Association of Rapid Early Weight Loss with One-Year Hepatic Steatosis Improvement After Sleeve Gastrectomy: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Perioperative Management
2.3. Data Collection and Variables
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Longitudinal Changes in Metabolic Parameters
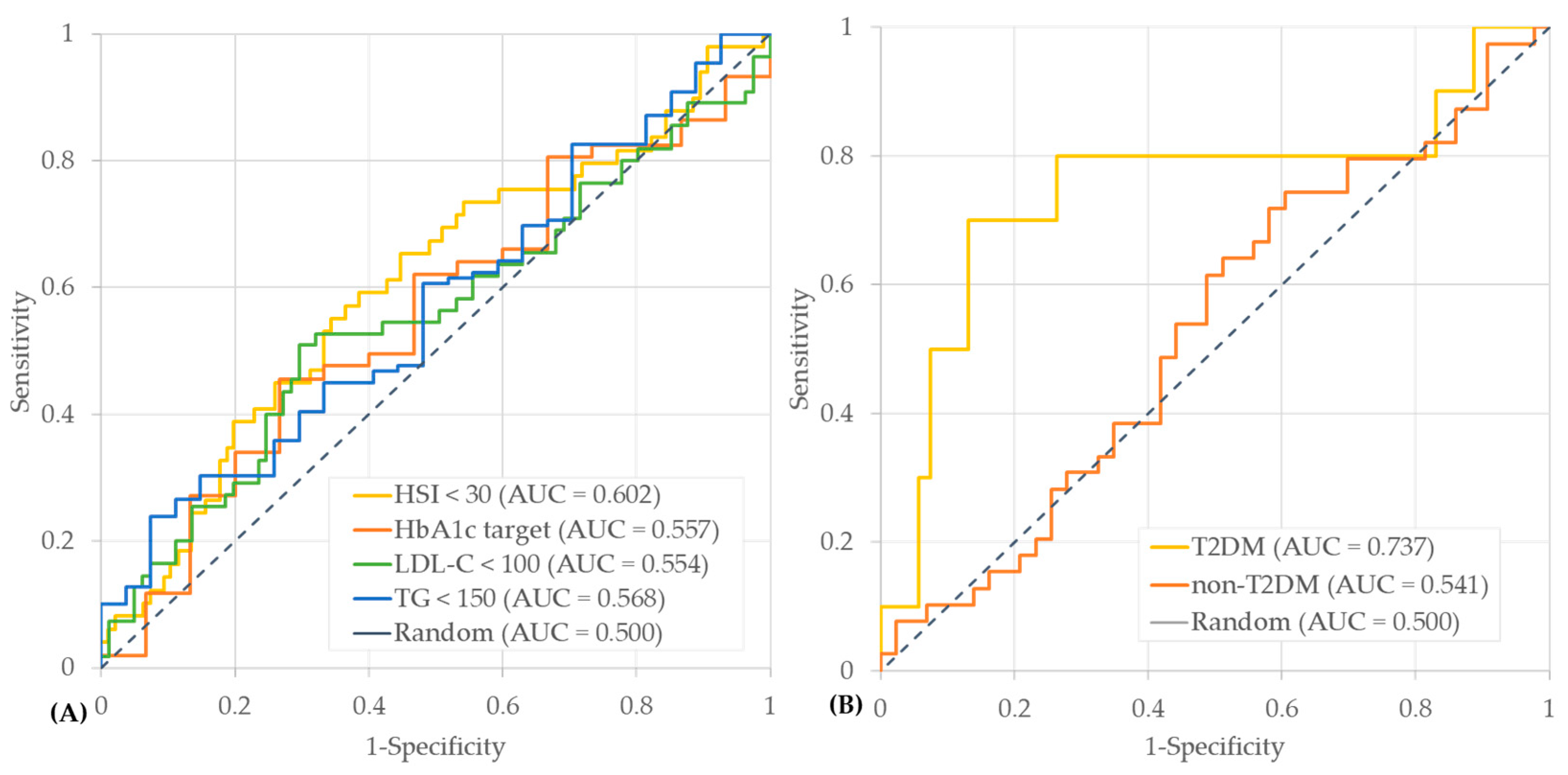
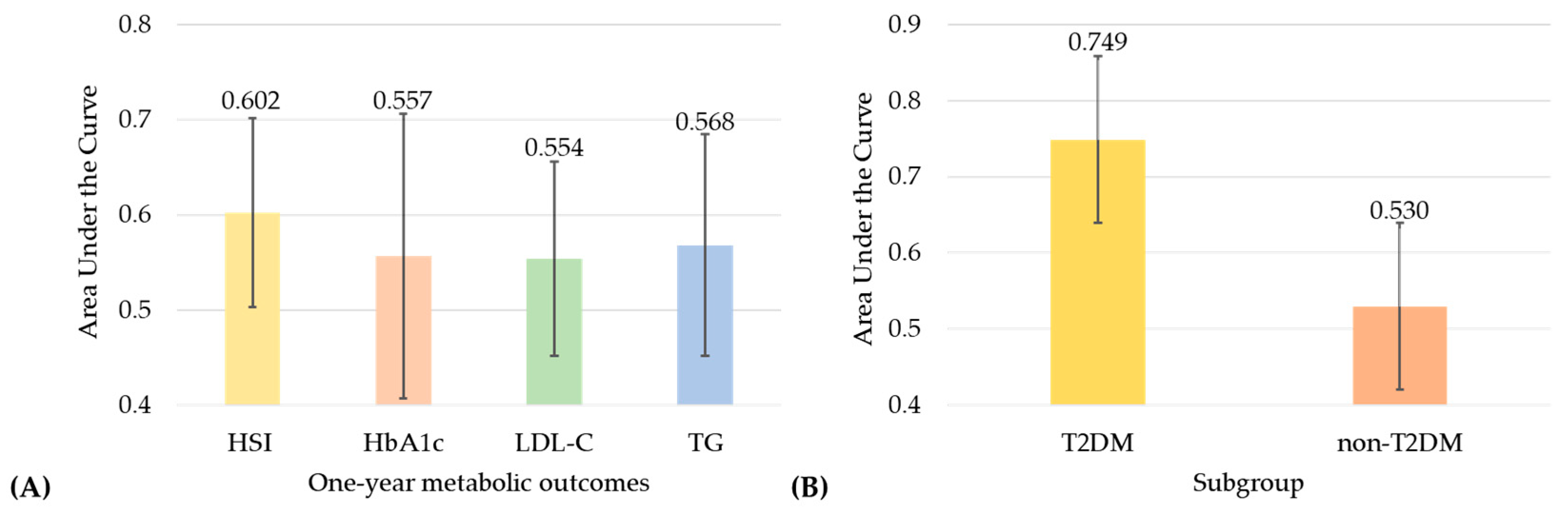
3.3. Predictive Performance of Early Postoperative Weight Loss for One-Year Outcomes
3.4. Association Between Early Weight Loss and One-Year Metabolic Improvements
3.5. Subgroup Analyses by Hepatic Steatosis Severity and Diabetes Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.F.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Brown, O.I.; Drozd, M.; McGowan, H.; Giannoudi, M.; Conning-Rowland, M.; Gierula, J.; Straw, S.; Wheatcroft, S.B.; Bridge, K.; Roberts, L.D.; et al. Relationship Among Diabetes, Obesity, and Cardiovascular Disease Phenotypes: A UK Biobank Cohort Study. Diabetes Care 2023, 46, 1531–1540. [Google Scholar] [CrossRef]
- Formisano, E.; Proietti, E.; Borgarelli, C.; Sukkar, S.G.; Albertelli, M.; Boschetti, M.; Pisciotta, L. The impact of overweight on lipid phenotype in different forms of dyslipidemia: A retrospective cohort study. J. Endocrinol. Investig. 2024, 47, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 2022, 71, 1867–1875. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, M.; Zeng, N.; Liu, Y.; Bai, R.; Zhang, N.; Song, J.; Zhang, P.; Yao, Q.; Yang, Z.; et al. Bariatric surgery for non-alcoholic fatty liver disease in individuals with obesity (Base-NAFLD): Protocol of a prospective multicenter observational follow-up study. BMC Surg. 2021, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48, S167–S180. [Google Scholar] [CrossRef]
- Brown, W.A.; Liem, R.; Al-Sabah, S.; Anvari, M.; Boza, C.; Cohen, R.V.; Ghaferi, A.; Våge, V.; Himpens, J.; Kow, L.; et al. Metabolic Bariatric Surgery Across the IFSO Chapters: Key Insights on the Baseline Patient Demographics, Procedure Types, and Mortality from the Eighth IFSO Global Registry Report. Obes. Surg. 2024, 34, 1764–1777. [Google Scholar] [CrossRef]
- Biter, L.U.; t Hart, J.W.; Noordman, B.J.; Smulders, J.F.; Nienhuijs, S.; Dunkelgrün, M.; Zengerink, J.F.; Birnie, E.; Friskes, I.A.; Mannaerts, G.H.; et al. Long-term effect of sleeve gastrectomy vs Roux-en-Y gastric bypass in people living with severe obesity: A phase III multicentre randomised controlled trial (SleeveBypass). Lancet Reg. Health Eur. 2024, 38, 100836. [Google Scholar] [CrossRef] [PubMed]
- Tettero, O.M.; Monpellier, V.M.; Janssen, I.M.C.; Steenhuis, I.H.M.; van Stralen, M.M. Early Postoperative Weight Loss Predicts Weight Loss up to 5 Years After Roux-En-Y Gastric Bypass, Banded Roux-En-Y Gastric Bypass, and Sleeve Gastrectomy. Obes. Surg. 2022, 32, 2891–2902. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.; Pucci, A.; Carter, N.C.; Elkalaawy, M.; Querci, G.; Magno, S.; Tamberi, A.; Finer, N.; Fiennes, A.G.; Hashemi, M.; et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg. Endosc. 2015, 29, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e1295. [Google Scholar] [CrossRef]
- Navarro-Masip, È.; Mestres, N.; Zorzano-Martínez, M.; Salinas-Roca, B.; Sánchez, E.; López-Cano, C.; Herrerías, F.; de la Fuente, M.C.; Santamaría, M.; León-Mengíbar, J.; et al. Mid-term Effects of Bariatric Surgery on Metabolic Dysfunction-Associated Fatty Liver Disease Remission and Predictive Factors: A Prospective Study with a Focus on Non-invasive Diagnosis. Obes. Surg. 2024, 34, 841–849. [Google Scholar] [CrossRef]
- Turquetil, A.; Morello, R.; Joubert, M.; Le Roux, Y.; Reznik, Y. Early continuous glucose monitoring for predicting remission of type 2 diabetes 1 year after bariatric surgery. Diabetes Metab. 2021, 47, 101255. [Google Scholar] [CrossRef]
- Gummesson, A.; Nyman, E.; Knutsson, M.; Karpefors, M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1295–1305. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48, S27–S49. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, H.W.; Yoo, J.J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; M, S.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Reinshagen, M.; Kabisch, S.; Pfeiffer, A.F.H.; Spranger, J. Liver Fat Scores for Noninvasive Diagnosis and Monitoring of Nonalcoholic Fatty Liver Disease in Epidemiological and Clinical Studies. J. Clin. Transl. Hepatol. 2023, 11, 1212–1227. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Kwon, Y.J.; Lee, E.; Lee, H.S.; Youn, Y.H.; Baik, S.J.; Shin, H.J.; Chae, H.W. Optimal Cutoffs of Fatty Liver Index and Hepatic Steatosis Index in Diagnosing Pediatric Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Gastroenterol. Hepatol. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Kim, H.L.; Kim, S.H.; Moon, M.K. Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement. J. Lipid Atheroscler. 2023, 12, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Moore, M.P.; Cunningham, R.P.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ganga, R.R.; Spencer, N.M.; Pitt, J.B.; Diaz-Arias, A.; Swi, A.I.A.; et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology 2022, 76, 1452–1465. [Google Scholar] [CrossRef]
- Colosimo, S.; Mitra, S.K.; Chaudhury, T.; Marchesini, G. Insulin resistance and metabolic flexibility as drivers of liver and cardiac disease in T2DM. Diabetes Res. Clin. Pract. 2023, 206, 111016. [Google Scholar] [CrossRef]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Aditya, I.; Gmora, S.; Anvari, M.; Hong, D. Laparoscopic Sleeve Gastrectomy Versus Laparoscopic Roux-en-Y Gastric Bypass: A Systematic Review and Meta-analysis of Weight Loss, Comorbidities, and Biochemical Outcomes From Randomized Controlled Trials. Ann. Surg. 2021, 273, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.A.Z.; Tan, I.J.-W.; Ng, H.J.H.; Lomanto, D.; So, J.; Shabbir, A. Early weight loss after laparoscopic sleeve gastrectomy predicts midterm weight loss in morbidly obese Asians. Surg. Obes. Relat. Dis. 2017, 13, 1966–1972. [Google Scholar] [CrossRef]
- Su, Y.T.; Su, Y.H.; Tam, K.W.; Yen, Y.C.; Wang, W.; Huang, M.T.; Wang, S.Y.; Pai, F.Y.; Kuo, C.Y.; Shen, S.C. Prediction of 5-Year Weight Loss and Weight Regain According to Early Weight Loss after Sleeve Gastrectomy. Obes. Surg. 2023, 33, 1366–1372. [Google Scholar] [CrossRef]
- Boustani, P.; Sheidaei, A.; Mokhber, S.; Pazouki, A. Early prediction of suboptimal clinical response after bariatric surgery using total weight loss percentiles. Sci. Rep. 2025, 15, 33173. [Google Scholar] [CrossRef]
- Tasabehji, D.; Saleh, S.; Mokadem, M. Impact of Bariatric Surgery and Endoscopic Therapies on Liver Health in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. J. Clin. Med. 2025, 14, 4012. [Google Scholar] [CrossRef] [PubMed]
- Geerts, A.; Lefere, S. Bariatric surgery for non-alcoholic fatty liver disease: Indications and post-operative management. Clin. Mol. Hepatol. 2023, 29, S276–S285. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, A.; Rabizadeh, S.; Reyhan, S.K.; Hobaby, S.; Afshari, S.A.; Shomalzadeh, M.; Nabipoorashrafi, S.A.; Soran, N.A.; Yadegar, A.; Mohammadi, F.; et al. Impact of bariatric surgery on liver fibrosis indices among type 2 diabetes patients in a national cohort. Sci. Rep. 2025, 15, 1235. [Google Scholar] [CrossRef]
- Baldwin, D.; Chennakesavalu, M.; Gangemi, A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg. Obes. Relat. Dis. 2019, 15, 2123–2130. [Google Scholar] [CrossRef]
- Johansson, H.-E.; Edholm, D.; Kullberg, J.; Rosqvist, F.; Rudling, M.; Straniero, S.; Karlsson, F.A.; Ahlström, H.; Sundbom, M.; Risérus, U. Energy restriction in obese women suggest linear reduction of hepatic fat content and time-dependent metabolic improvements. Nutr. Diabetes 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018, 28, 547–556.e543. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef]
- Tu, J.; Wang, Y.; Jin, L.; Huang, W. Bile acids, gut microbiota and metabolic surgery. Front. Endocrinol. 2022, 13, 929530. [Google Scholar] [CrossRef]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef] [PubMed]
- Katafuchi, T.; Makishima, M. Molecular Basis of Bile Acid-FXR-FGF15/19 Signaling Axis. Int. J. Mol. Sci. 2022, 23, 6046. [Google Scholar] [CrossRef]
- Wahlström, A.; Aydin, Ö.; Olsson, L.M.; Sjöland, W.; Henricsson, M.; Lundqvist, A.; Marschall, H.U.; Franken, R.; van de Laar, A.; Gerdes, V.; et al. Alterations in bile acid kinetics after bariatric surgery in patients with obesity with or without type 2 diabetes. EBioMedicine 2024, 106, 105265. [Google Scholar] [CrossRef]
- Huang, H.H.; Lee, W.J.; Chen, S.C.; Chen, T.F.; Lee, S.D.; Chen, C.Y. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J. Clin. Med. 2019, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos-Roman, M.A.; Valdez, M.J.; Browning, J.D.; Rogers, T.; Parks, E.J. Weight loss in MASLD restores the balance of liver fatty acid sources. J. Clin. Investig. 2025, 135, e174233. [Google Scholar] [CrossRef] [PubMed]
| Overall Cohort | T2DM | Non-T2DM | p-Value | |
|---|---|---|---|---|
| Age (years) | 37.01 ± 9.38 | 38.03 ± 10.30 | 36.21 ± 8.48 | 0.212 |
| Male | 73 (41.2) | 38 (48.7) | 35 (35.4) | 0.073 |
| Comorbidities | ||||
| T2DM | 79 (44.6) | |||
| Hypertension | 141 (79.7) | 66 (84.6) | 75 (75.8) | 0.146 |
| Dyslipidemia | 107 (60.5) | 56 (71.8) | 51 (51.5) | 0.006 |
| Fatty liver | 110 (62.1) | 57 (73.1) | 53 (53.5) | 0.008 |
| Clinical parameters | ||||
| Body weight (kg) | 113.23 ± 23.71 | 113.71 ± 23.39 | 112.86 ± 24.07 | 0.814 |
| BMI (kg/m2) | 39.91 ± 6.61 | 39.60 ± 6.52 | 40.17 ± 6.70 | 0.565 |
| SBP (mmHg) | 145.87 ± 16.55 | 146.72 ± 17.01 | 145.20 ± 16.23 | 0.549 |
| DBP (mmHg) | 88.87 ± 11.81 | 88.03 ± 11.16 | 89.54 ± 12.33 | 0.394 |
| Biological parameters | ||||
| FPG (mg/dL) | 119.15 ± 38.40 | 139.21 ± 48.48 | 103.35 ± 15.14 | <0.001 |
| HbA1c (%) | 6.37 ± 1.34 | 7.34 ± 1.45 | 5.59 ± 0.42 | <0.001 |
| C-peptide (ng/mL) | 4.28 ± 2.26 | 4.70 ± 2.86 | 3.95 ± 1.54 | 0.043 |
| TC (mg/dL) | 180.19 ± 38.62 | 169.37 ± 41.66 | 188.70 ± 33.88 | 0.001 |
| TG (mg/dL) | 176.70 ± 108.63 | 199.50 ± 111.31 | 158.36 ± 103.37 | 0.013 |
| HDL-C (mg/dL) | 44.61 ± 9.64 | 42.22 ± 8.47 | 46.54 ± 10.12 | 0.002 |
| LDL-C (mg/dL) | 113.92 ± 35.18 | 104.58 ± 37.35 | 121.43 ± 31.56 | 0.002 |
| AST (U/L) | 36.11 ± 26.70 | 41.97 ± 33.01 | 31.50 ± 19.36 | 0.014 |
| ALT (U/L) | 44.23 ± 37.22 | 50.68 ± 43.77 | 39.14 ± 30.39 | 0.050 |
| Creatinine (mg/dL) | 0.86 ± 0.73 | 0.96 ± 1.09 | 0.79 ± 0.16 | 0.179 |
| eGFR (mL/min/1.73 m2) | 104.75 ± 18.74 | 104.18 ± 23.24 | 105.20 ± 14.35 | 0.734 |
| HSI | 51.50 ± 7.86 | 52.24 ± 7.22 | 50.92 ± 8.32 | 0.263 |
| FIB-4 | 0.79 ± 0.55 | 0.92 ± 0.68 | 0.69 ± 0.40 | 0.008 |
| Pre-OP | Post-OP 2 W | Post-OP 3 M | p-Value | Post-OP 6 M | p-Value | Post-OP 1 Year | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Body weight | 113.33 ± 23.70 | 104.28 ± 23.67 | 93.75 ± 20.35 | <0.001 ** | 88.17 ± 20.13 | <0.001 ** | 84.85 ± 19.31 | <0.001 ** |
| BMI | 39.91 ± 6.62 | 30.92 ± 6.18 | 27.83 ± 5.31 | <0.001 ** | 26.11 ± 5.27 | <0.001 ** | 25.15 ± 5.03 | <0.001 ** |
| %TWL_2W | 7.90 ± 6.62 | |||||||
| FBS | 119.15 ± 38.3 | 102.41 ± 22.77 | <0.001 ** | 97.53 ± 16.26 | <0.001 ** | 98.67 ± 23.18 | <0.001 ** | |
| HbA1c | 6.37 ± 1.34 | 5.61 ± 0.86 | <0.001 ** | 5.54 ± 0.74 | <0.001 ** | 5.59 ± 0.85 | <0.001 ** | |
| TC | 180.19 ± 38.61 | 179.60 ± 35.19 | 0.724 | 183.67 ± 39.11 | 0.391 | 181.38 ± 33.67 | 0.990 | |
| TG | 176.70 ± 108.62 | 123.37 ± 54.31 | <0.001 ** | 113.66 ± 59.10 | <0.001 ** | 109.45 ± 53.34 | <0.001 ** | |
| HDL-C | 44.61 ± 9.63 | 48.75 ± 33.62 | 0.167 | 51.75 ± 10.56 | <0.001 ** | 57.67 ± 13.11 | <0.001 ** | |
| LDL-C | 113.92 ± 35.17 | 116.31 ± 30.74 | 0.199 | 115.46 ± 33.60 | 0.447 | 107.38 ± 29.53 | 0.143 | |
| AST | 36.11 ± 26.69 | 29.02 ± 65.53 | 0.279 | 20.93 ± 7.11 | <0.001 ** | 20.65 ± 6.75 | <0.001 ** | |
| ALT | 44.23 ± 37.22 | 26.58 ± 73.02 | 0.009 * | 15.41 ± 9.65 | <0.001 ** | 15.91 ± 10.53 | <0.001 ** | |
| HSI | 51.50 ± 7.85 | 36.22 ± 6.86 | <0.001 ** | 33.84 ± 6.44 | <0.001 ** | 33.15 ± 5.82 | <0.001 ** | |
| FIB-4 | 0.79 ± 0.55 | 0.80 ± 0.47 | 0.761 | 0.83 ± 0.46 | 0.549 | 0.88 ± 0.45 | 0.064 |
| Outcome | OR (95% CI) | p-Value |
|---|---|---|
| HSI | 2.34 (1.16–4.73) | 0.0176 * |
| LDL-C | 2.46 (1.21–5.02) | 0.0131 * |
| HbA1c | 2.31 (0.69–7.73) | 0.1749 |
| TG | 3.92 (0.87–17.66) | 0.0757 |
| Baseline HSI | OR (95% CI) | p-Value |
|---|---|---|
| 35–44 | 1.52 (0.29–8.03) | 0.6254 |
| 45–54 | 3.56 (1.26–10.04) | 0.0167 * |
| ≥55 | 0.94 (0.08–11.15) | 0.9596 |
| Group | OR (95% CI) | p-Value | Model |
|---|---|---|---|
| T2DM | 13.42 (2.86–63.02) | 0.0010 ** | unadjusted |
| 9.12 (1.39–59.82) | 0.0212 * | adjusted | |
| Non-T2DM | 1.11 (0.42–2.95) | 0.8386 | unadjusted |
| 0.70 (0.18–2.77) | 0.6108 | adjusted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, M.K.; Yoon, S.Y.; An, J.A.; Kim, J.S.; Kim, M.-S.; Lee, J.A.; Ko, C.S.; Min, S.H. Association of Rapid Early Weight Loss with One-Year Hepatic Steatosis Improvement After Sleeve Gastrectomy: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 7284. https://doi.org/10.3390/jcm14207284
Jang MK, Yoon SY, An JA, Kim JS, Kim M-S, Lee JA, Ko CS, Min SH. Association of Rapid Early Weight Loss with One-Year Hepatic Steatosis Improvement After Sleeve Gastrectomy: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(20):7284. https://doi.org/10.3390/jcm14207284
Chicago/Turabian StyleJang, Min Kyoung, Si Yeol Yoon, Jin A. An, Ji Soo Kim, Min-Seon Kim, Jung Ah Lee, Chang Seok Ko, and Se Hee Min. 2025. "Association of Rapid Early Weight Loss with One-Year Hepatic Steatosis Improvement After Sleeve Gastrectomy: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 20: 7284. https://doi.org/10.3390/jcm14207284
APA StyleJang, M. K., Yoon, S. Y., An, J. A., Kim, J. S., Kim, M.-S., Lee, J. A., Ko, C. S., & Min, S. H. (2025). Association of Rapid Early Weight Loss with One-Year Hepatic Steatosis Improvement After Sleeve Gastrectomy: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(20), 7284. https://doi.org/10.3390/jcm14207284






