Systematic Notchplasty in Primary ACL Reconstruction Using Hamstring Autografts: A Prospective Cohort Study on the Rate of Secondary Arthrolysis
Abstract
1. Introduction
2. Materials and Methods
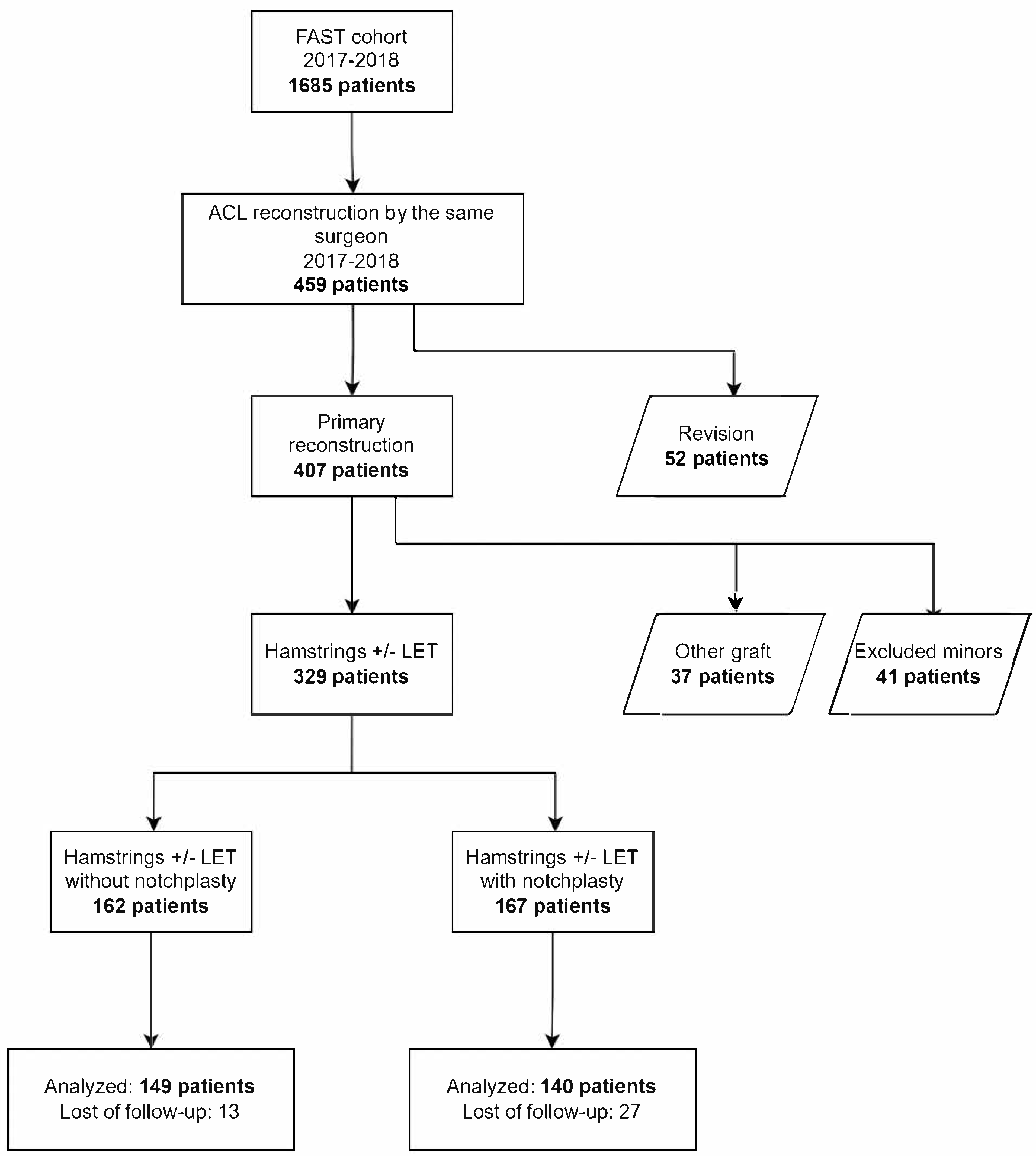
2.1. Study Population
2.2. Patient Characteristics
2.3. Study Protocol, Surgical Technique
2.4. Statistical Analysis
3. Results
3.1. Rate of Arthrolysis and Other Complications
3.2. Functional Outcome and Return to Sport
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerra-Pinto, F.; Thaunat, M.; Daggett, M.; Kajetanek, C.; Marques, T.; Guimaraes, T.; Quelard, B.; Sonnery-Cottet, B. Hamstring Contracture After ACL Reconstruction Is Associated With an Increased Risk of Cyclops Syndrome. Orthop. J. Sports Med. 2017, 5, 2325967116684121. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.; Cristiani, R.; Edman, G.; Forssblad, M.; Stålman, A. One sixth of primary anterior cruciate ligament reconstructions may undergo reoperation due to complications or new injuries within 2 years. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Noailles, T.; Chalopin, A.; Boissard, M.; Lopes, R.; Bouguennec, N.; Hardy, A. Incidence and risk factors for cyclops syndrome after anterior cruciate ligament reconstruction: A systematic literature review. Orthop. Traumatol. Surg. Res. 2019, 105, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Panisset, J.C.; Pailhé, R.; Schlatterer, B.; Sigwalt, L.; Sonnery-Cottet, B.; Lutz, C.; Lustig, S.; Batailler, C.; Bertiaux, S.; Ehkirch, F.P.; et al. Short-term complications in intra- and extra-articular anterior cruciate ligament reconstruction. Comparison with the literature on isolated intra-articular reconstruction. A multicenter study by the French Arthroscopy Society. Orthop. Traumatol. Surg. Res. 2017, 103, S231–S236. [Google Scholar] [CrossRef]
- Sanders, T.L.; Kremers, H.M.; Bryan, A.J.; Kremers, W.K.; Stuart, M.J.; Krych, A.J. Procedural intervention for arthrofibrosis after ACL reconstruction: Trends over two decades. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 532–537. [Google Scholar] [CrossRef]
- Hussein, M.; van Eck, C.F.; Cretnik, A.; Dinevski, D.; Fu, F.H. Biomechanical Evaluation of Different Anterior Cruciate Ligament Reconstruction Techniques and Risk of Graft Impingement. Am. J. Sports Med. 2012, 40, 815–823. [Google Scholar] [CrossRef]
- Orsi, A.D.; Canavan, P.K.; Vaziri, A.; Goebel, R.; Kapasi, O.A.; Nayeb-Hashemi, H. The effects of graft size and insertion site location during anterior cruciate ligament reconstruction on intercondylar notch impingement. Knee 2017, 24, 525–535. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Archbold, P.; Cucurulo, T.; Fayard, J.-M.; Bortolletto, J.; Thaunat, M.; Prost, T.; Chambat, P. The influence of the tibial slope and the size of the intercondylar notch on rupture of the anterior cruciate ligament. J. Bone Jt. Surg. Br. Vol. 2011, 93, 1475–1478. [Google Scholar] [CrossRef]
- Wilson, W.T.; Hopper, G.P.; O’Boyle, M.; Henderson, L.; Blyth, M.J.G. Quantifying graft impingement in anterior cruciate ligament reconstruction. Knee 2022, 34, 270–278. [Google Scholar] [CrossRef]
- Keklikci, K.; Yapici, C.; Kim, D.; Linde-Rosen, M.; Smolinski, P.; Fu, F.H. The effect of notchplasty in anterior cruciate ligament reconstruction: A biomechanical study in the porcine knee. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1915–1921. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Yoo, Y.-S.; Kim, Y.S.; Jang, S.-W.; Song, S.Y.; Hyun, Y.S.; Smolinski, P.; Fu, F.H. The Effect of Notchplasty on Tunnel Widening in Anterior Cruciate Ligament Reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 739–746. [Google Scholar] [CrossRef]
- Ranuccio, F.; Familiari, F.; Tedesco, G.; La Camera, F.; Gasparini, G. Effects of Notchplasty on Anterior Cruciate Ligament Reconstruction: A Systematic Review. Joints 2017, 5, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. A Systematic Review of the Psychological Factors Associated With Returning to Sport Following Injury. Br. J. Sports Med. 2013, 47, 1120–1126. [Google Scholar] [CrossRef]
- Rossi, M.J.; Lubowitz, J.H.; Guttmann, D. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am. J. Sports Med. 2002, 30, 152. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.K.; Kocher, M.S.; Rodkey, W.G.; Steadman, J.R. Reliability, validity, and responsiveness of the Lysholm knee score and Tegner activity scale for patients with meniscal injury of the knee. J. Bone Jt. Surg. Am. 2006, 88, 698–705. [Google Scholar]
- Collins, N.J.; Prinsen Ca, C.; Christensen, R.; Bartels, E.M.; Terwee, C.B.; Roos, E.M. Knee Injury and Osteoarthritis Outcome Score (Knee injury and Osteoarthritis Outcome Score (KOOS)): Systematic review and meta-analysis of measurement properties. Osteoarthr. Cartil. 2016, 24, 1317–1329. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Yagishita, K.; Watanabe, T.; Mochizuki, T.; Horie, M.; Nakamura, T.; Sekiya, I. Effect of Notchplasty in Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2014, 42, 1813–1821. [Google Scholar] [CrossRef]
- May, D.A.; Snearly, W.N.; Bents, R.; Jones, R. MR imaging findings in anterior cruciate ligament reconstruction: Evaluation of notchplasty. AJR Am. J. Roentgenol. 1997, 169, 217–222. [Google Scholar] [CrossRef]
- Kitridis, D.; Tsifountoudis, I.; Georgiannos, D.; Tsikopoulos, K.; Givissis, P.; Bisbinas, I. Does Bone Regrow After Notchplasty in ACL Reconstruction? A Prospective Computed Tomography Study With 2-Year Follow-up. Orthop. J Sports Med. 2021, 9, 23259671211029228. [Google Scholar] [CrossRef]
- Kanamiya, T.; Hara, M.; Naito, M. Magnetic resonance imaging evaluation after re-notchplasty at second-look arthroscopy. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 584–588. [Google Scholar] [CrossRef]
- Murray, M.M.; Kiapour, A.M.; Kalish, L.A.; Ecklund, K.; The BEAR Trial Team; Freiberger, C.; Henderson, R.; Kramer, D.; Micheli, L.; Yen, Y.-M.; et al. Predictors of Healing Ligament Size and Magnetic Resonance Signal Intensity at 6 Months After Bridge-Enhanced Anterior Cruciate Ligament Repair. Am. J. Sports Med. 2019, 47, 1361–1369. [Google Scholar] [CrossRef]
- van Eck, C.F.; Martins, C.A.Q.; Vyas, S.M.; Celentano, U.; van Dijk, C.N.; Fu, F.H. Femoral intercondylar notch shape and dimensions in ACL-injured patients. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, R.; Meredith, S.J.; Lian, J.; Li, R.; Nickoli, M.; Fu, F.H.; Musahl, V. Intercondylar Notch Measurement During Arthroscopy and on Preoperative Magnetic Resonance Imaging. Arthrosc. Tech. 2019, 8, e1263–e1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, X.; Hu, X.Q.; Jiang, Y.F.; Ao, Y.F. Association between time from anterior cruciate ligament injury to reconstruction and morphological changes of the intercondylar notch using MRI and arthroscopy. Knee 2021, 31, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, G.; Fang, Z.; Zhang, X.; Huangfu, X.; Zhao, J. Assessment of relationship between three dimensional femoral notch volume and anterior cruciate ligament injury in Chinese Han adults: A retrospective MRI study. Int. Orthop. 2019, 43, 1231–1237. [Google Scholar] [CrossRef]
- Delaloye, J.R.; Murar, J.; Vieira, T.D.; Franck, F.; Pioger, C.; Helfer, L.; Saithna, A.; Sonnery-Cottet, B. Knee Extension Deficit in the Early Postoperative Period Predisposes to Cyclops Syndrome After Anterior Cruciate Ligament Reconstruction: A Risk Factor Analysis in 3633 Patients From the SANTI Study Group Database. Am. J. Sports Med. 2020, 48, 565–572. [Google Scholar] [CrossRef]
- Pape, D.; Seil, R.; Adam, F.; Gödde, S.; Georg, T.; Rupp, S.; Kohn, D. Blood loss in anterior cruciate ligament (ACL) reconstruction with and without intercondylar notchplasty: Does it affect the clinical outcome? Arch. Orthop. Trauma Surg. 2001, 121, 574–577. [Google Scholar] [CrossRef]
| Total | Without Notchplasty | With Notchplasty | p Value | |
|---|---|---|---|---|
| Number (%) | 289 | 149 (51.6) | 140 (48.4) | |
| Men (%) | 177 (61.2) | 92 (61.7) | 85 (60.7) | n.s. |
| Age at surgery (years) | 30.7 | 30.7 | 30.7 | n.s. |
| BMI | 25.3 | 25.5 | 25.2 | n.s. |
| Practice of sport | n.s. | |||
| 3 (1.0) | 1 (0.7) | 2 (1.4) | |
| 25 (8.7) | 17 (11.4) | 8 (5.7) | |
| 123 (42.6) | 65 (43.6) | 58 (41.4) | |
| 122 (42.2) | 58 (38.9) | 64 (45.7) | |
| 16 (5.5) | 8 (5.4) | 8 (5.7) | |
| Type of sport | n.s. | |||
| 2 (0.7) | 0 (0.0) | 2 (1.4) | |
| 34 (11.8) | 19 (12.8) | 15 (10.7) | |
| 53 (18.3) | 31 (20.8) | 22 (15.7) | |
| 200 (69.2) | 99 (66.4) | 101 (72.1) | |
| Lachman | n.s. | |||
| 3 (1.0) | 3 (2.0) | 0 (0.0) | |
| 204 (70.6) | 97 (65.1) | 107 (76.4) | |
| 42 (14.5) | 27 (18.1) | 15 (10.7) | |
| 40 (13.8) | 22 (14.8) | 18 (12.9) | |
| Pivot shift | n.s. | |||
| 156 (54.0) | 68 (45.6) | 88 (62.9) | |
| 76 (26.3) | 56 (37.6) | 20 (14.3) | |
| 22 (7.6) | 13 (8.7) | 9 (6.4) | |
| 35 (12.1) | 12 (8.1) | 23 (16.4) | |
| IKDC objective | n.s. | |||
| 201 (69.6) | 94 (63.1) | 107 (76.4) | |
| 57 (19.7) | 46 (30.9) | 11 (7.9) | |
| 31 (10.7) | 9 (6.0) | 22 (15.7) | |
| IKDC subjective | 59.2 | 57.5 | 60.9 | n.s. |
| KOOS | 60 | 58.7 | 61.4 | n.s. |
| Lysholm | 70.2 | 69.5 | 71.0 | n.s. |
| Tegner, median (range values) | 7.2 (7) | 7.2 (7) | 7.3 (7) | n.s. |
| ACL-RSI | 38.7 | 39.9 | 37.5 | n.s. |
| Subjective instability | ||||
| 53 (18.3) | 31 (20.8) | 22 (15.7) | |
| 82 (28.4) | 39 (26.2) | 43 (30.7) | |
| 54 (18.7) | 23 (15.4) | 31 (22.1) | |
| 88 (30.4) | 48 (32.2) | 40 (28.6) | |
| 12 (4.2) | 8 (5.4) | 4 (2.9) |
| Total | Without Notchplasty | With Notchplasty | p Value | |
|---|---|---|---|---|
| Number (%) | 289 | 149 (51.6) | 140 (48.4) | |
| Time since 1st accident/surgery (months) | 17.7 | 17.2 | 18.2 | n.s. |
| With extralateral tenodesis | 141 (48.8) | 80 (53.7) | 61 (43.6) | n.s. |
| Chondropathy, n (%) | 12 (4.2) | 7 (4.7) | 5 (3.6) | n.s. |
| 2 | 1 | 1 | |
| 6 | 5 | 1 | |
| 4 | 1 | 3 | |
| Medial meniscal lesions, n (%) | 74 (25.6) | 38 (25.5) | 36 (25.7) | n.s. |
| 10 | 7 | 3 | |
| 52 | 24 | 28 | |
| 12 | 7 | 5 | |
| Lateral meniscal lesions, n (%) | 42 (14.5) | 23 (15.4) | 19 (13.6) | n.s. |
| 6 | 4 | 2 | |
| 21 | 13 | 10 | |
| 15 | 6 | 7 | |
| Femoral tunnel diameter (mm) | 7.8 | 7.8 | 7.8 | n.s. |
| Tibial tunnel diameter (mm) | 7.8 | 7.8 | 7.8 | n.s. |
| Total | Without Notchplasty | With Notchplasty | p Value | |
|---|---|---|---|---|
| Number of patients (%) | 289 | 149 (51.6) | 140 (48.4) | |
| Stiffness with arthrolysis, n (%) | 13 (4.5) | 6 (4.0) | 7 (5.0) | n.s. |
| Retear, n (%) | 3 (1.0) | 2 (1.3) | 1 (0.7) | n.s. |
| Post-reconstruction meniscectomy, n (%) | 5 (1.7) | 3 (2.0) | 2 (1.4) | n.s. |
| Post-reconstruction chondral repair, n (%) | 3 (1.0) | 2 (1.3) | 1 (0.7) | n.s. |
| Other complications | 4 (1.4) | 2 (1.3) | 2 (1.4) | n.s. |
| 2 | 1 | 1 | |
| 0 | 0 | 0 | |
| 1 | 0 | 1 | |
| 1 | 1 | 0 | |
| 5 (1.7) | 5 (3.4) | 0 (0.0) | n.s. |
| Total | Without Notchplasty | With Notchplasty | p Value | |
| Number (%) | 289 | 128 (51.8) | 119 (48.2) | |
| Mean follow-up (months) | 31.8 | 35.4 (11.1) | 27.4 (4.5) | <0.0001 |
| Subjective IKDC | 86.5 | 86.5 | 86.4 | n.s. |
| KOOS | 87.5 | 87.2 | 87.9 | n.s. |
| Lysholm | 90.9 | 90.8 | 90.9 | n.s. |
| Tegner | 6.4 (10) | 6.5 (10) | 6.3 (10) | n.s. |
| ACLR-RSI | 70.0 | 70.3 | 69.8 | n.s. |
| Satisfaction | n.s. | |||
| 2 (0.7) | 2 (1.3) | 0 (0.0) | |
| 20 (6.9) | 11 (7.4) | 9 (6.4) | |
| 86 (29.8) | 50 (33.6) | 36 (25.7) | |
| 181 (62.6) | 86 (57.7) | 95 (67.9) | |
| Subjective instability | n.s. | |||
| 214 (74.0) | 110 (73.8) | 104 (74.3) | |
| 56 (19.4) | 30 (20.1) | 26 (18.6) | |
| 5 (1.7) | 1 (0.7) | 4 (2.9) | |
| 13 (4.5) | 7 (4.7) | 6 (4.3) | |
| 1 (0.3) | 1 (0.7) | 0 (0.0) | |
| Return to running, n (%) | 217 (75.1) | 114 (76.5) | 103 (73.6) | n.s. |
| 10.0 | 9.3 | 11 | n.s. |
| 185 (64.0) | 103 (69.1) | 82 (58.6) | n.s. |
| 14.4 | 13.2 | 15 | n.s. |
| Return to competitive pivot sport, n (%) | 93 (32.2) | 50 (33.6) | 43 (30.7) | n.s. |
| 15.1 | 15.1 | 15 | n.s. |
| 164 (56.7) | 84 (56.4) | 80 (57.1) | n.s. |
| 13.5 | 12.9 | 14 | n.s. |
| Performance level | n.s. | |||
| 10 (3.5) | 3 (2.0) | 7 (5.0) | |
| 41 (14.2) | 17 (11.4) | 24 (17.1) | |
| 129 (44.6) | 70 (47.0) | 59 (42.1) | |
| 80 (27.7) | 42 (28.2) | 38 (27.1) | |
| 29 (10.0) | 17 (11.4) | 12 (8.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saint-Etienne, A.; Hardy, A.; Miele, A.; Lefevre, N.; Grimaud, O.; Meyer, A.; Bohu, Y. Systematic Notchplasty in Primary ACL Reconstruction Using Hamstring Autografts: A Prospective Cohort Study on the Rate of Secondary Arthrolysis. J. Clin. Med. 2025, 14, 7285. https://doi.org/10.3390/jcm14207285
Saint-Etienne A, Hardy A, Miele A, Lefevre N, Grimaud O, Meyer A, Bohu Y. Systematic Notchplasty in Primary ACL Reconstruction Using Hamstring Autografts: A Prospective Cohort Study on the Rate of Secondary Arthrolysis. Journal of Clinical Medicine. 2025; 14(20):7285. https://doi.org/10.3390/jcm14207285
Chicago/Turabian StyleSaint-Etienne, Adrien, Alexandre Hardy, Antonio Miele, Nicolas Lefevre, Olivier Grimaud, Alain Meyer, and Yoann Bohu. 2025. "Systematic Notchplasty in Primary ACL Reconstruction Using Hamstring Autografts: A Prospective Cohort Study on the Rate of Secondary Arthrolysis" Journal of Clinical Medicine 14, no. 20: 7285. https://doi.org/10.3390/jcm14207285
APA StyleSaint-Etienne, A., Hardy, A., Miele, A., Lefevre, N., Grimaud, O., Meyer, A., & Bohu, Y. (2025). Systematic Notchplasty in Primary ACL Reconstruction Using Hamstring Autografts: A Prospective Cohort Study on the Rate of Secondary Arthrolysis. Journal of Clinical Medicine, 14(20), 7285. https://doi.org/10.3390/jcm14207285






