte, T.; Quezada-Feijoo, M.; Ramos, M.; Toro, R. Ischemic dilated cardiomyopathy pathophysiology through microRNA-16-5p. Rev. Esp. Cardiol. 2021, 74, 740–749. [Google Scholar] [CrossRef]
- Ferreira, L.R.P.; Frade, A.F.; Santos, R.H.B.; Teixeira, P.C.; Baron, M.A.; Navarro, I.C.; Benvenuti, L.A.; Fiorelli, A.I.; Bocchi, E.A.; Stolf, N.A.; et al. MicroRNAs are dysregulated in Chronic Chagas disease Cardiomyopathy. Int. J. Cardiol. 2014, 175, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hailu, F.T.; Karimpour-Fard, A.; Toni, L.S.; Bristow, M.R.; Miyamoto, S.D.; Stauffer, B.L.; Sucharov, C.C. Integrated analysis of miRNA-mRNA interaction in pediatric dilated cardiomyopathy. Pediatr. Res. 2022, 92, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, A.; Oikonomou, E.; Vogiatzi, G.; Antonopoulos, A.; Tsalamandris, S.; Georgakopoulos, C.; Papanikolaou, P.; Lazaros, G.; Charalambous, G.; Siasos, G.; et al. MicroRNAs as Biomarkers in Hypertrophic Cardiomyopathy: Current State of the Art. Curr. Med. Chem. 2021, 28, 7400–7412. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wei, Y.; He, J.; Feng, B.; Chen, Y.; Guo, R.; Griffin, M.D.; Hynes, S.O.; Shen, S.; Liu, Y.; et al. Human umbilical cord-derived mesenchymal stromal cells improve myocardial fibrosis and restore miRNA-133a expression in diabetic cardiomyopathy. Stem. Cell Res. Ther. 2024, 15, 120. [Google Scholar] [CrossRef]
- Houyel, L.; Meilhac, S.M. Heart Development and Congenital Structural Heart Defects. Annu. Rev. Genomics Hum. Genet. 2021, 22, 257–284. [Google Scholar] [CrossRef]
- Gorla, S.R.; Chakraborty, A.; Garg, A.; Gugol, R.A.; Kardon, R.E.; Swaminathan, S. Emerging trends in the prenatal diagnosis of complex CHD and its influence on infant mortality in this cohort. Cardiol. Young 2019, 29, 270–276. [Google Scholar] [CrossRef]
- Pass, R.H.; Cohen, J. Tetralogy of fallot. In Pediatric Cardiology: Fetal, Pediatric, and Adult Congenital Heart Diseases; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1727–1749. [Google Scholar]
- Abumehdi, M.; Al Nasef, M.; Mehta, C.; Botha, P.; McMahon, C.; Oslizlok, P.; Walsh, K.P.; McCrossan, B.; Kenny, D.; Stümper, O. Short to medium term outcomes of right ventricular outflow tract stenting as initial palliation for symptomatic infants with complete atrioventricular septal defect with associated tetralogy of Fallot. Catheter. Cardiovasc. Interv. 2020, 96, 1445–1453. [Google Scholar] [CrossRef]
- Olney, R.S.; Ailes, E.C.; Sontag, M.K. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Semin. Perinatol. 2015, 39, 230–237. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Brueckner, M.; Chung, W.K.; Garg, V.; Lacro, R.V.; McGuire, A.L.; Mital, S.; Priest, J.R.; Pu, W.T.; Roberts, A.; et al. Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e653–e711. [Google Scholar] [CrossRef]
- Stark, C.M.; Hughes, B.N.; Schacht, J.P.; Urbina, T.M. Decoding Hearts: Genetic Insights and Clinical Strategies in Congenital Heart Disease. Neoreviews 2025, 26, e73–e88. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.; Decker, E.S.; Plank, A.C.; Mestermann, S.; Purbojo, A.; Cesnjevar, R.A.; Kratz, O.; Eichler, A. Long-Term Effects of Child Early Surgical Ventricular Septal Defect Repair on Maternal Stress. Children 2023, 10, 1832. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, Z.; Li, H.; Yao, X.; Peng, C.; Qi, Y.; Wu, B.; Zhao, T.; Li, C.; Shen, J.; et al. Elevated microRNA-187 causes cardiac endothelial dysplasia to promote congenital heart disease through inhibition of NIPBL. J. Clin. Investig. 2024, 135, 1. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, F.; Khatami, M.; Heidari, M.M.; Bragança, J.; Tatari, H.; Namnabat, M.; Hadadzadeh, M.; Navabi Shirazi, M.A. Novel and deleterious nucleotide variations in the HAND1 gene probably affect miRNA target sites and protein function in pediatric patients with congenital heart disease. Mol. Biol. Rep. 2024, 51, 468. [Google Scholar] [CrossRef]
- Li, D.; Ji, L.; Liu, L.; Liu, Y.; Hou, H.; Yu, K.; Sun, Q.; Zhao, Z. Characterization of circulating microRNA expression in patients with a ventricular septal defect. PLoS ONE 2014, 9, e106318. [Google Scholar] [CrossRef]
- González-Moyotl, N.; Huesca-Gómez, C.; Torres-Paz, Y.E.; Fuentevilla-Álvarez, G.; Romero-Maldonado, S.; Sámano, R.; Soto, M.E.; Martínez-Rosas, M.; Domínguez-López, A.; Gamboa, R. Paediatrics congenital heart disease is associated with plasma miRNAs. Pediatr. Res. 2024, 96, 1220–1227. [Google Scholar] [CrossRef]
- Wang, L.; Tian, D.; Hu, J.; Xing, H.; Sun, M.; Wang, J.; Jian, Q.; Yang, H. MiRNA-145 regulates the development of congenital heart disease through targeting FXN. Pediatr. Cardiol. 2016, 37, 629–636. [Google Scholar] [CrossRef]
- Wu, K.-H.; Xiao, Q.-R.; Yang, Y.; Xu, J.-L.; Zhang, F.; Liu, C.-M.; Zhang, Z.-M.; Lu, Y.-Q.; Huang, N.-P. MicroRNA-34a modulates the Notch signaling pathway in mice with congenital heart disease and its role in heart development. J. Mol. Cell Cardiol. 2018, 114, 300–308. [Google Scholar] [CrossRef]
- Kang, Z.; Li, Z.; Huang, P.; Luo, J.; Liu, P.; Wang, Y.; Xia, T.; Zhou, Y. Remote ischemic preconditioning upregulates microRNA-21 to protect the kidney in children with congenital heart disease undergoing cardiopulmonary bypass. Pediatr. Nephrol. 2018, 33, 911–919. [Google Scholar] [CrossRef]
- Zhu, S.; Cao, L.; Zhu, J.; Kong, L.; Jin, J.; Qian, L.; Zhu, C.; Hu, X.; Li, M.; Guo, X. Identification of maternal serum microRNAs as novel non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Clin. Chim. Acta 2013, 424, 66–72. [Google Scholar] [CrossRef]
- Ramachandran, V.; Bhagavatheeswaran, S.; Shanmugam, S.; Vasudevan, M.; Ragunathan, M.; Cherian, K.M.; Munirajan, A.K.; Ravi, S.; Balakrishnan, A. Deep sequencing unveils altered cardiac miRNome in congenital heart disease. Mol. Genet. Genom. 2022, 297, 1123–1139. [Google Scholar] [CrossRef]
- Hu, C.; Huang, S.; Wu, F.; Ding, H. MicroRNA-219-5p participates in cyanotic congenital heart disease progression by regulating cardiomyocyte apoptosis. Exp. Ther. Med. 2021, 21, 36. [Google Scholar] [CrossRef]
- Chang, W.T.; Lee, W.C.; Lin, Y.W.; Shih, J.Y.; Hong, C.S.; Chen, Z.C.; Chu, C.Y.; Hsu, C.H. Transpulmonary Expression of Exosomal microRNAs in Idiopathic and Congenital Heart Disease–Related Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2023, 12, e031435. [Google Scholar] [CrossRef]
- Kong, D.; Li, H.; Ren, C.; Yang, W.; Zhang, Z. MicroRNAs in Fetal umbilical cord blood as a prenatal screening tool for congenital heart disease. Ann. Clin. Lab. Sci. 2021, 51, 705–712. [Google Scholar]
- Shen, Y.; Liao, D.; Shangguan, W.; Chen, L. Variation and significance of serum microRNA-21 level in pediatric pulmonary artery hypertension associated with congenital heart disease. Front. Cardiovasc. Med. 2024, 11, 1424679. [Google Scholar] [CrossRef] [PubMed]
- Grunert, M.; Appelt, S.; Dunkel, I.; Berger, F.; Sperling, S.R. Altered microRNA and target gene expression related to Tetralogy of Fallot. Sci. Rep. 2019, 9, 19063. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Hu, D.Q.; Zhao, M.; Ichimura, S.; Barnes, E.A.; Cornfield, D.N.; Alejandre Alcázar, M.A.; Spiekerkoetter, E.; Fajardo, G.; Bernstein, D. MicroRNA-34a-Dependent Attenuation of Angiogenesis in Right Ventricular Failure. J. Am. Heart Assoc. 2024, 13, e029427. [Google Scholar] [CrossRef]
- Bittel, D.C.; Kibiryeva, N.; Marshall, J.A.; O’Brien, J.E., Jr. MicroRNA-421 dysregulation is associated with tetralogy of fallot. Cells 2014, 3, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Yan, Z.; Huang, K.; Jiang, Y.; Zhang, L. MicroRNA expression, target genes, and signaling pathways in infants with a ventricular septal defect. Mol. Cell Biochem. 2018, 439, 171–187. [Google Scholar] [CrossRef]
- Zhang, W.; Hua, Y.; Zheng, D.; Chen, Q.; Huang, R.; Wang, W.; Li, X. Expression and clinical significance of miR-8078 in patients with congenital heart disease-associated pulmonary arterial hypertension. Gene 2024, 896, 147964. [Google Scholar] [CrossRef]
- Paul, M.H.; Harvey, R.P.; Wegner, M.; Sock, E. Cardiac outflow tract development relies on the complex function of Sox4 and Sox11 in multiple cell types. Cell Mol. Life Sci. 2014, 71, 2931–2945. [Google Scholar] [CrossRef]
- Knowles, R.L.; Bull, C.; Wren, C.; Dezateux, C. Mortality with congenital heart defects in England and Wales, 1959-2009: Exploring technological change through period and birth cohort analysis. Arch. Dis. Child 2012, 97, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S.; Liberthson, R.R. Prevalence of congenital heart disease. Am. Heart J. 2004, 147, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Karakikes, I.; Chaanine, A.H.; Kang, S.J.; Mukete, B.N.; Jeong, D.T.; Zhang, S.H.; Hajjar, R.J.; Lebeche, D. Therapeutic Cardiac-Targeted Delivery of Reverses Pressure Overload-Induced Cardiac Hypertrophy and Attenuates Pathological Remodeling. J. Am. Heart Assoc. 2013, 2, e000078. [Google Scholar] [CrossRef] [PubMed]
- Callis, T.E.; Pandya, K.; Seok, H.Y.; Tang, R.-H.; Tatsuguchi, M.; Huang, Z.-P.; Chen, J.-F.; Deng, Z.; Gunn, B.; Shumate, J. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar] [CrossRef]
- Deacon, D.C.; Nevis, K.R.; Cashman, T.J.; Zhou, Y.; Zhao, L.; Washko, D.; Guner-Ataman, B.; Burns, C.G.; Burns, C.E. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development 2010, 137, 1887–1896. [Google Scholar] [CrossRef]
- Liang, D.; Xu, X.; Deng, F.; Feng, J.; Zhang, H.; Liu, Y.; Zhang, Y.; Pan, L.; Liu, Y.; Zhang, D. mi RNA-940 reduction contributes to human Tetralogy of Fallot development. J. Cell Mol. Med. 2014, 18, 1830–1839. [Google Scholar] [CrossRef]
- Liu, J.; van Mil, A.; Vrijsen, K.; Zhao, J.; Gao, L.; Metz, C.H.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J. Cell Mol. Med. 2011, 15, 1474–1482. [Google Scholar] [CrossRef]
- Rao, P.K.; Toyama, Y.; Chiang, H.R.; Gupta, S.; Bauer, M.; Medvid, R.; Reinhardt, F.; Liao, R.; Krieger, M.; Jaenisch, R.; et al. Loss of Cardiac microRNA-Mediated Regulation Leads to Dilated Cardiomyopathy and Heart Failure. Circ. Res. 2009, 105, 585–594. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Meese, E.; Keller, A.; Abdul-Khaliq, H.; Rädle-Hurst, T. Analysis of circulating microRNAs in patients with repaired Tetralogy of Fallot with and without heart failure. J. Transl. Med. 2017, 15, 156. [Google Scholar] [CrossRef]
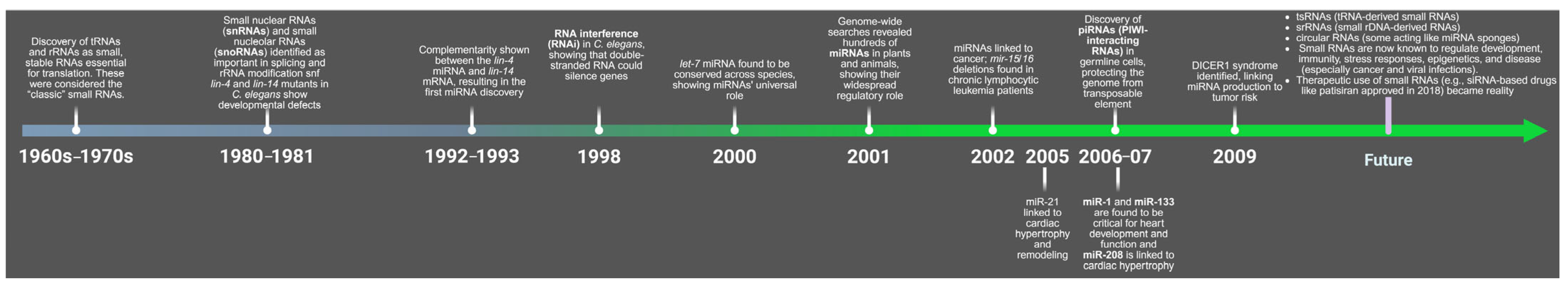
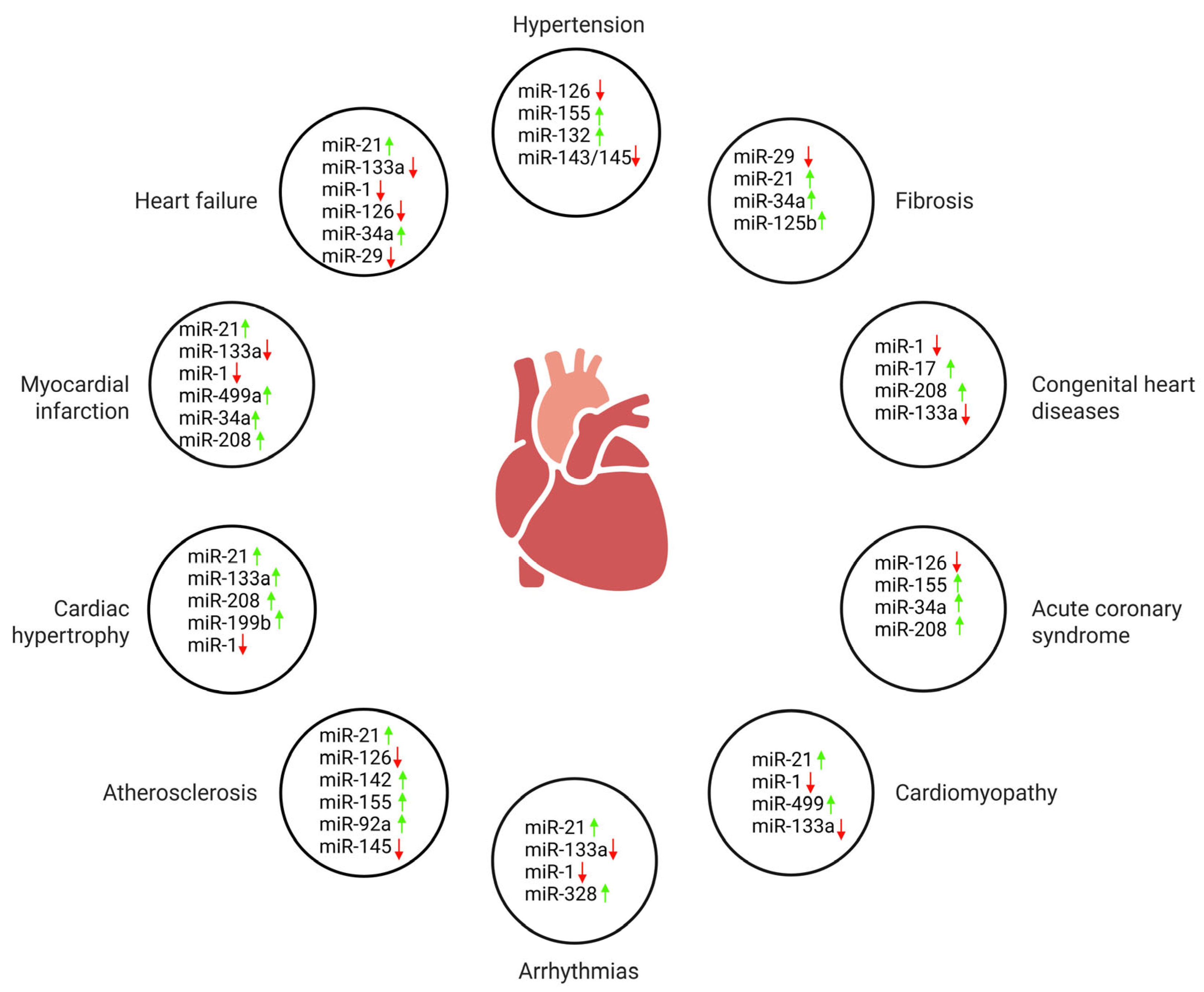
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Neonatal rat cardiomyocytes | ↑ miR-30b targets ↓ Bcl-2 | [20] | Sprague–Dawley rat cardiomyocytes | ↑ miR-15b targets ↓ Bcl-2 affects cytochrome c and caspase-3/9. | [21] | H9c2 rat cardiomyocytes | ↓ miR-101 inhibition leads to ↑ RAB5A →↑ autophagy →↓ apoptosis | [22] |
| Neonatal rat cardiomyocytes | ↑ miR-21 targets ↓ PDCD4 | [23] | Rat cardiomyocytes | ↑ miR-133 targets ↓ caspase-9, ↓ caspase-3 | [24] | Cardiac myocytes | ↓ miR-199a targets ↑ Hif-1α, ↑ Sirt1 | [25] |
| Mouse cardiomyocytes | ↓ miR-24 in MI (↑ when treated) targets ↓ Bim | [26] | Rat (in vivo ischemia model and H9c2 | ↓ miR-378 in ischemia (↑ when treated) targets ↓ caspase-3. | [27] | Rats and neonatal rat cardiomyocytes | ↓ miR-1, ↓ miR-133a in IR (↑ with IPost); miR-133a targets ↓ CASP9 | [28] |
| Cultured cardiac myocytes | ↑ miR-21 targets ↓ PDCD4 → affects AP-1 signaling | [29] | Isolated cardiomyocytes and in vivo models (mouse/rat) | ↓ miR-30 targets ↓ β1AR, β2AR, Giα-2, ↓ BNIP3L/NIX | [30] | H9c2 rat cardiomyocytes | ↑ miR-34a targets ↓ Bcl-2 | [31] |
| Rat model and H9C2 cardiomyocytes | ↑ miR-21 targets ↓ PTEN →↑ Akt →↑ Bcl-2/Bax →↓ Caspase-3 | [32] | Neonatal mouse cardiomyocytes | ↑ miR-100 targets ↓ IGF1R; ↓ miR-100 →↑ IGF1R →↓ apoptosis | [33] | H9C2 rat cardiomyocytes | ↑ miR-1 targets ↓ IGF-1 | [34] |
| Cultured cells | ↓ miR-30 targets ↓ p53 →↓ Drp1 →↓ mitochondrial fission | [35] | Rat I/R model and cultured cardiomyocytes | ↑ miR-1 targets ↓ Bcl-2 | [36] | Cultured cardiomyocytes | ↑ HIF-1α →↑ miR-21 (feedback loop); miR-21 modulates ↓ PTEN →↑ Akt | [37] |
| Cultured cardiomyocytes | ↑ miR-145 targets ↓ CaMKIIδ | [38] | Neonatal rat cardiomyocytes | ↑ miR-20a (targets ↓ Egln3/PHD3) | [39] | Rat H9c2 cardiomyocytes | ↓ miR-92a (targets ↑ Smad7 →↓ NF-κB p65 →↓ apoptosis) | [40] |
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Human | ↓ miR-126 in CAD patients; ↑ PLGF in UAP and AMI patients. | [55] | Human | ↓ miR-133b and ↑ miR-21 in CAD patients; both correlated with disease severity. | [56] | Human | ↑ miR-208a (p = 0.006) and ↑ miR-370 (p = 0.003) in patients. | [57] |
| Human | miR-146a rs2910164 GC and CC genotypes ↑ CAD risk and ↑ mature miRNA expression. | [58] | Human | ↓ miR-503 in both CCC groups vs. controls; lowest in good CCC group; ↑ VEGF-A in good CCC group. | [59] | Human, Mouse (ApoE−/−) and HUVECs | ↑ miR-126 reduced atherosclerotic plaques, ↓ TNF-α, ↓ IL-1β, ↑ IL-10; miR-126 targets S1PR2. | [60] |
| Mouse (apoE−/−), Human | ↑ miR-34a, miR-21 and miR-23a in CAD patients; differentially expressed miRNAs confirmed in apoE−/− mice. | [61] | Human | ↓ miR-214 in patients; ↑ PLGF in UAP and AMI but not SAP; positive correlation between miR-214 and PLGF in AMI. | [62] | Human | ↓ miR-145, ↓ miR-155, and ↓ let-7c in patients; the combination of three miRNAs provided a diagnostic signature. | [63] |
| Human | miR-149 rs2292832 and miR-196a2 rs11614913 are significantly associated with CAD. | [64] | Human | ↑ miR-122 and miR-370 in hyperlipidemia; positively correlated with TC, TG, LDL-C and Gensini score. | [65] | Human | ↑ miR-133a-3p, ↑ miR-451a, ↑ miR-584-5p, ↑ miR-21-5p, ↑ miR-221-3p; strongest markers. | [66] |
| Human | ↑ miR-146a in good CCC, ↓ in poor CCC; VEGF- | [67] | Human, in vitro (macrophage model) | ↓ miR-128-3p in patients; no change in miR-195-5p; miR-128-3p impairs cholesterol efflux in macrophages. | [68] | Human (postmortem) | ↓ miR-126-5p and ↓ miR-499a-5p in CAD-SCD; no change in miR-134-5p. | [69] |
| Human | ↑ miR-144 in patients; levels correlated with STEMI and higher SYNTAX scores. | [70] | Human | ↑ SGPP1 (target of miR-133b); ↓ ATG5 and ↓ LRP6 (targets of miR-21); expression modulated by miRNA inhibitors. | [71] | Human | ↑ miR-137 (up to 1198-fold) and ↑ miR-106b-5p (up to 122-fold) in CAD patients. | [72] |
| Human | ↑ miR-146a/b, TLR4, IRAK1, TRAF6 in patients; all decreased after treatment, especially with ARB. | [73] | Human | ↓ miR-96-5p and ↑ BCL2L13 in patients and hypoxic cells; | [74] | Human | 159 miRNAs DE (119 ↑, 40 ↓); top ↑: miR-144-3p, miR-34a-5p. | [75] |
| Human | ↑ miR-27a, ↓ miR-146a; no sig. change in miR-34a and miR-149; correlations with lipid profile and BMI. | [76] | Human | 33 miRNAs changed significantly post-exercise; 16 differed by gender; 9 miRNAs showed significant gender-dependent responses. | [77] | Human and cell lines | ↑ miR-6721-5p and ↓ meta-VCL in CAD; miR-6721-5p targets meta-VCL; IL-10 and TNF-α. | [78] |
| Human | ↓ miR-5187-5p, ↑ miR-199a-5p, miR-182-5p. | [79] | Human | ↑ miR-17-5p, ↑ miR-21-5p, ↑ miR-210-3p, ↑ miR-29b-3p, ↑ miR-7-5p and ↑ miR-99a-5p (>2-fold) in patients vs. controls. | [80] | Human | Plasma miR-378 was significantly downregulated in CAD patients and negatively correlated with Gensini score. | [81] |
| Human | miR-106b-5p expression is significantly elevated in CAD group compared to controls. | [82] | Human | 7 miRNAs (miR-10b-5p, miR-29c-3p, miR-142-5p, miR-320b, miR-451a, miR-486-3p, miR-625-3p) differentially expressed. | [83] | Human | Serum miR-299-3p was significantly upregulated in CAD patients compared to controls. | [84] |
| Human | miRNA146, miRNA21 and MMP1 significantly increased in STEMI vs. SIHD. miRNA126 negatively correlated with LV and arterial function parameters. MMPs correlated with stiffness. | [85] | Human | Higher MMP-9 in oCAD; higher TNF-α in INOCA; miR-145 predictive for INOCA/ANOCA. | [86] | Human | AMPKα1 and let-7g-5p are differentially expressed in EAT vs. VAT; TNFA is upregulated in both tissues in CAD; miR-1247-5p and miR-326 downregulated are in EAT with obesity. | [87] |
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse | miR-1 ↑; trafficking-related genes (Stx6, Braf, Ube3a, etc.) ↓ | [123] | Human and Rat | miR-1 ↓, miR-328 ↓, miR-664 ↑, etc.; miR-1 inhibits CACNB2 expression →↓ Ca2+ influx | [124] | Mouse | miR-206 ↑→ Cx43 ↓; caused arrhythmias, abnormal heart rate and PR interval; | [125] |
| Rat | Tanshinone IIA ↓ miR-1 ↑ (in MI); restored Kir2.1 and IK1 current; | [126] | Human | CAF ↑ miR-21 ↑→↓ CACNA1C and CACNB2 | [127] | Mouse | ZFHX3 KD → miR-133a/b ↓, miR-184 ↑ | [128] |
| Human | Variants found in miR-1-2 and miR-133a-2 did not affect mature miRNA. | [129] | Human and Mouse | miR-106b-25 cluster ↓→ RyR2 ↑ (42%); ↑ Ca2+ spark and SR leak; ↑ | [130] | Mouse | miR-206 inhibitor ↓ arrhythmia severity; ↓ CKMB and cTnI | [131] |
| Mouse | HFD ↑ miR-27b ↑→ Cx40 ↓; slowed atrial conduction, increased atrial tachycardia vulnerability | [132] | Human (Pediatric) | SVa: miR-1 ↑, miR-133a ↑; Va & SVa: miR-133b ↓ vs. controls; miR-133a higher in SVa vs. Va | [133] | Human | miR-365 ↑→ APD ↑ in Short-QT; miR-365 inhibition → APD normalization in Long-QT | [134] |
| Mouse | miR-130a ↑→ Cx43 ↓ (>90%); caused atrial and ventricular tachyarrhythmias | [135] | Human | AT1R-1166CC genotype →↑ ICD therapies (ATP + shocks); miR-155 ↓. | [136] | Human and Rat | miR-1231 ↑ in MI; targets CACNA2D2 ↓; overexpression → arrhythmia ↑; inhibition → arrhythmia ↓ | [137] |
| Rat | ART ↓ VAS; 16 DEMs altered with ART; MAPK, Wnt, Hippo pathways implicated; rno-miR-370-3p and rno-miR-6319 ↓. | [138] | Rat and Human | miR-1 ↑ in CAD; ↑ arrhythmias; ↓ KCNJ2 (Kir2.1) and GJA1 (Cx43) | [139] | Rat | novel-miR-17 ↑→ Gja1 ↓→ Cx43 ↓→↓ conduction velocity; activates PKC/c-Jun pathway | [140] |
| Dog, Human and Mouse | miR-328 ↑ in AF; CACNA1C and CACNB1 ↓ | [141] | Human | miR-483-5p ↑ (atrial and serum); predictive of POAF with 78% accuracy; miR-208a ↓ in POAF tissue | [142] | Human | miR-15a-5p ↑, miR-16-5p ↑, miR-92a-3p ↑ in ARVC | [143] |
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Human and Rat | miR-210 ↑ associated with hypoxia/oxygen metabolism | [154] | Human | Higher miR-132 was linked to HF severity, but lower levels predicted HF hospitalizations independently. | [155] | Human | CDR132L caused dose-dependent miR-132 ↓, NT-proBNP ↓ | [156] |
| Mouse | 69 miRNAs ↑ and 2 ↓ in early HF; 16 ↑ and 6 ↓ in late HF; miR-696, miR-532, miR-690 and miR-345-3p enriched in early HF mitochondria. | [157] | Human | miR-21 levels (PV and CS) were higher in HF, correlated with EF and BNP; predicted rehospitalization | [158] | Rat | miR-27a ↑ in CHF hearts and EVs; targets PDLIM5 ↓. | [159] |
| Human | 8-miRNA panel effectively identified HF; improved specificity and accuracy when combined with NT-proBNP | [160] | Human | miR-145 ↓ in AMI and HF; negatively correlated with BNP and troponin T; positively with EF | [161] | Rat | miR-21 ↑ in fibroblasts and HFpEF model; upregulates Bcl-2 | [162] |
| Rat | MI rats showed ↑ miR-21, atrial fibrosis, and AF; KD21 reduced miR-21 levels, fibrosis, and AF duration. | [163] | Human | miR423-5p enriched in HF blood; AUC 0.91 (p < 0.001); 5 other miRNAs less specific | [164] | Rat | miR-133a ↓ in HF; overexpression improved LV function and reduced fibrosis; Akt inhibition reversed these effects. | [165] |
| Human and Canine | miR-30d is associated with CRT response, enriched in high-stress myocardium and reduces apoptosis in vitro. | [166] | Mouse and Human | ↓ miR-18a, miR-19a/b ↓ linked with ↑ CTGF, TSP-1 in failure-prone hearts; opposite in failure-resistant | [167] | Human | miR-150-5p significantly ↓ in AHF vs. HS and MHF; associated with maladaptive remodeling and disease severity | [168] |
| Human | miR-155 ↑ is associated with poor cardiac function post-MI. | [169] | Human and Rat | miR-181b ↓ anti-inflammatory; regulates TNF-α, IL-1β, IL-6 | [170] | Human | Higher miR-21 levels are significantly associated with HFpEF development. | [171] |
| Human, Mouse and Rat | miR-221/222 ↓ targets TGF-βR1, TGF-βR2, JNK1 and ETS-1; inhibits SMAD2-mediated signaling | [172] | Human | miR-181c↑ targets PRKN and SMAD7. | [173] | Human and Rat | miR-125b-5p downregulated in DCM-HF | [174] |
| Rat | miR-132 ↑ targets PTEN, suppresses PI3K/Akt pathway and fibrotic gene expression. | [175] | Rat and Cell Line | miR-152-3p ↓ targets ETS1; ETS1 → RhoH ↑; mitophagy inhibited | [176] | Human | miR-210-3p, NT-proBNP, sST2, and galectin-3 levels were significantly higher in HFrEF than HFpEF. | [177] |
| Rat | miR-155 ↑ targets BDNF and is regulated by Sirt1/NF-κB p65. | [178] | Mouse | Silencing MEG3 improved cardiac dysfunction, reduced hypertrophy, oxidative stress, apoptosis, autophagy, and fibrosis. | [179] |
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Human | miR-205-3p ↓ → IL-1β ↑; miR-3909 ↓ → IL1R1 ↑ | [183] | Human | miR-500, miR-3174, miR-17, etc. ↔ → DCN, ACAN, FMOD, ACTA2, ECM2, DES, ESM1, PECAM1 (in silico predicted; inverse correlations) | [193] | Human | miR-21 ↑ → Myocardial fibrosis ↑; LV strain ↓ | [194] |
| Human | miR-15a ↓ → PUMA ↓; miR-29a ↓ → DRP1 ↓; miR-214 ↑ → ARC ↓ | [195] | Human | miR-30b ↓ → Runx2 ↑, Smad1 ↑, caspase-3 ↑ | [196] | Canine | miR-30b-5p ↑ in B1 < 3y, B1 3–7y, B1 > 7y vs. A | [197] |
| Human | miR-222 ↑ → IL-6 ↑, hs-CRP ↑, NT-proBNP ↑ | [198] | Human | miR-140-3p ↑, miR-150-5p ↑, miR-210-3p ↑, miR-451a ↑, miR-487a-3p ↑; miR-223-3p ↓, miR-323a-3p ↓, miR-340-5p ↓, miR-361-5p ↓ | [199] | Human | miR-139-5p ↓ → RUNX2 ↑, FZD4 ↑, CTNNB1 ↑ → Osteogenesis ↑ | [200] |
| Canine | let-7c ↓, miR-17 ↓, miR-20a ↓ → myofibroblast differentiation/senescence ↑; miR-30d ↓ → apoptosis ↑ | [201] | Human | miR-146a ↑ → TLR4/IRAK1 | [202] | Human & Mouse | miR-214 ↑ → TWIST1 ↓ → VIC calcification ↑ | [203] |
| Human (in vitro) | miR-195 ↓ → VWF ↑ → p38-MAPK ↑ → ALP/Runx2/OCN ↑ → Calcification ↑ | [204] | Human (in vitro) | miR-22 ↑ → CAB39 ↓ → AMPK/mTOR dysregulation → Osteogenesis ↑ | [205] | Human & Porcine | miR-141 ↓ → BMP-2 ↑ → ALP ↑ → Calcification ↑ | [206] |
| Human & Mouse | miR-204 ↓ → Runx2 ↑, Osx ↑ → ALP ↑ → Calcification ↑ | [207] | Human (in vitro) | miR-629-3p ↓ → TAGLN ↑ → Calcification ↑ | [208] | Human | let-7e-5p ↑, miR-196a-5p ↑ → Aortic dilation ↑; miR-17a-5p ↓ (NS) | [209] |
| Human & Mouse | miR-122-5p ↑ → BCL2 ↓ → Cardiomyocyte viability ↓ → LVEF ↓ | [210] | Human | miR-125b ↑ → CCL4 ↓ (targeted in THP-1 macrophages) | [211] | Human (in vitro) | miR-93-5p ↑, miR-374a-5p ↑ → BMP2 ↓ → Smad1/5 ↓ → Runx2 ↓ → Calcification ↓ | [212] |
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse | miR-223-3p ↑ → NLRP3 ↓ | [226] | Human | miR-29a → associated with both hypertrophy & fibrosis | [227] | Human | mir-142-5p, ↑ mir-143-3p, ↑ mir-27b-3p, ↑ mir-126-3p, ↑ let-7g ↑ → LVEF ↓ | [228] |
| Human and Mouse | miR-146a ↑ → NRAS, ↓ Erbb4, ↓ Notch1, ↓ Irak1 ↓ | [229] | Rat and Cell Line | miR-30d ↑ → foxo3a, ↓ ARC ↓, caspase-1, ↑ IL-1β, ↑ IL-18 ↑ | [230] | Human | miR-18a-5p, miR-146a-5p, miR-30d-5p, miR-17-5p, miR-200a-3p, miR-19b-3p, miR-21-5p, miR-193-5p, miR-10b-5p, miR-15a-5p, miR-29a-3p ↑ | [231] |
| Human | miR-208 ↑ → β-MHC, ↑ collagen volume ↑ | [232] | Human | miR-16 ↑, miR-26a ↑, let-7f ↑, miR-1 ↑, miR-133a ↑, miR-125a-5p ↓ → ET-1 ↑ | [233] | Human | ↑ miR-204 → TRPM3 (trend) ↑, troponin I phosphorylation ↓ | [234] |
| Human | miR-135b, ↑ miR-155, ↑ miR-190, ↑ miR-422a, ↑ miR-489, ↑ miR-590, ↑ miR-601, ↑ miR-1290 ↑ → immune gene suppression | [235] | Human | miR-218-5p ↑ → DDX6, ↓ TTC39C, ↓ SEMA4A ↓; miR-494-3p ↑ → SGMS2 ↓ | [236] | Human and Cell Line | miR-20a-5p ↑ → MFN2 ↓ → ANP → ↑ hypertrophy ↑ | [237] |
| Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference | Organism | miRNA and/or Targets | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse and Human | miR-187 ↑ → NIPBL ↓ | [268] | Human | Altered miRNA target sites in HAND1 3′UTR (gain/loss of binding sites) | [269] | Human | let-7e-5p, miR-222-3p, miR-433 → NOTCH1, HAND1, ZFPM2, GATA3 | [270] |
| Human | miR-21-5p ↑, miR-221-3p ↑, miR-26a-5p ↑, miR-15-5p ↑ | [271] | Human and cell lines | miR-145 ↑ → FXN ↓ | [272] | Mouse (pregnant D7 mice and embryonic endocardial cells) | miR-34a ↑ → NOTCH-1 ↓ → altered Notch signaling | [273] |
| Human (children with CHD) | miR-21 ↑ → TNF-α ↓ | [274] | Human (pregnant women) | miR-19b ↑, miR-22 ↑, miR-29c ↑, miR-375 ↑ | [275] | Human | miR-221-3p ↑, miR-218-5p ↑, miR-873-5p ↑ (associated with cardiogenesis and dysfunction) | [276] |
| Human and rat cardiomyocyte cell line (H9C2) | miR-219-5p ↑ → LRH-1 → ↓ Wnt/β-catenin pathway ↓ | [277] | Human | miR-21 ↑ → (RAS/PI3K/AKT pathway ↓ under shear stress; suppression reverses this) | [278] | Human (fetuses via umbilical cord blood) | miR-1 ↓, miR-208 ↓, miR-499 ↓ | [279] |
| Human | miR-21 ↑ | [280] | Human | miR-1, miR-133 → KCNJ2, FBN2, SLC38A3, TNNI1 | [281] | Mouse and Human (CHD-related RVF tissue) | miR-34a ↑ → HIF-1α, VEGFA, VEGFB, VEGFR2, SIRT1 ↓ | [282] |
| Human (infants with and without TOF) | miR-421 ↑ → SOX4 (Notch signaling) ↓ | [283] | Human | 22 miRNAs (mostly ↓) → mGLUR, Gq, PLC, PKC, ECM, FAK, PI3K, PDK1 ↑ | [284] | Human | miR-8078 ↑ (targets implicated in MAPK cascade, ion transport, atrioventricular valve morphogenesis) | [285] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, M.; Ozkan, U.; Budak, M. The Heart’s Small Molecules: The Importance of MicroRNAs in Cardiovascular Health. J. Clin. Med. 2025, 14, 7454. https://doi.org/10.3390/jcm14217454
Yildiz M, Ozkan U, Budak M. The Heart’s Small Molecules: The Importance of MicroRNAs in Cardiovascular Health. Journal of Clinical Medicine. 2025; 14(21):7454. https://doi.org/10.3390/jcm14217454
Chicago/Turabian StyleYildiz, Mustafa, Ugur Ozkan, and Metin Budak. 2025. "The Heart’s Small Molecules: The Importance of MicroRNAs in Cardiovascular Health" Journal of Clinical Medicine 14, no. 21: 7454. https://doi.org/10.3390/jcm14217454
APA StyleYildiz, M., Ozkan, U., & Budak, M. (2025). The Heart’s Small Molecules: The Importance of MicroRNAs in Cardiovascular Health. Journal of Clinical Medicine, 14(21), 7454. https://doi.org/10.3390/jcm14217454





