Abstract
Background: Point-of-care ultrasonography (POCUS) is becoming an increasingly relevant tool in hospital medicine, but its effective application in inpatient perioperative medicine remains to be determined. Much of the POCUS literature describes its use by anesthesiologists to evaluate cardiac function, volume status, pulmonary findings, and gastric volume. Objective: To identify, evaluate, and synthesize all available literature investigating the use of point-of-care ultrasound and associated clinical outcomes in inpatient perioperative medical management. Patients and Methods: A systematic review was designed using the PRISMA guidelines with sources of literature including Ovid, PubMed, Scopus, and the Web of Science. Literature search was conducted for published works between 1 January 2002 to 8 February 2024. Results: Three hundred sixty-seven abstracts were identified in our search, and, ultimately, 24 studies were included in this review. Most studies were done by anesthesiology evaluating cardiopulmonary and gastric POCUS. Studies supported using POCUS to expedite cardiac examination, promptly diagnose postoperative pulmonary complications, and optimize surgical timing. Conclusions: POCUS is a versatile tool in the perioperative setting; however, few studies were powered to assess clinical outcomes, and even fewer showed conclusive evidence of improved clinical outcomes. Furthermore, only two studies evaluated the use of POCUS specifically by acute care providers; more extensive studies are needed from their perspective as they take on increasing perioperative responsibilities.
1. Introduction
Point-of-care ultrasonography (POCUS) has emerged as a popular tool in hospital medicine due to its portability, ease of use, and ability to provide real-time, dynamic imaging at the patient’s bedside [1]. Its versatility has revolutionized the diagnostic process, offering rapid and non-invasive insights that aid in timely decision-making [2]. Typical applications of POCUS include assessing cardiac function, identifying fluid collections, and guiding procedures such as central line placement and thoracentesis. Additionally, POCUS plays a pivotal role in diagnosing pathologies such as systolic heart failure, pericardial tamponade, pleural effusions and consolidations, venous thromboembolisms, and abdominal aortic aneurysms [3]. POCUS for bedside diagnosis has been well studied with many RCTs and clinical guidelines supporting its use [4,5,6]. POCUS certifications are available through multiple medical societies, including the Society of Hospital Medicine, the American College of Physicians, the American College of Chest Physicians, the Alliance for Physician Certification and Advancement, and the American Academy of Physician Assistants [7,8,9,10,11].
Multiple specialty societies, such as the American Society of Echocardiography, American College of Emergency Physicians, and European Society of Anesthesiology, offer suggestions for the use of POCUS in perioperative cardiac function assessment, identification of fluid status, and regional anesthesia guidance [4,12,13]. These serve as a crucial framework for harnessing the full potential of POCUS in perioperative medicine. While POCUS adoption has increased significantly amongst anesthesiologists, a direct correlation between perioperative decisions guided by POCUS and demonstrable changes in patient outcomes remains largely unestablished. Further research is needed to quantify the impact of POCUS-informed decisions in this setting. This systematic review attempts to synthesize the evidence regarding the impact of POCUS on clinical outcomes in the inpatient perioperative setting to help guide clinicians in the medical management of perioperative patients.
2. Methods
This study utilized a systematic review design performed in compliance with the Preferred Reporting in Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14,15]. Literature search of publications within the past two decades was conducted with the assistance of a medical librarian on 8 February 2024. Sources included Ovid (Embase & Cochrane), PubMed (MEDLINE), Scopus (Elsevier), and Web of Science with query terms including point-of-care, ultrasound, POCUS, adult, and perioperative. Inclusion criteria included adult patients, planned/performed surgical intervention, use of POCUS during evaluation, and studies with measured clinical outcomes. For this study, clinical outcomes were broadly defined as patient and clinician-reported outcomes, including but not limited to changes in symptoms, clinical status, mortality, comorbidities, and hospital metrics. Studies were excluded if they involved only pediatric patients, outpatient POCUS evaluation, intra-operative use of POCUS, or were non-peer-reviewed publications, letters to the editor, case reports, or in a non-English language (Figure 1). Each abstract, full text, data abstraction, and bias assessment was completed by two different reviewers, with a third reviewer as arbitrator. Bias assessment for the non-randomized cohort and case-control studies included was accomplished using the Newcastle–Ottawa grading scale, with 1 point for each star on the cohort studies scale for a maximum of 9 points. Studies totaling 7–9 points were graded as “good,” 4–6 points as “moderate,” and <4 points as “poor” [16]. Randomized control studies (RCTs) were assessed for bias using the Cochrane Risk of Bias 2.0 tool [17]. Operator ultrasound experience was determined to be “expert” if there was mention of certification in ultrasonography, “experienced” if there was mention of the operator being experienced or formally trained in US, “limited training” if there was specific mention of limited or brief training in US, and “unknown” if there was no mention of US experience or level of experience was unclear. The web-based Covidence platform was used to screen the literature and extract data, and integrated functions were used to determine proportionate agreement [18]. Proportionate agreement was calculated by dividing the total number of agreements by the total number of items for the respective screening stages. A meta-analysis was not conducted due to the significant variation in study methods, including patient populations, interventions, including different types of POCUS, and the different primary and secondary outcomes studied within the limited number of articles that were included in the review.
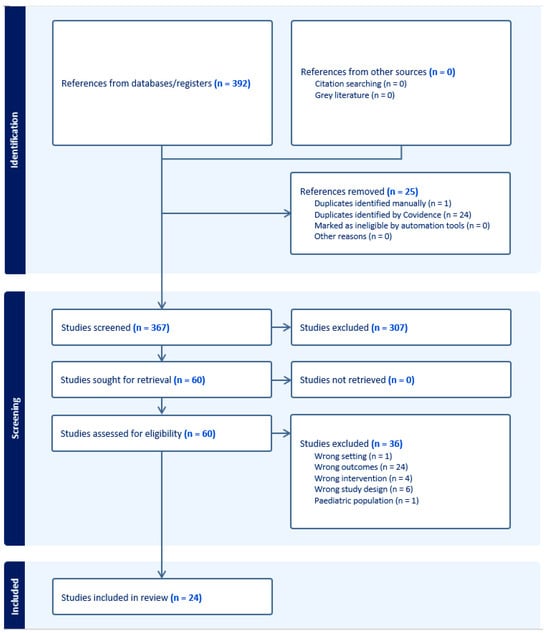
Figure 1.
PRISMA diagram for systematic review.
3. Results
Our literature search strategy yielded 367 abstracts, of which 60 met the inclusion criteria (Supplementary Materials). After excluding articles for lack of clinical outcomes (n = 24), incorrect study design (n = 6), lack of POCUS as an intervention (n = 4), outpatient setting (n = 1), and pediatric population (n = 1), 24 studies were included in the review [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Most of the studies were cohort or case-control studies except for Szabo (2023), Ravetti (2023), and Cavallari (2015) [25,37,39], which were RCTs. Five reviewers screened titles and abstracts with a proportionate agreement equal to 84%, and seven reviewers screened full texts with a proportionate agreement equal to 82%. Based on the Newcastle–Ottawa grading scale, all studies were of “good” quality except for Cowie (2011) [27], which was of “moderate” quality (Supplementary Materials). The intention of utilizing a numerical system was to tabulate the authors’ bias evaluation for ease of presentation, and it is not intended to compare studies to one another. The authors’ general assessment of bias in the studies mimics the quality of the study (from a bias perspective) represented by the tabulated results from using the Newcastle–Ottawa scale. All studies, except for Cowie (2011) [27], had low bias based on the authors’ critical assessment.
The organ systems studied include cardiac (8), pulmonary (7), gastrointestinal (4), vascular (4), renal (3), musculoskeletal (1), and genitourinary (1) in the perioperative setting of various surgeries, with all but 2 being non-cardiac, and orthopedic surgery being the most common of the non-cardiac surgeries. Five studies showed some evidence of impact on clinical outcomes, four showed no impact on clinical outcomes, five studies showed an impact on diagnostic accuracy, five studies showed an impact on anesthetic plan or intraoperative management, and six studies showed an association between US findings and clinical parameters without changes to clinical outcomes. Baseline patient characteristics for each study are listed in Table 1. Sample sizes ranged from 20 to 512 patients, with an average of 112 patients. 12 studies had fewer than 100 patients, and 12 had 100 patients or greater. The average age ranged from 33 to 82 years, with a mean of 62 years, the percent male ranged from 34 to 93, with a mean of 54, and the average body mass index (BMI) ranged from 23.4 to 28.6, with a mean of 25.8. American Society of Anesthesiologists (ASA) classification information was presented in 13 out of the 24 studies, with class 2 being the most common.

Table 1.
Baseline patient characteristics. BMI (body mass index), ASA (American Society of Anesthesiology), n/a (not available).
A summary of study methods for each study is listed in Table 2. Most studies were done in the United States (5), followed by Canada (3) and Italy (3). The ultrasound operator specialties across the 24 studies were anesthesia (15), cardiology (2), surgery (1), medicine/critical care (2), nursing (1), and not specified in 4 studies. Most of these operators were “experienced” with POCUS, with a few being “experts” or having “limited training”. Ultrasound devices varied greatly between studies, but nearly all devices were either cart-based or laptop design, except for devices used in Ramsingh (2021), Cavallari (2015), and Cutright (2011), which were hand-held [25,29,36]. Chui (2023) [26] did not have a listed device.

Table 2.
Summary of methods. Abbreviations: GE (General Electric), USA (United States of America), ENT (ear, nose, throat), PA (pulmonary artery), CT (computed topography), TTE (transthoracic echocardiogram), mmHg (millimeters of mercury), PACU (post-anesthesia care unit).
The specific aims, measurements, and conclusions of each study varied substantially, even when assessing similar organ systems (Table 3). Cardiac ultrasound was utilized to assess baseline cardiac function, presence of a patent foramen ovale, and volume status [19,20,25,27,30]. Lung ultrasound was utilized to assess for atelectasis and pulmonary congestion [21,31,38,41]. Gastric ultrasound was utilized predominantly to assess gastric volume [28,32,34,40]. Vascular and renal ultrasound was utilized to assess volume status and risk for acute kidney injury [33,35,39]. Multisystem evaluations, including the heart, lungs, and/or inferior vena cava, were utilized in four studies to assess volume status [26,36,37,39]. Results regarding conclusions from each study and their implications are considered in the discussion section.

Table 3.
Summary of objectives and conclusions.
4. Discussion
Limited research has been conducted on outcomes-based investigations that employ point-of-care ultrasonography in inpatient perioperative medicine. Notably, the existing literature lacks perspectives from acute care providers. This comprehensive review reveals that the use of POCUS in perioperative medicine derives primarily from the anesthesia standpoint, predominantly concerning its impact on anesthetic plans and post-anesthesia care. The studies within this domain largely revolve around cardiac, pulmonary, and gastric POCUS.
Most of the study patients are classified as American Society of Anesthesiologists (ASA) class 1 or 2. In contrast, inpatient medicine consults for presurgical evaluation generally involve ASA class 3 and 4 patients [43,44]. Furthermore, the ultrasound operators in these studies predominantly hail from anesthesia and cardiology specialties, both of which undergo formal ultrasound and POCUS training during their respective training programs. This specialized training might elevate the significance of their findings compared to studies involving general medicine providers with varied levels of POCUS training. However, there is evidence, including studies beyond the scope of this review, validating the ability of novice POCUS users to accurately assess cardiac function and make common diagnoses after limited training [45,46]. Therefore, cardiac POCUS is a potential starting point for assessing the impact of acute care providers using POCUS in inpatient preoperative evaluation on patient outcomes. The following sections highlight the application of POCUS in inpatient perioperative medical management of the five most studied organ systems.
4.1. Cardiac
Andruszkiew (2015) and Cowie (2011) [20,27] demonstrate that even when performed by anesthesiologists with basic training, focused cardiac POCUS can significantly alter perioperative decisions. Additionally, Cowie (2011) and Cavallari (2015) [25,27], an RCT, highlight that focused transthoracic echocardiography (TTE) and hand-held TTE devices, respectively, can provide satisfactory diagnostic quality with the advantage of shorter wait times and expedited exam performance. In contrast, Chui (2023) [26] indicates that while POCUS does not necessarily change anesthetic plans, its use may avert surgical delays and assist in assessing severe cardiopulmonary conditions. These studies suggest cardiac POCUS may be a more practical exam than formal TTE for most perioperative patients.
Furthermore, targeted applications—such as using the velocity–time integral of the left ventricular outflow tract-passive leg raise to predict post-induction hypotension (Aissaoui, 2022) [19] or detecting patent foramen ovales potentially linked to postoperative delirium (Gai, 2018) [30]—underscore the evolving role of POCUS in risk stratification. Lastly, the integration of combined cardiac and lung assessments, as reported by Ramsingh (2021) [36], appears beneficial in reducing post-anesthesia care unit length of stay in vitally unstable patients.
4.2. Pulmonary
Basumatary (2023) and Wu (2023) [21,41] highlight that lung ultrasound can identify lung congestion, atelectasis, and aeration before the manifestation of clinical signs and symptoms during the early postoperative period. A more quantitative approach using the lung ultrasound score has proven to be a valuable metric in identifying patients at risk of or in the early phase of postoperative pulmonary complications (Szabó, 2021) and correlates with prolonged weaning time for respiratory support (Goel, 2020) [31,38]. These studies show that point-of-care lung ultrasound is a vital tool in the early detection and management of pulmonary complications in the perioperative setting.
4.3. Gastrointestinal
Cozza (2021) [28] demonstrates that a preoperative dilated antrum identified via POCUS is significantly related to adverse postoperative outcomes, suggesting that targeted ultrasound follow-up may be useful in optimizing postoperative nutrition and antiemetic therapy. Van de Putte (2017) [40] finds that patients may present with full stomachs despite adhering to recommended fasting guidelines, which indicates a prime opportunity for the use of POCUS in pre-anesthetic management to mitigate risks. Post-operatively, Lamm (2022) [34] reports that patients with full stomachs on postoperative day one after colorectal surgery experience a delayed recovery of gastrointestinal function, as indicated by prolonged GI3 recovery, and Haskins (2017) [32] observes that intra-abdominal fluid extravasation following hip arthroscopy is correlated with increased postoperative pain, thereby providing predictive insight into a patient’s clinical course. Collectively, these studies underline the critical role of perioperative gastrointestinal evaluation in tailoring management strategies. This could be particularly important for patients on GLP-1 receptor agonists, which can slow gastric emptying and increase the risk of retained gastric contents and aspiration [47].
4.4. Vascular/Renal
Studies addressing vascular ultrasound evaluation are largely focused on the correlation between vasculature, fluid status, and renal function. Kaydu (2019) [33] demonstrates no relationship between inferior vena cava parameters and blood-urea-nitrogen/creatinine ratio to predict perioperative dehydration, but Szabo (2023) [39], an RCT, successfully implemented a preoperative, ultrasound-based fluid administration protocol that prevents early intraoperative hypotension and guides timing of fluid administration. Consistent with this, Beaubien-Souligny (2018) [22] shows an association between a decrease in the renal resistance index and an increase in cardiac output following passive leg raise after cardiac surgery.
Several studies looked at the impact of vascular POCUS on the development of AKI. Pettey (2022) and Yamanaka (2022) [35,42] utilize hepatic vein flow wave ratios and renal artery pulsatility index, respectively, to show an association between these parameters and perioperative AKI. Brusasco (2023) [23] refines this association to predict perioperative AKI using intra-renal venous flow patterns. A multivariate model focused on IVC, renal, and hepatic vasculature may have more promising outcomes on AKI prediction and prevention. However, Ravetti (2023) [37], an RCT focused on assessing the impact of bedside lung, IVC, and cardiac ultrasound on hemodynamic management in the immediate post-operative period, did not show benefits in the incidence of post-operative AKI.
Many of the POCUS applications reviewed by the system above may be applicable to inpatient medicine; accordingly, inpatient practice would benefit from studies exploring the use of POCUS specifically by acute care providers. Future studies on perioperative medical management utilizing POCUS should focus on patient outcomes while meticulously defining baseline patient characteristics and clinical measurements in addition to POCUS parameters. Essential patient characteristics for data collection include age, sex distribution, BMI, ASA class, planned surgery, and highest metabolic equivalent level, as well as results of commonly used perioperative risk calculators, including the National Surgical Quality Improvement Project, myocardial infarction-cardiac arrest, revised cardiac risk index, and the ARISCAT score for post-operative pulmonary complications. For methodology, ultrasound operator specialty, operator training, ultrasonography device, ultrasound views, and parameter data, clinical translation of imaging findings (e.g., cardiac function vs. change in left ventricle diameter), and most importantly, clinical outcomes should be considered. These may include length of stay (PACU, ICU, or hospital), morbidity and mortality, diagnostic accuracy, time to diagnosis or intervention, complication rates, and readmission rates. The standardized reporting of baseline data and clinical measurements would improve comparability between studies by allowing researchers and readers to easily compare patient populations, interventions, and outcomes, along with allowing more rigorous review of the literature via meta-analysis. This will facilitate the application of future POCUS research in the clinical setting, propelling the field of perioperative medicine forward.
Limitations of this systematic review include the heterogeneity of the studies included, which makes it challenging to synthesize results and draw meaningful conclusions. The studies vary in design and methods, operator experience or training, patient characteristics, consistency of findings (most studies focus on cardiopulmonary POCUS and evaluation), and clinical relevance in terms of the studied patient outcomes. Inherent to their study design, the three RCTs provide the most robust evidence on comparing formal echocardiography with cardiac POCUS and managing intraoperative fluid administration. Given the multitude of different methods and outcomes studied amongst the included studies, no subgroup analysis was conducted. Broad variations in patient demographics may affect the generalizability of the findings; thus, findings from each study should be taken in the context of their respective patient population. The review is also susceptible to publication and reporting biases, given the tendency for positive studies to be published more frequently than those with negative or inconclusive results. Small sample sizes for some studies may have led to imprecise estimation of the effect. In addition, direct application of these studies on inpatient management will likely vary based on the practice setting, available resources, and staff experience at each institution. Limitations in this review’s methods include a lack of registration with PROSPERO, an international systematic review registry, and intentional exclusion of grey literature. These limitations in methods are thought to have a negligible impact on the results and implications of this review. Grey literature is generally not peer-reviewed and thus introduces additional bias and inconsistency within systematic reviews, so the authors felt that inclusion of grey literature would negatively impact the quality of the study while providing limited information on patient outcomes. However, grey literature can help reduce publication bias and provide unique or more up-to-date information, so its exclusion is a noted limitation. Lack of PROSPERO registration does restrict the review’s openness and reproducibility, particularly while a study is in progress; however, a thorough literature search was completed with the assistance of a medical librarian to ensure that similar reviews were not previously published, and we believe the transparent methodology, including the search strategy included in Supplementary Materials, provides the resources for reproducibility if needed.
5. Conclusions
This review demonstrates the versatility of POCUS in perioperative management. Cardiopulmonary and gastric POCUS imaging have the most data to guide management for post-operative ICU care and appropriate surgical timing, respectively. Very few studies were powered to assess clinical outcomes, and even fewer showed overlapping evidence to strongly suggest improvement in clinical outcomes secondary to the use of perioperative POCUS. Additionally, more extensive studies are needed to truly evaluate the benefits or harms of using POCUS in inpatient perioperative medical management, especially from the acute care provider perspective, as they undertake more perioperative responsibilities. Future studies should focus on clinical questions answerable with POCUS for preoperative medical evaluation and non-ICU post-operative care in the less-controlled environment of inpatient wards, with an emphasis on evaluating associated clinical outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14207429/s1, Table S1: Systematic Review Search Strategies; Table S2: Quality and Bias Assessment Consensus Data; Table S3: PRISMA 2020 Checklist.
Author Contributions
D.M.J.: Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft, Funding Acquisition. M.J.B.: Investigation, Writing—Review & Editing. G.J.K.: Investigation, Writing—Review & Editing. M.I.: Investigation, Data Curation, Writing—Review & Editing. R.K.: Investigation, Writing—Review & Editing. M.N.B.: Investigation, Writing—Review & Editing. C.J.C.: Methodology, Validation, Investigation, Writing—Review & Editing. R.W.K.: Conceptualization, Methodology, Investigation, Data Curation, Writing—Review & Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Conflicts of Interest
Author Ryan Kingsley owns <$200 of individually owned company stock in Butterfly Network. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
AKI, acute kidney injury; ASA, American Society of Anesthesiologists; POCUS, point-of-care ultrasound; RCT, randomized clinical trial; TTE, transthoracic echocardiogram
References
- Soni, N.J.; Schnobrich, D.; Mathews, B.K.; Tierney, D.M.; Jensen, T.P.; Dancel, R.; Cho, J.; Dversdal, R.K.; Mints, G.; Bhagra, A.; et al. Point-of-Care Ultrasound for Hospitalists: A Position Statement of the Society of Hospital Medicine. J. Hosp. Med. 2019, 14, E1–E6. [Google Scholar] [CrossRef]
- Thind, G.S.; Fox, S.; Gupta, M.; Chahar, P.; Jones, R.; Dugar, S. Point-of-care ultrasonography for the hospitalist. Cleve Clin. J. Med. 2021, 88, 345–359. [Google Scholar] [CrossRef]
- Hashim, A.; Tahir, M.J.; Ullah, I.; Asghar, M.S.; Siddiqi, H.; Yousaf, Z. The utility of point of care ultrasonography (POCUS). Ann. Med. Surg. 2021, 71, 102982. [Google Scholar] [CrossRef]
- Qaseem, A.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Kansagara, D.; Fitterman, N.; Wilt, T.J.; Clinical Guidelines Committee of the American College of Physicians; Batur, P.; Cooney, T.G.; Crandall, C.J.; et al. Appropriate Use of Point-of-Care Ultrasonography in Patients with Acute Dyspnea in Emergency Department or Inpatient Settings: A Clinical Guideline from the American College of Physicians. Ann. Intern. Med. 2021, 174, 985–993. [Google Scholar] [CrossRef]
- Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann. Emerg. Med. 2017, 69, e27–e54. [CrossRef] [PubMed]
- Basmaji, J.; Arntfield, R.; Desai, K.; Lau, V.I.; Lewis, K.; Rochwerg, B.; Fiorini, K.; Honarmand, K.; Slessarev, M.; Leligdowicz, A.; et al. The Impact of Point-of-Care Ultrasound-Guided Resuscitation on Clinical Outcomes in Patients with Shock: A Systematic Review and Meta-Analysis. Crit. Care Med. 2024, 52, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- POCUS Certificate of Completion. Society of Hospital Medicine. Available online: https://www.hospitalmedicine.org/clinical-topics/ultrasound/pocus-certificate-of-completion/ (accessed on 1 March 2024).
- POCUS for Physician Assistants. Point-of-Care Ultrasound Certification Academy. Available online: https://www.pocus.org/pocus-for-physician-assistants/ (accessed on 1 March 2024).
- Point of Care Ultrasound (POCUS) Pathway for Internal Medicine. American College of Physicians. Available online: https://www.acponline.org/meetings-courses/focused-topics/point-of-care-ultrasound-pocus-for-internal-medicine (accessed on 1 March 2024).
- Point-of-Care Ultrasound Certificate of Completion. Chest. Available online: https://www.chestnet.org/learning-and-events/learning/certificate-of-completion/pocus (accessed on 1 March 2024).
- Point-of-Care Ultrasound Certification Academy. Alliance for Physician Certification and Advancement. Available online: https://www.apca.org/point-of-care-ultrasound-pocus/ (accessed on 1 March 2024).
- Lamperti, M.; Biasucci, D.G.; Disma, N.; Pittiruti, M.; Breschan, C.; Vailati, D.; Subert, M.; Traskaite, V.; Macas, A.; Estebe, J.P.; et al. European Society of Anaesthesiology guidelines on peri-operative use of ultrasound-guided for vascular access (PERSEUS vascular access). Eur. J. Anaesthesiol. 2020, 37, 344–376. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Grimm, R.; Johri, A.M.; Kimura, B.J.; Kort, S.; Labovitz, A.J.; Lanspa, M.; Phillip, S.; Raza, S.; Thorson, K. Recommendations for echocardiography laboratories participating in cardiac point of care cardiac ultrasound (POCUS) and critical care echocardiography training: Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 409–422, e404. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269, W64. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 March 2024).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Covidence systematic review software; Veritas Health Innovation: Melbourne, Australia. Available online: www.covidence.org (accessed on 1 March 2024).
- Aissaoui, Y.; Jozwiak, M.; Bahi, M.; Belhadj, A.; Alaoui, H.; Qamous, Y.; Serghini, I.; Seddiki, R. Prediction of post-induction hypotension by point-of-care echocardiography: A prospective observational study. Anaesth. Crit. Care Pain. Med. 2022, 41, 101090. [Google Scholar] [CrossRef]
- Andruszkiewicz, P.; Sobczyk, D.; Gorkiewicz-Kot, I.; Kowalik, I.; Gelo, R.; Stach, O. Reliability of focused cardiac ultrasound by novice sonographer in preoperative anaesthetic assessment: An observational study. Cardiovasc. Ultrasound 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, K.; Dey, S.; Neema, P.K.; Mujahid, O.M.; Arora, P.; Kalbande, J. Incidence of postoperative pulmonary congestion as diagnosed by lung ultrasound in surgeries performed under general anaesthesia: A prospective, observational study. Indian. J. Anaesth. 2023, 67, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Huard, G.; Bouchard, J.; Lamarche, Y.; Denault, A.; Albert, M. Doppler Renal Resistance Index for the Prediction of Response to Passive Leg-Raising Following Cardiac Surgery. J. Clin. Ultrasound 2018, 46, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, C.; Tavazzi, G.; Cucciolini, G.; Di Nicolo, P.; Wong, A.; Di Domenico, A.; Germinale, F.; Dotta, F.; Micali, M.; Coccolini, F.; et al. Perioperative Renal Ultrasonography of Arterio-to-Venous Coupling Predicts Postoperative Complications after Major Laparoscopic Urologic Surgery. J. Clin. Med. 2023, 12, 5013. [Google Scholar] [CrossRef]
- Canales, C.; Mazor, E.; Coy, H.; Grogan, T.R.; Duval, V.; Raman, S.; Cannesson, M.; Singh, S.P. Preoperative Point-of-Care Ultrasound to Identify Frailty and Predict Postoperative Outcomes: A Diagnostic Accuracy Study. Anesthesiology 2022, 136, 268–278. [Google Scholar] [CrossRef]
- Cavallari, I.; Mega, S.; Goffredo, C.; Patti, G.; Chello, M.; Di Sciascio, G. Hand-held echocardiography in the setting of pre-operative cardiac evaluation of patients undergoing non-cardiac surgery: Results from a randomized pilot study. Int. J. Cardiovasc. Imaging 2015, 31, 995–1000. [Google Scholar] [CrossRef]
- Chui, J.; Hegazy, A.F.; German, M.; Arango-Ferreira, C.; Fochesato, L.A.; Lavi, R.; Bainbridge, D. Point-of-care lung and cardiac ultrasound (LUCAS) study in hip fracture patients: A prospective cohort study. Can. J. Anaesth. 2023, 70, 1474–1485. [Google Scholar] [CrossRef]
- Cowie, B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia 2011, 66, 268–273. [Google Scholar] [CrossRef]
- Cozza, V.; Barberis, L.; Altieri, G.; Donatelli, M.; Sganga, G.; La Greca, A. Prediction of postoperative nausea and vomiting by point-of-care gastric ultrasound: Can we improve complications and length of stay in emergency surgery? A cohort study. BMC Anesth. 2021, 21, 211. [Google Scholar] [CrossRef]
- Cutright, J. The effect of the bladder scanner policy on the number of urinary catheters inserted. J. Wound Ostomy Cont. Nurs. 2011, 38, 71–76. [Google Scholar] [CrossRef]
- Gai, N.; Lavi, R.; Jones, P.M.; Lee, H.; Naudie, D.; Bainbridge, D. The use of point-of-care ultrasound to diagnose patent foramen ovale in elective hip and knee arthroplasty patients and its association with postoperative delirium. Can. J. Anaesth. 2018, 65, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Sen, I.M.; Bakshi, J. Lung ultrasonography as a tool to guide perioperative atelectasis treatment bundle in head and neck cancer patients undergoing free flap reconstructive surgeries: A preliminary observational study. Braz. J. Otorhinolaryngol. 2022, 88, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Haskins, S.C.; Desai, N.A.; Fields, K.G.; Nejim, J.A.; Cheng, S.; Coleman, S.H.; Nawabi, D.H.; Kelly, B.T. Diagnosis of Intraabdominal Fluid Extravasation After Hip Arthroscopy With Point-of-Care Ultrasonography Can Identify Patients at an Increased Risk for Postoperative Pain. Anesth. Analg. 2017, 124, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Kaydu, A.; Gokcek, E. Inferior vena cava diameter measurements and BUN/creatinine values to determine dehydration in patients with hip fractures preoperatively: A prospective observational study. Medicine 2019, 98, e15197. [Google Scholar] [CrossRef]
- Lamm, R.; Bloom, J.; Collins, M.; Goldman, D.; Beausang, D.; Costanzo, C.; Schwenk, E.S.; Phillips, B. A Role for Gastric Point of Care Ultrasound in Postoperative Delayed Gastrointestinal Functioning. J. Surg. Res. 2022, 276, 92–99. [Google Scholar] [CrossRef]
- Pettey, G.; Hermansen, J.L.; Nel, S.; Moutlana, H.J.; Muteba, M.; Juhl-Olsen, P.; Tsabedze, N.; Chakane, P.M. Ultrasound Hepatic Vein Ratios Are Associated with the Development of Acute Kidney Injury After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1326–1335. [Google Scholar] [CrossRef]
- Ramsingh, D.; Singh, S.; Canales, C.; Guran, E.; Taylor, Z.; Antongiorgi, Z.; Cannesson, M.; Martin, R. The Evaluation Point-of-Care Ultrasound in the Post-Anesthesia Unit-A Multicenter Prospective Observational Study. J. Clin. Med. 2021, 10, 2389. [Google Scholar] [CrossRef]
- Ravetti, C.G.; Vassallo, P.F.; Ataide, T.B.L.S.; Braganca, R.D.; dos Santos, A.C.S.; Lima Bastos, F.; Rocha, G.C.; Muniz, M.R.; Borges, I.N.; Marinho, C.C.; et al. Impact of bedside ultrasound to reduce the incidence of acute renal injury in high-risk surgical patients: A randomized clinical trial. J. Ultrasound 2023, 26, 449–457. [Google Scholar] [CrossRef]
- Szabó, M.; Bozó, A.; Darvas, K.; Soós, S.; Őzse, M.; Iványi, Z.D. The role of ultrasonographic lung aeration score in the prediction of postoperative pulmonary complications: An observational study. BMC Anesth. 2021, 21, 19. [Google Scholar] [CrossRef]
- Szabo, M.; Pleck, A.P.; Soos, S.A.; Keczer, B.; Varga, B.; Szell, J. A preoperative ultrasound-based protocol for optimisation of fluid therapy to prevent early intraoperative hypotension: A randomised controlled study. Perioper. Med. 2023, 12, 30. [Google Scholar] [CrossRef]
- Van De Putte, P.; Vernieuwe, L.; Jerjir, A.; Verschueren, L.; Tacken, M.; Perlas, A. When fasted is not empty: A retrospective cohort study of gastric content in fasted surgical patients. Br. J. Anaesth. 2017, 118, 363–371. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Yin, Y.; Yang, L.; Sun, X.; Zhang, J. Lung ultrasound for evaluating perioperative atelectasis and aeration in the post-anesthesia care unit. J. Clin. Monit. Comput. 2023, 37, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Sugimoto, H.; Yokoyama, H.; Mochizuki, Y.; Taniguchi, K. The renal artery pulsatility index enables real-time monitoring of acute kidney injury after digestive surgery. Surgery 2022, 171, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.J.; Henderson, W.G.; Dyas, A.R.; Bronsert, M.R.; Colborn, K.L.; Lambert-Kerzner, A.; Meguid, R.A. Inpatient Versus Outpatient Surgery: A Comparison of Postoperative Mortality and Morbidity in Elective Operations. World J. Surg. 2023, 47, 627–639. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Godil, S.S.; Asher, A.L.; Parker, S.L.; Devin, C.J. Quality analysis of anterior cervical discectomy and fusion in the outpatient versus inpatient setting: Analysis of 7288 patients from the NSQIP database. Neurosurg Focus 2015, 39, E9. [Google Scholar] [CrossRef]
- Cooper, M.C.; Jones, J.; Pascual, M.; Field, S.; Rendon, J.M.; Kulstad, C.; Dixon, B.; Pham Tu, K.; Narayan, A.; Pyle, H.; et al. Can Medical Students Learn and Perform POCUS in the Pediatric Emergency Department? Implementation of a Short Curriculum. POCUS J. 2022, 7, 171–178. [Google Scholar] [CrossRef]
- Russell, F.M.; Herbert, A.; Peterson, D.; Wallach, P.M.; Ferre, R.M. Assessment of Medical Students’ Ability to Integrate Point-of-Care Cardiac Ultrasound Into a Case-Based Simulation After a Short Intervention. Cureus 2022, 14, e27513. [Google Scholar] [CrossRef]
- McIsaac, D.I.; Bryson, G.L. Glucagon-like peptide-1 receptor agonists and aspiration risk. BMJ 2024, 387, q1986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).