High-Fidelity MicroCT Reconstructions of Cardiac Devices Enable Patient-Specific Simulation for Structural Heart Interventions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Device Samples
2.3. Image Acquisition
2.4. Image Processing and Reverse Modeling
2.5. Preprocedural Simulations
2.6. Statistical Analysis
3. Results
3.1. Agreement Between Simulated and Postprocedural Measurements
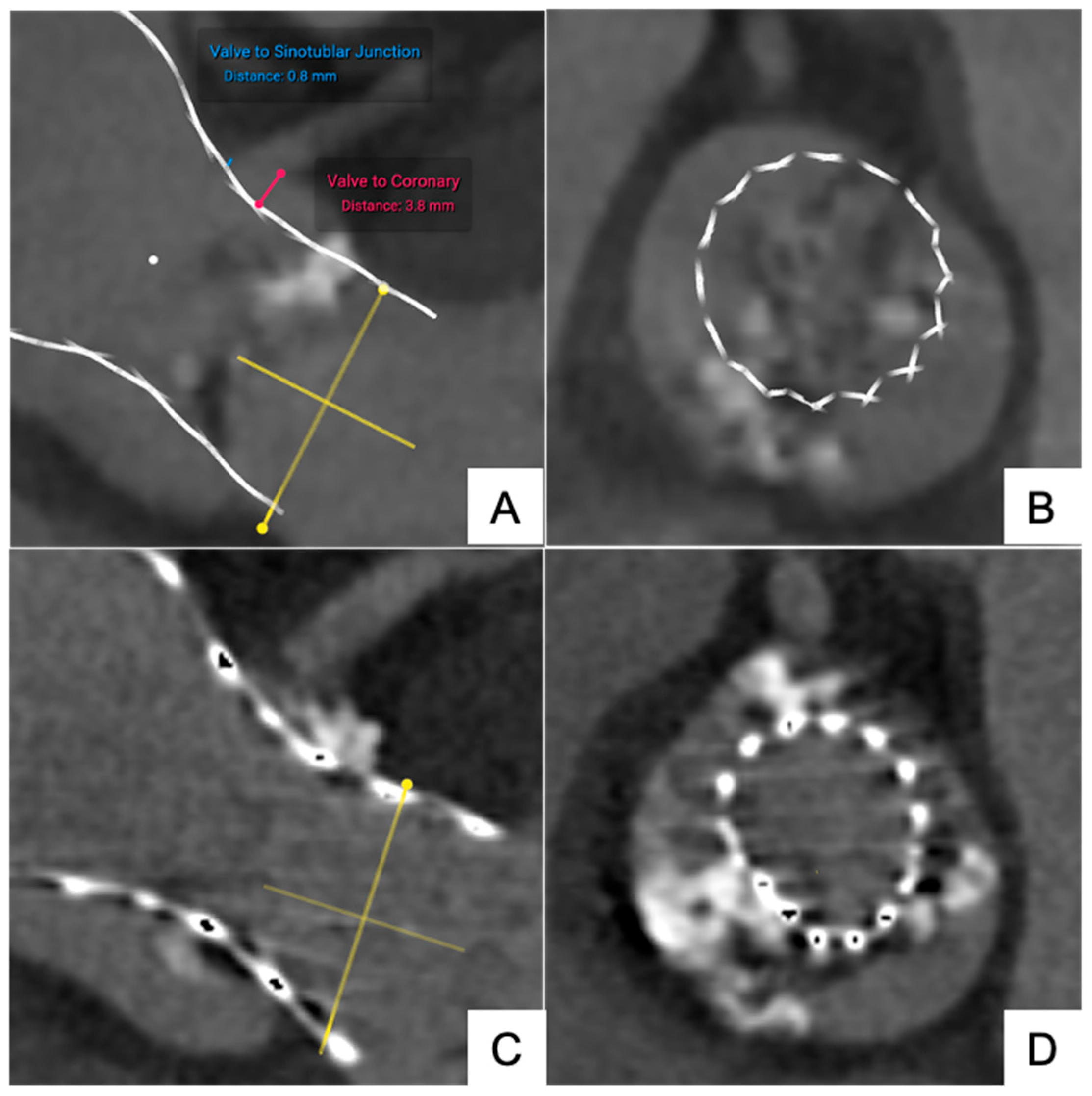
3.1.1. Transfemoral Self-Expanding Transcatheter Aortic Valve
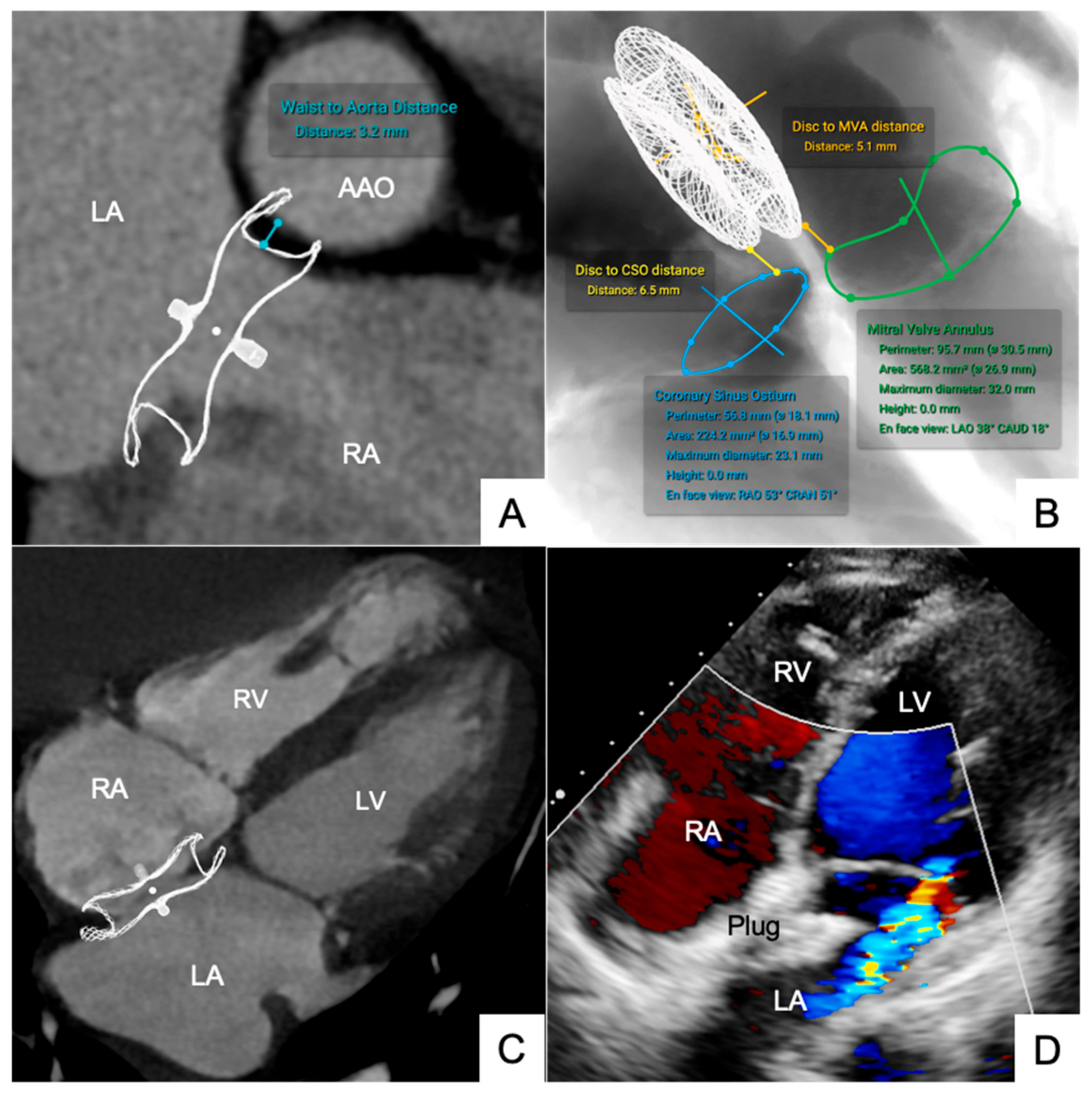
3.1.2. Occluders for ASD/VSD and PDA
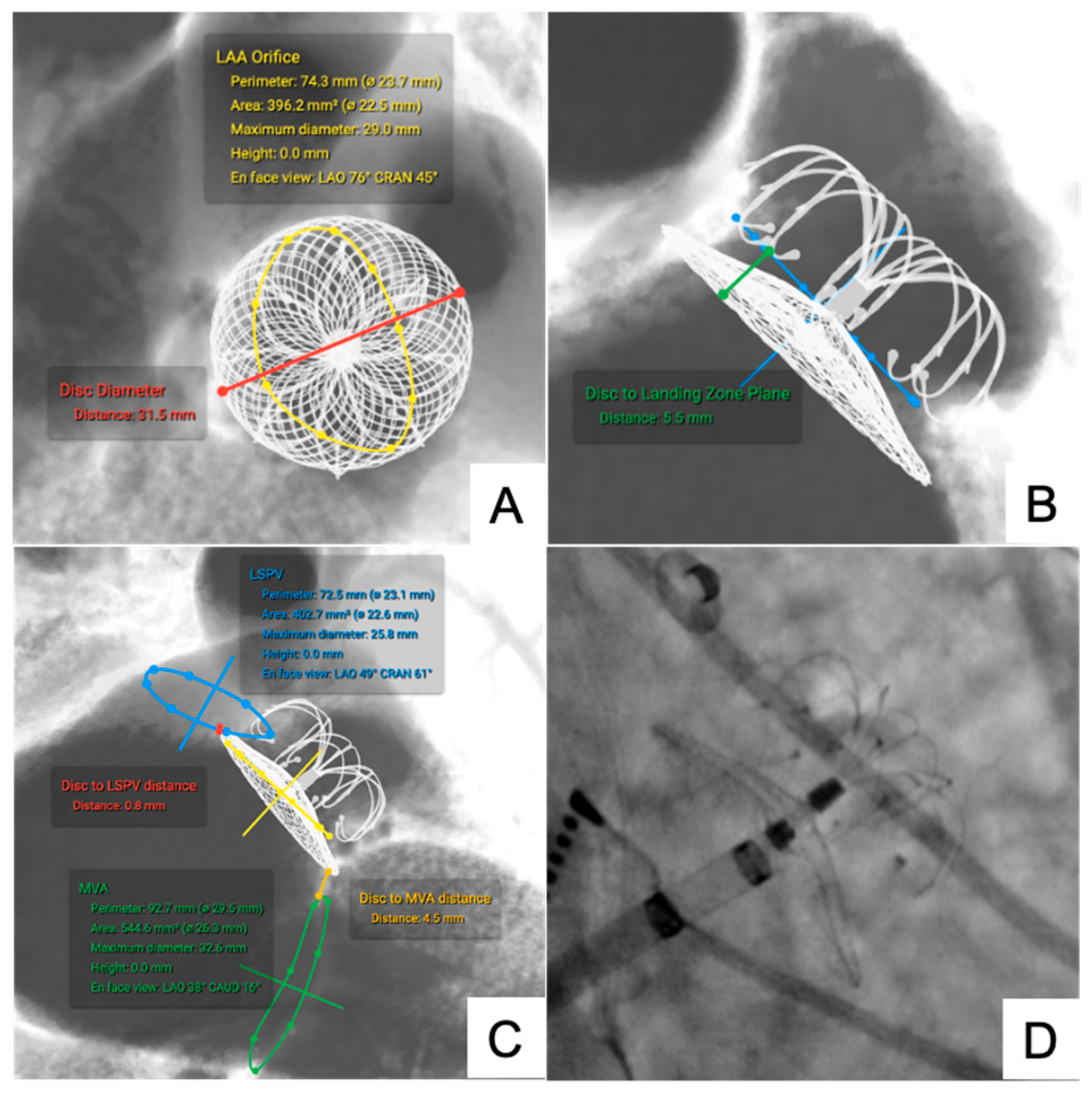
3.1.3. LAA Occluder
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLA | Stereolithography |
| CT | Computed tomography |
| DICOM | Digital imaging and communications in medicine |
| THV | Transcatheter heart valve |
| MSCT | Multi-slice computed tomography |
| microCT | Micro-computed tomography |
| ASD | Atrial septal defect |
| VSD | Ventricular septal defect |
| PDA | Patent ductus arteriosus |
| LAA | Left atrial appendage |
| ICC | Intra-class correlation coefficients |
Appendix A

References
- Xu, B.; Betancor, J.; Sato, K.; Harb, S.; Rehman, K.A.; Patel, K.; Kumar, A.; Cremer, P.C.; Jaber, W.; Rodriguez, L.L.; et al. Computed tomography measurement of the left atrial appendage for optimal sizing of the Watchman device. J. Cardiovasc. Comput. Tomogr. 2018, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Faza, N.N.; Harb, S.C.; Wang, D.D.; van den Dorpel, M.M.P.; Van Mieghem, N.; Little, S.H. Physical and Computational Modeling for Transcatheter Structural Heart Interventions. JACC Cardiovasc. Imaging 2024, 17, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Eng, M.; Kupsky, D.; Myers, E.; Forbes, M.; Rahman, M.; Zaidan, M.; Parikh, S.; Wyman, J.; Pantelic, M.; et al. Application of 3-Dimensional Computed Tomographic Image Guidance to WATCHMAN Implantation and Impact on Early Operator Learning Curve: Single-Center Experience. JACC Cardiovasc. Interv. 2016, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Atala, A. Printing Technologies for Medical Applications. Trends Mol. Med. 2016, 22, 254–265. [Google Scholar] [CrossRef] [PubMed]
- El Sabbagh, A.; Eleid, M.F.; Al-Hijji, M.; Anavekar, N.S.; Holmes, D.R.; Nkomo, V.T.; Oderich, G.S.; Cassivi, S.D.; Said, S.M.; Rihal, C.S.; et al. The Various Applications of 3D Printing in Cardiovascular Diseases. Curr. Cardiol. Rep. 2018, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Van De Bruaene, A.; Farooqi, K.; Valverde, I.; Jung, C.; Votta, E.; Sturla, F.; Diller, G.P.; Brida, M.; Sun, Z.; et al. Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: An exciting future. Eur. Heart J. 2022, 43, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, X.; Han, F.; Zhao, Q.; Xie, T.; Wu, J.; Zhang, Y. 3D printing of self-healing personalized liver models for surgical training and preoperative planning. Nat. Commun. 2023, 14, 8447. [Google Scholar] [CrossRef] [PubMed]
- Borges-Rosa, J.; Oliveira-Santos, M.; Paiva, L.; Puga, L.; Botelho, A.; Costa, M.; Gonçalves, L. Guiding Rescue LAMPOON Through Personalised 3D Simulators: The Role of 3D Printed Models in Complex Cardiac Interventions. Can. J. Cardiol. 2024, 40, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.X.; Geng, J.W.; Luan, W.Y.; Zhang, F.; Miao, Y.D. Bibliometric analysis of three-dimensional printing applications in cardiovascular diseases: A correspondence. Int. J. Surg. 2024, 110, 1322–1324. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, Y.; Yu, K.; Liu, L.; Fu, J.; Yao, X.; Zhang, A.; He, Y. 3D Printing of Physical Organ Models: Recent Developments and Challenges. Adv. Sci. 2021, 8, e2101394. [Google Scholar] [CrossRef] [PubMed]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef] [PubMed]
- Harb, S.C.; Rodriguez, L.L.; Vukicevic, M.; Kapadia, S.R.; Little, S.H. Three-Dimensional Printing Applications in Percutaneous Structural Heart Interventions. Circ. Cardiovasc. Imaging 2019, 12, e009014. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.A.; Mitsouras, D.; Yoo, S.J.; Liu, P.P.; Chatzizisis, Y.S.; Rybicki, F.J. Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 2016, 13, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2020, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Capellini, K.; Ait-Ali, L.; Pak, V.; Cantinotti, M.; Murzi, M.; Vignali, E.; Fanni, B.M.; Clemente, A.; Celi, S.; Gasparotti, E. Three-dimensional printed models as an effective tool for the management of complex congenital heart disease. Front. Bioeng. Biotechnol. 2024, 12, 1369514. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, M.; Russo, G.; De Siati, M.; Tomao, F.; Massaro, G.; Benedetto, D.; Longoni, M.; Matteucci, A.; Maffi, V.; Mariano, E.G.; et al. Holographic mixed reality for planning transcatheter aortic valve replacement. Int. J. Cardiol. 2024, 412, 132330. [Google Scholar] [CrossRef] [PubMed]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Singh, G.K.; Varughese, J.; Nguyen, H.; Billadello, J.J.; Sheybani, E.F.; Woodard, P.K.; Manning, P.; Eghtesady, P. 3D Printing in Complex Congenital Heart Disease: Across a Spectrum of Age, Pathology, and Imaging Techniques. JACC Cardiovasc. Imaging 2017, 10, 953–956. [Google Scholar] [CrossRef] [PubMed]

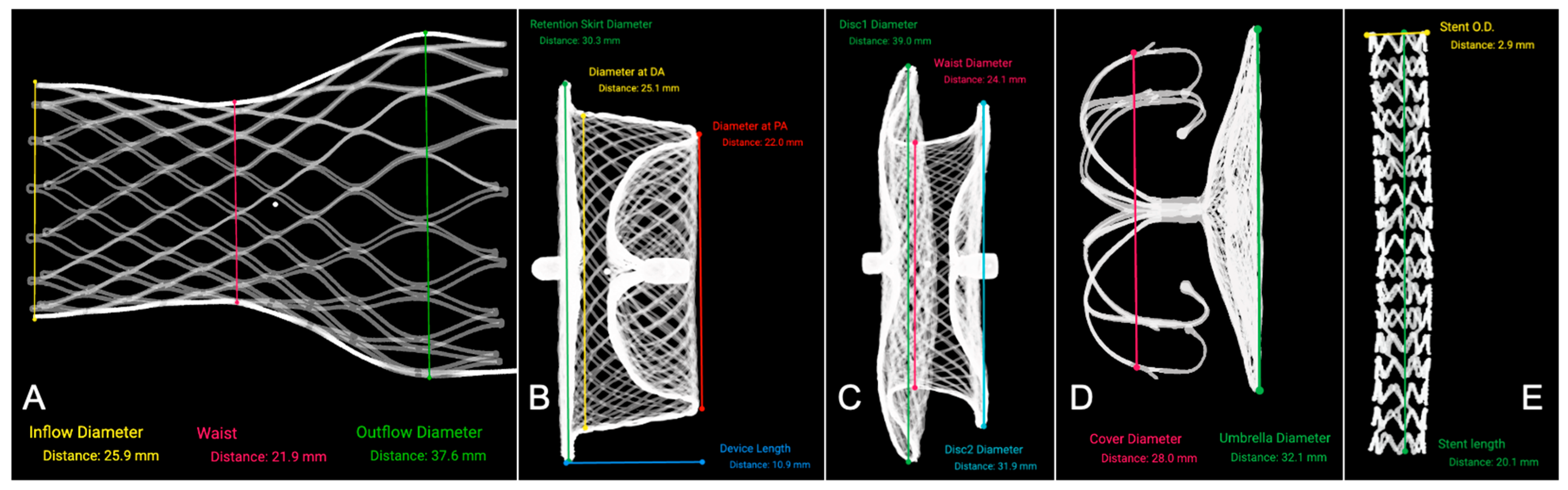
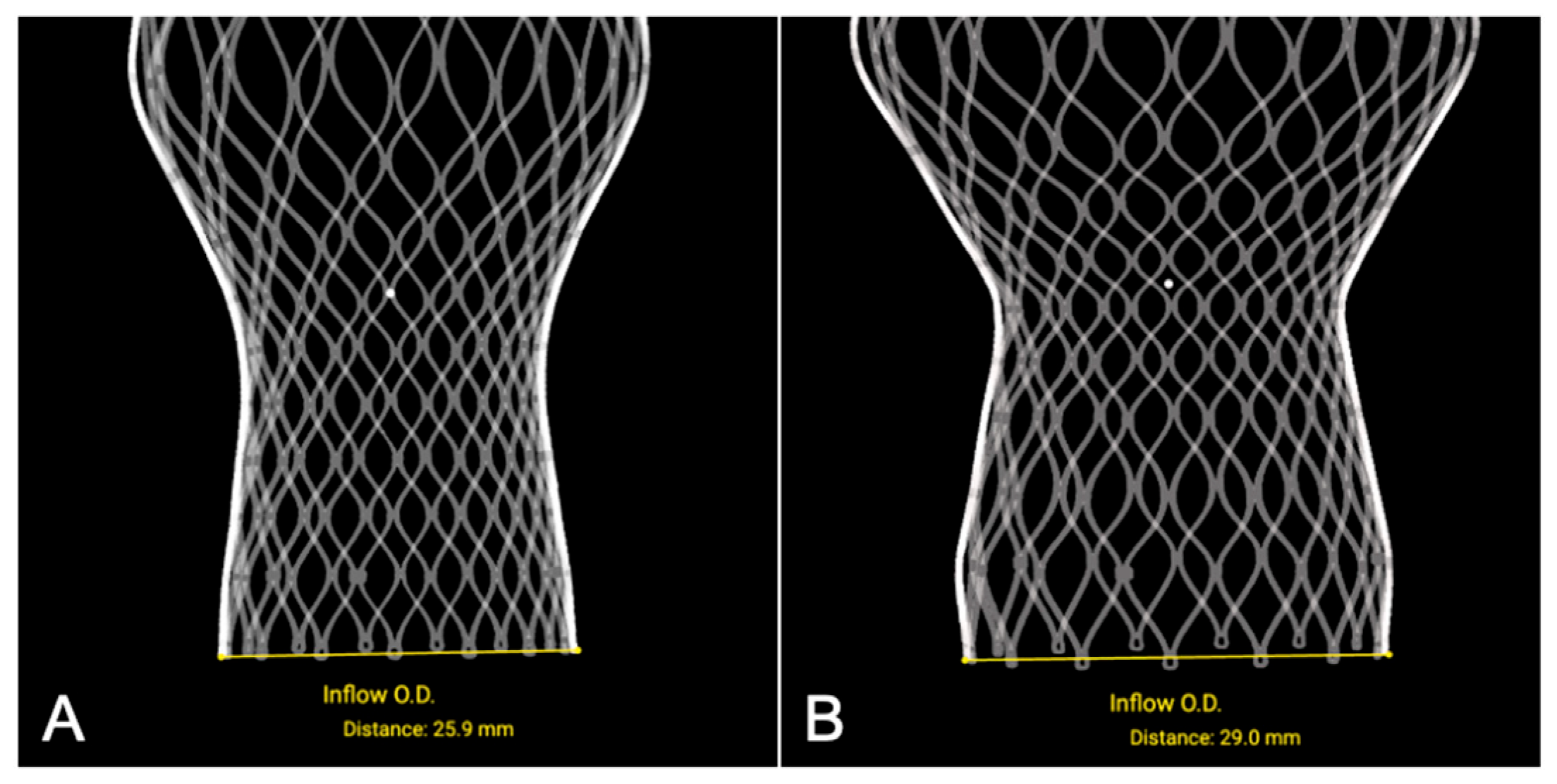
| Device Type | Nominal Parameter (mm) | N | Reconstructed Parameter (mm) | ΔDistance (mm) |
|---|---|---|---|---|
| THV (Inflow) | 23 | 3 | 22.9 ± 0.26 | −0.10 ± 0.26 |
| 26 | 3 | 25.9 ± 0.30 | −0.10 ± 0.30 | |
| 29 | 2 | 29.1 ± 0.14 | +0.10 ± 0.14 | |
| ASD Occluder (Left Disc) | 18 | 1 | 17.8 | −0.20 |
| 20 | 2 | 20.1 ± 0.14 | +0.10 ± 0.14 | |
| 24 | 1 | 23.9 | −0.10 | |
| VSD Occluder (Left Disc) | 10 | 1 | 10.1 | +0.10 |
| 12 | 1 | 11.8 | −0.20 | |
| LAA Occluder (Umbrella) | 28 | 2 | 28.1 ± 0.14 | +0.10 ± 0.14 |
| 32 | 2 | 31.8 ± 0.28 | −0.20 ± 0.28 | |
| 34 | 1 | 33.9 | −0.10 | |
| PDA Occluder (Left Disc) | 8 | 1 | 7.9 | −0.10 |
| 10 | 1 | 10.1 | +0.10 | |
| 22 | 1 | 12.2 | +0.20 | |
| Coronary Stent | 3.0 × 20.0 | 1 | 2.9 × 20.1 | +0.01 |
| Device Type | Measurement Parameter | Simulated (mm) | Postprocedural (mm) | ΔDistance (%) | ICC | Bias (mm) | 95% LoA (mm) |
|---|---|---|---|---|---|---|---|
| THV (n = 27) | VTLC Distance | 3.18 ± 0.58 | 3.42 ± 0.60 | 7.25 ± 1.84 | 0.94 | 0.24 | −0.10 to +0.58 |
| VTRC Distance | 4.57 ± 0.66 | 4.80 ± 0.68 | 2.42 ± 0.88 | 0.92 | 0.23 | −0.15 to +0.61 | |
| LCC Depth | 6.34 ± 0.70 | 7.60 ± 0.72 | 4.05 ± 1.02 | 0.93 | 0.26 | −0.13 to +0.65 | |
| NCC Depth | 5.22 ± 0.28 | 5.43 ± 0.30 | 3.81 ± 0.80 | 0.94 | 0.21 | −0.09 to +0.51 | |
| ASD Occluder (n = 6) | Disc to MVA | 9.48 ± 2.20 | 10.70 ± 2.32 | 3.15 ± 0.68 | 0.95 | 0.22 | −0.10 to +0.54 |
| Disc to CSO | 7.28 ± 2.82 | 9.05 ± 3.04 | 5.10 ± 1.16 | 0.96 | 0.21 | −0.12 to +0.50 | |
| Waist to AAO | 5.48 ± 2.25 | 6.65 ± 2.30 | 3.12 ± 0.70 | 0.95 | 0.23 | −0.11 to +0.56 | |
| LAA Occluder (n = 6) | Disc to MVA | 6.10 ± 2.20 | 6.74 ± 2.30 | 3.88 ± 0.76 | 0.93 | 0.24 | −0.11 to +0.59 |
| Disc to LSPV | 3.82 ± 1.70 | 4.25 ± 2.20 | 2.60 ± 1.05 | 0.91 | 0.22 | −0.10 to +0.55 | |
| Disc to LZP | 6.78 ± 2.05 | 7.20 ± 2.54 | 4.56 ± 1.64 | 0.92 | 0.21 | −0.12 to +0.56 | |
| VSD Occluder (n = 3) | Center to AVA | 3.96 ± 1.40 | 5.15 ± 0.48 | 3.70 ± 0.90 | 0.91 | 0.22 | −0.10 to +0.53 |
| Center to AVN | 13.78 ± 3.40 | 11.00 ± 4.20 | 12.50 ± 4.05 | 0.91 | 0.22 | −0.11 to +0.54 | |
| PDA Occluder (n = 4) | Disc to AAM | 4.06 ± 0.88 | 3.98 ± 1.90 | 2.45 ± 1.08 | 0.93 | 0.24 | −0.13 to +0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhou, Y.; Chen, Y.; Peng, Y.; Chen, M.; Feng, Y. High-Fidelity MicroCT Reconstructions of Cardiac Devices Enable Patient-Specific Simulation for Structural Heart Interventions. J. Clin. Med. 2025, 14, 7341. https://doi.org/10.3390/jcm14207341
Zhu Z, Zhou Y, Chen Y, Peng Y, Chen M, Feng Y. High-Fidelity MicroCT Reconstructions of Cardiac Devices Enable Patient-Specific Simulation for Structural Heart Interventions. Journal of Clinical Medicine. 2025; 14(20):7341. https://doi.org/10.3390/jcm14207341
Chicago/Turabian StyleZhu, Zhongkai, Yaojia Zhou, Yong Chen, Yong Peng, Mao Chen, and Yuan Feng. 2025. "High-Fidelity MicroCT Reconstructions of Cardiac Devices Enable Patient-Specific Simulation for Structural Heart Interventions" Journal of Clinical Medicine 14, no. 20: 7341. https://doi.org/10.3390/jcm14207341
APA StyleZhu, Z., Zhou, Y., Chen, Y., Peng, Y., Chen, M., & Feng, Y. (2025). High-Fidelity MicroCT Reconstructions of Cardiac Devices Enable Patient-Specific Simulation for Structural Heart Interventions. Journal of Clinical Medicine, 14(20), 7341. https://doi.org/10.3390/jcm14207341







